In Vitro Wear Behavior of Knee Implants at Different Load Levels: The Impact of the Test Fluid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Groups
2.2. Implant System
2.3. Wear Simulation
2.4. Analysis Methods
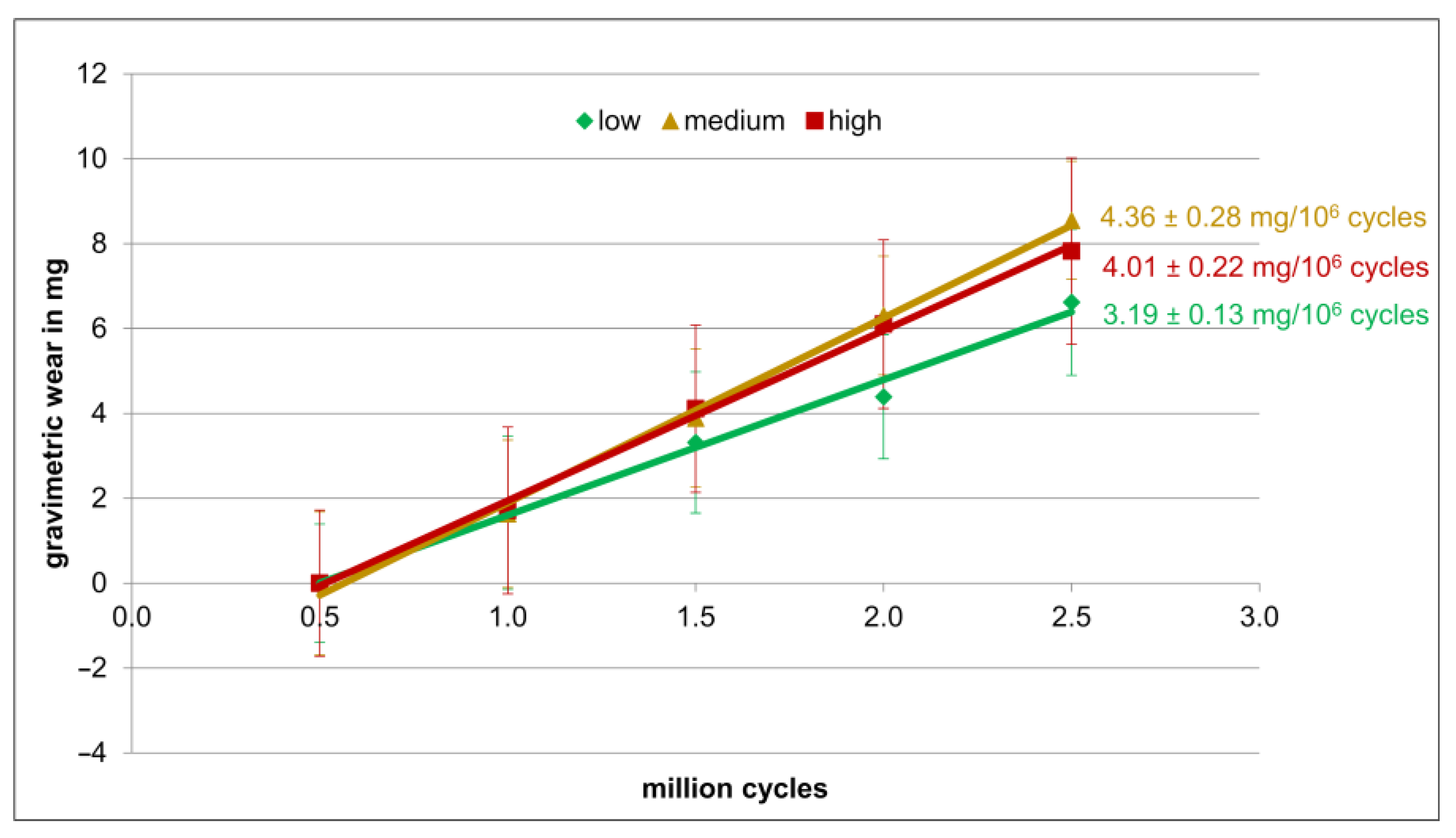
2.4.1. Gravimetric Wear Measurement
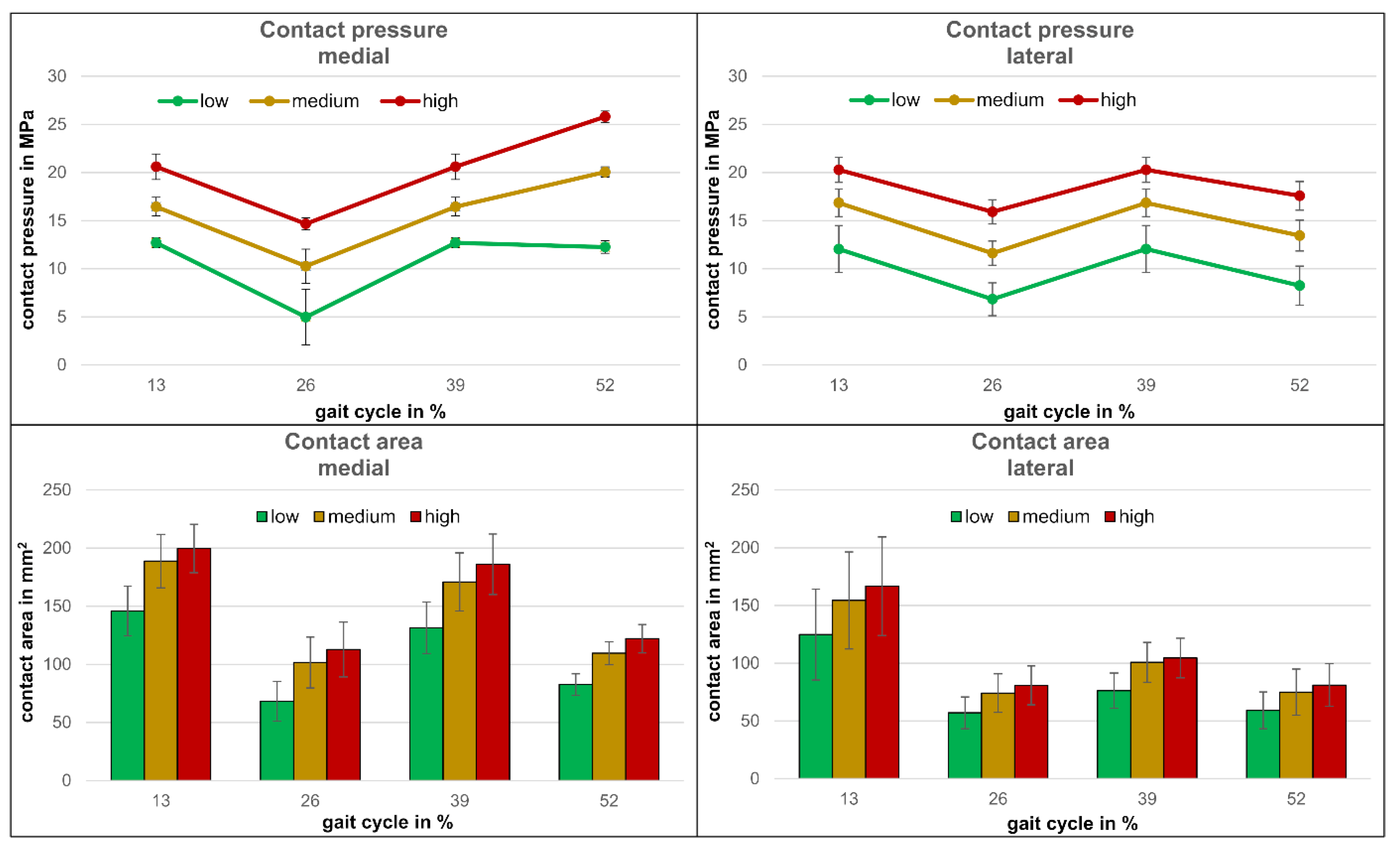
2.4.2. Pressure Measurements
2.4.3. Rheological Measurements
2.5. Statistics
3. Results
3.1. Gravimetric Wear Rates
3.2. Comparison of the Pressure Measurements
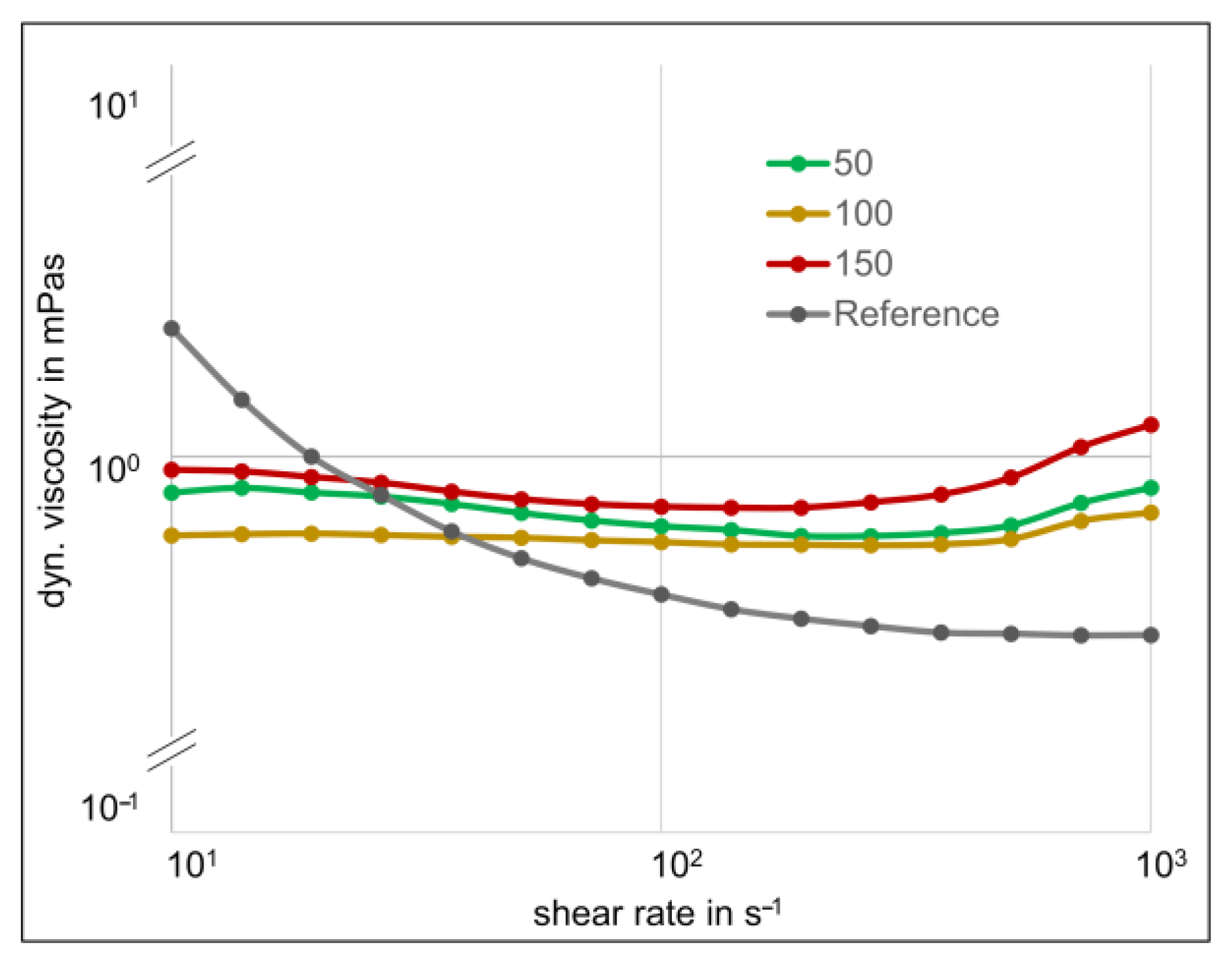
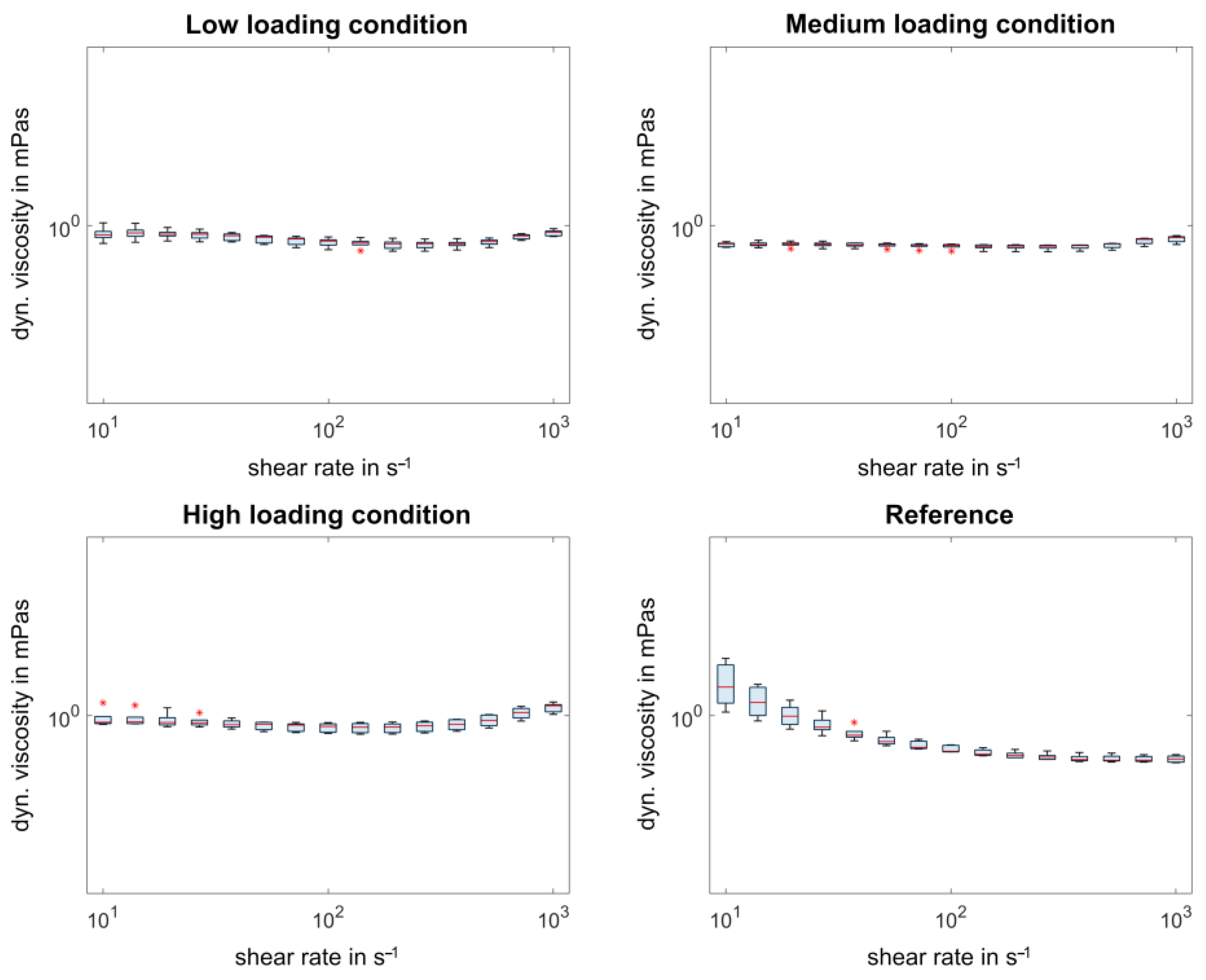
3.3. Comparison of the Rheological Measurements
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grupp, T.M.; Kaddick, C.; Schwiesau, J.; Maas, A.; Stulberg, S.D. Fixed and mobile bearing total knee arthroplasty--influence on wear generation, corresponding wear areas, knee kinematics and particle composition. Clin. Biomech. 