Optimizing Hyaluronan-Based Lubricants for Treating Thoracolumbar Fascia Pathologies: Insights from Tribological and Pharmacokinetic Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Lubricants
2.2. Tribological Setup
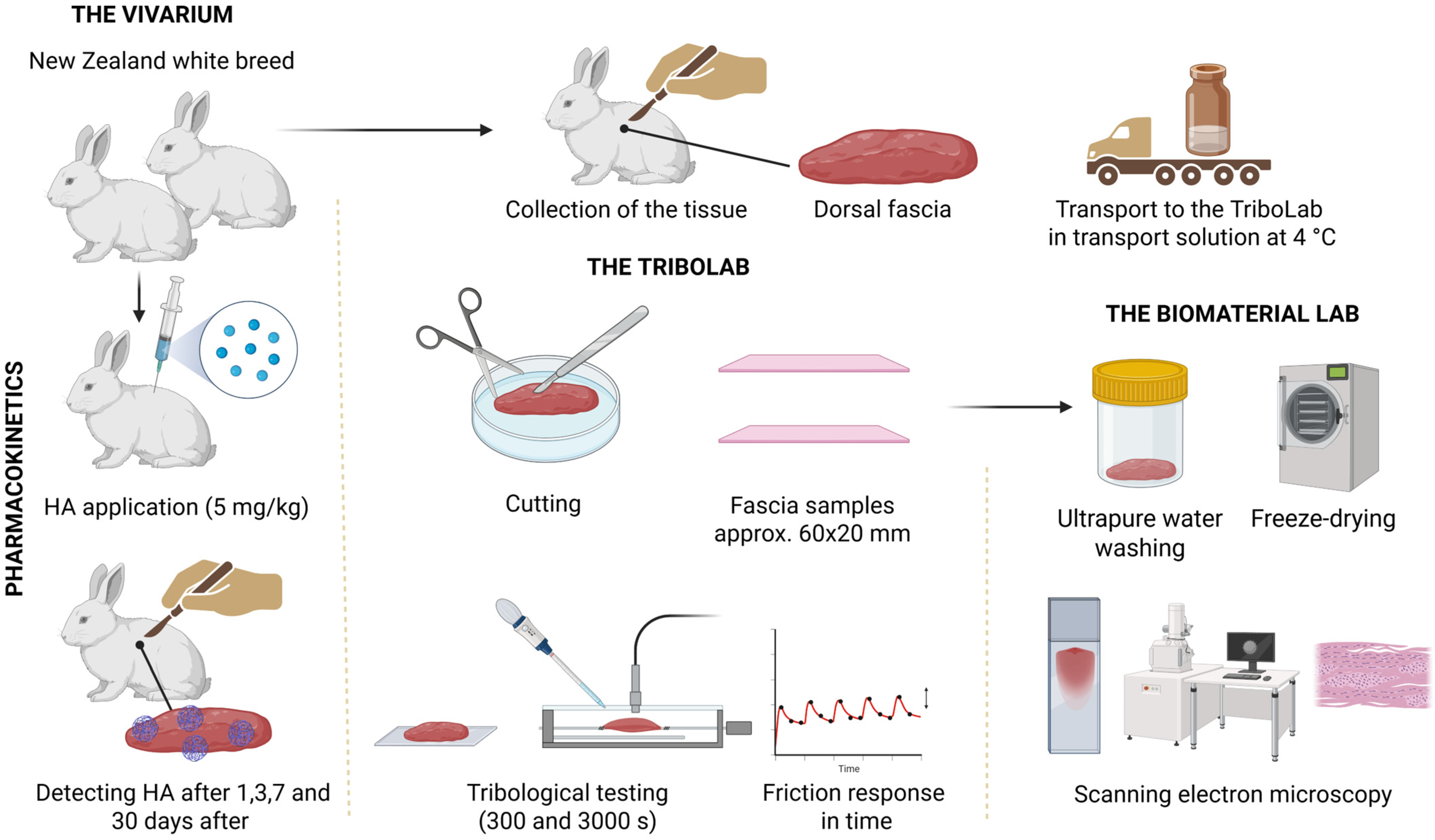
2.3. Preparation of Samples of Rabbit TLF
2.4. Pharmacokinetic Study
2.5. Determination of Isotopically Labelled HA and HA-RED in Rabbit Fascia and Muscle