2009, 24, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, S.; Harrasser, N.; Schroeder, C.; Mazoochian, F.; Jansson, V. Wear of contemporary total knee replacements—A knee simulator study of six current designs. Clin. Biomech. 2009, 24, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Maag, C.; Cracaoanu, I.; Langhorn, J.; Heldreth, M. Total knee replacement wear during simulated gait with mechanical and anatomic alignments. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine 2021, 235, 515–522. [Google Scholar] [CrossRef] [PubMed]
- ISO 14243-1:2009; Implants for Surgery—Wear of Total Knee-Joint Prostheses—Part 1: Loading and Displacement Parameters for Wear-Testing Machines with Load Control and Corresponding Environmental Conditions for Test. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 14243-2:2009; Implants for Surgery—Wear of Total Knee-Joint Prostheses—Part 2: Methods of Measurement. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 14243-3:2020; Implants for Surgery—Wear of Total Knee-Joint Prostheses — Part 3: Loading and Displacement Parameters for Wear-Testing Machines with Displacement Control and Corresponding Environmental Conditions for Test. International Organization for Standardization: Geneva, Switzerland, 2020.
- Harsha, A.P.; Joyce, T.J. Challenges associated with using bovine serum in wear testing orthopaedic biopolymers. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.M.; Briere, L.K.; Marr, J.; MacDonald, S.J.; Bourne, R.B.; Medley, J.B. Biochemical comparisons of osteoarthritic human synovial fluid with calf sera used in knee simulator wear testing. J. Biomed. Mater. Res. Part A 2010, 94, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Galandáková, A.; Ulrichová, J.; Langová, K.; Hanáková, A.; Vrbka, M.; Hartl, M.; Gallo, J. Characteristics of synovial fluid required for optimization of lubrication fluid for biotribological experiments. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Uhler, M.; Schonhoff, M.; Nees, T.A.; Wonerow, T.; Nuppnau, J.; Mantwill, F.; Kretzer, J.P.; Schroeder, S. Rheological Behavior of Different Calf Sera before, during and after Biomechanical Testing. Lubricants 2022, 10, 224. [Google Scholar] [CrossRef]
- Reinders, J.; Sonntag, R.; Kretzer, J.P. How do gait frequency and serum-replacement interval affect polyethylene wear in knee-wear simulator tests? Journal of materials science. Mater. Med. 2014, 25, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Reinders, J.; Sonntag, R.; Kretzer, J.P. Synovial fluid replication in knee wear testing: An investigation of the fluid volume. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2015, 33, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.M.; Charron, K.D.; Zhao, L.; MacDonald, S.J.; Medley, J.B. Commissioning of a displacement-controlled knee wear simulator and exploration of some issues related to the lubricant. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 736–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Essner, A.; Schmidig, G. The effects of lubricant composition on in vitro wear testing of polymeric acetabular components. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 68, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Abdelgaied, A.; Brockett, C.L.; Liu, F.; Jennings, L.M.; Fisher, J.; Jin, Z. Quantification of the effect of cross-shear and applied nominal contact pressure on the wear of moderately cross-linked polyethylene. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2013, 227, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Saikko, V. Effect of contact pressure on wear and friction of ultra-high molecular weight polyethylene in multidirectional sliding. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2006, 220, 723–731. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.T.; Luo, Y.; Brandt, J.-M. In-vitro and in-silico investigations on the influence of contact pressure on cross-linked polyethylene wear in total knee replacements. Wear 2015, 332–333, 687–693. [Google Scholar] [CrossRef]
- Wonerow, T.; Uhler, M.; Nuppnau, J.; Kretzer, J.P.; Mantwill, F. Rheologic Behavior of Bovine Calf Serum. Materials 2021, 14, 2538. [Google Scholar] [CrossRef] [PubMed]
- Brockett, C.L.; Carbone, S.; Fisher, J.; Jennings, L.M. Influence of conformity on the wear of total knee replacement: An experimental study. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Schwiesau, J.; Schilling, C.; Utzschneider, S.; Jansson, V.; Fritz, B.; Blomer, W.; Grupp, T.M. Knee wear simulation under conditions of highly demanding daily activities--influence on an unicompartmental fixed bearing knee design. Med. Eng. Phys. 2013, 35, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- McEwen, H.M.; Barnett, P.I.; Bell, C.J.; Farrar, R.; Auger, D.D.; Stone, M.H.; Fisher, J. The influence of design, materials and kinematics on the in vitro wear of total knee replacements. J. Biomech. 2005, 38, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Bortel, E.; Charbonnier, B.; Heuberger, R. Development of a Synthetic Synovial Fluid for Tribological Testing. Lubricants 2015, 3, 664–686. [Google Scholar] [CrossRef]
- Scholes, S.C.; Joyce, T.J. In vitro tests of substitute lubricants for wear testing orthopaedic biomaterials. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2013, 227, 693–703. [Google Scholar] [CrossRef] [PubMed]
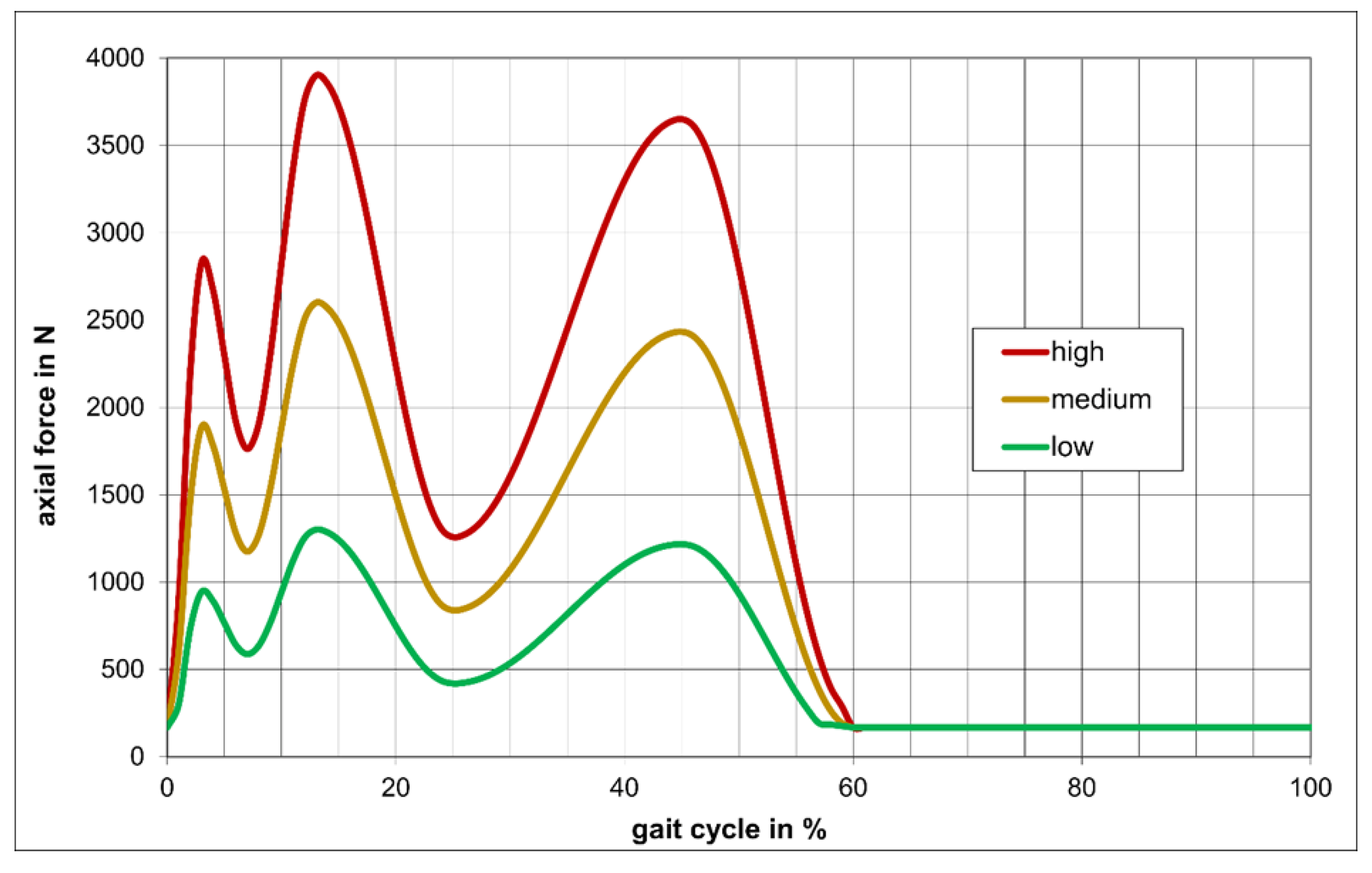
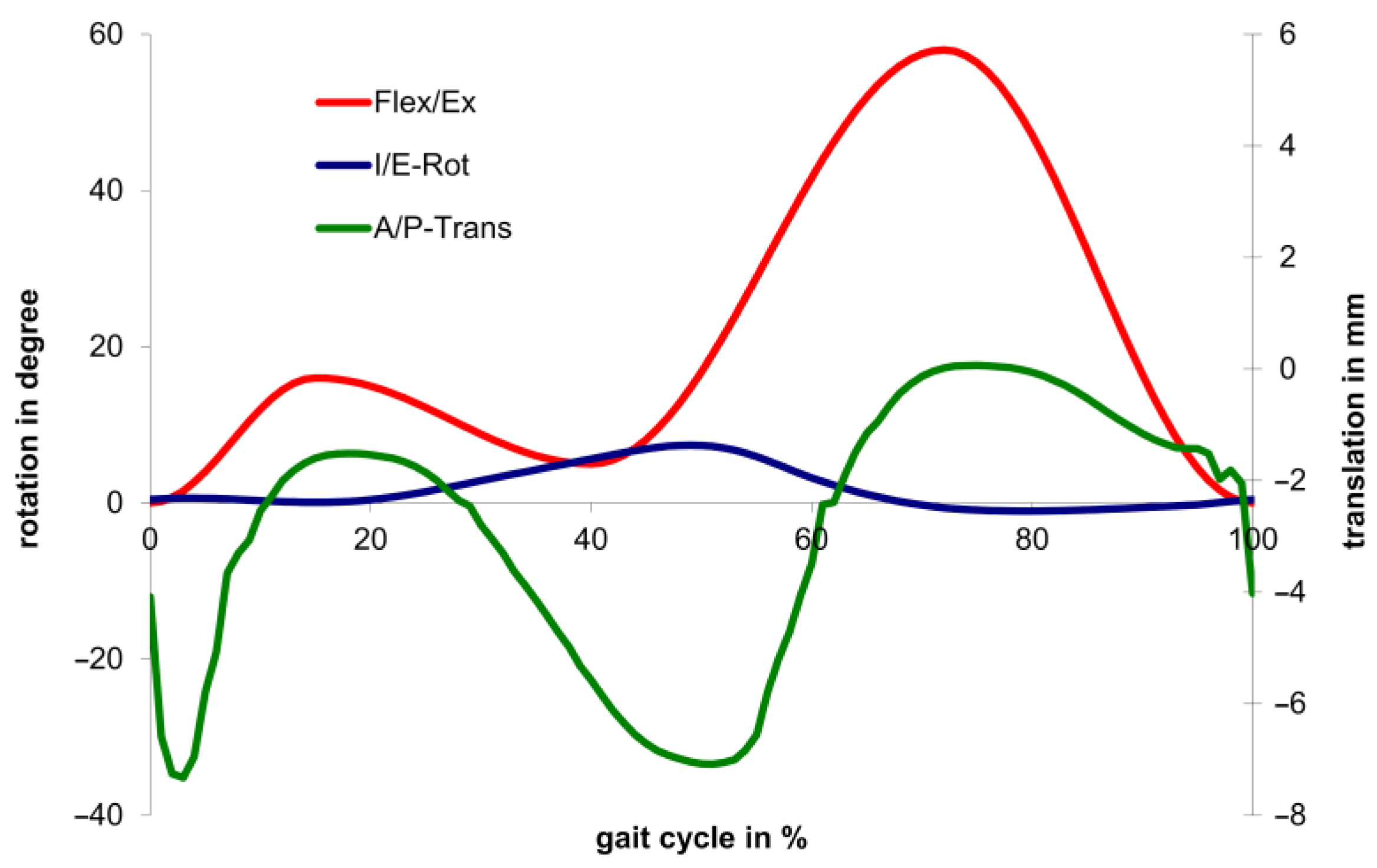
| Gait Cycle in % | Flex/Ex in ° | I/E-Rot in ° | A/P-Trans in mm | Axial Force in N (Low) | Axial Force in N (Medium) | Axial Force in N (High) |
|---|---|---|---|---|---|---|
| 13 | 15.3 | 0.1 | −1.8 | 1300.0 | 2600.0 | 3900.0 |
| 26 | 11.5 | 1.7 | −2.0 | 424.0 | 848.0 | 1272.0 |
| 39 | 5.00 | 5.4 | −5.3 | 1052.4 | 2104.7 | 3157.2 |
| 52 | 21.4 | 7.0 | −7.1 | 709.5 | 1419.0 | 2128.5 |
| Shear Rate in s−1 | Low | Medium | High | Reference |
|---|---|---|---|---|
| 101 | 0.97 ± 0.03 | 0.93 ± 0.01 | 0.99 ± 0.04 | 1.12 ± 0.10 |
| 102 | 0.94 ± 0.02 | 0.92 ± 0.01 | 0.95 ± 0.02 | 0.87 ± 0.01 |
| 103 | 0.97 ± 0.01 | 0.95 ± 0.01 | 1.03 ± 0.02 | 0.84 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schroeder, S.; Uhler, M.; Schonhoff, M.; Nees, T.A.; Wonerow, T.; Nuppnau, J.; Mantwill, F.; Kretzer, J.P. In Vitro Wear Behavior of Knee Implants at Different Load Levels: The Impact of the Test Fluid. Lubricants 2023, 11, 474. https://doi.org/10.3390/lubricants11110474
Schroeder S, Uhler M, Schonhoff M, Nees TA, Wonerow T, Nuppnau J, Mantwill F, Kretzer JP. In Vitro Wear Behavior of Knee Implants at Different Load Levels: The Impact of the Test Fluid. Lubricants. 2023; 11(11):474. https://doi.org/10.3390/lubricants11110474
Chicago/Turabian StyleSchroeder, Stefan, Maximilian Uhler, Mareike Schonhoff, Timo A. Nees, Tanja Wonerow, Jens Nuppnau, Frank Mantwill, and Jan Philippe Kretzer. 2023. "In Vitro Wear Behavior of Knee Implants at Different Load Levels: The Impact of the Test Fluid" Lubricants 11, no. 11: 474. https://doi.org/10.3390/lubricants11110474





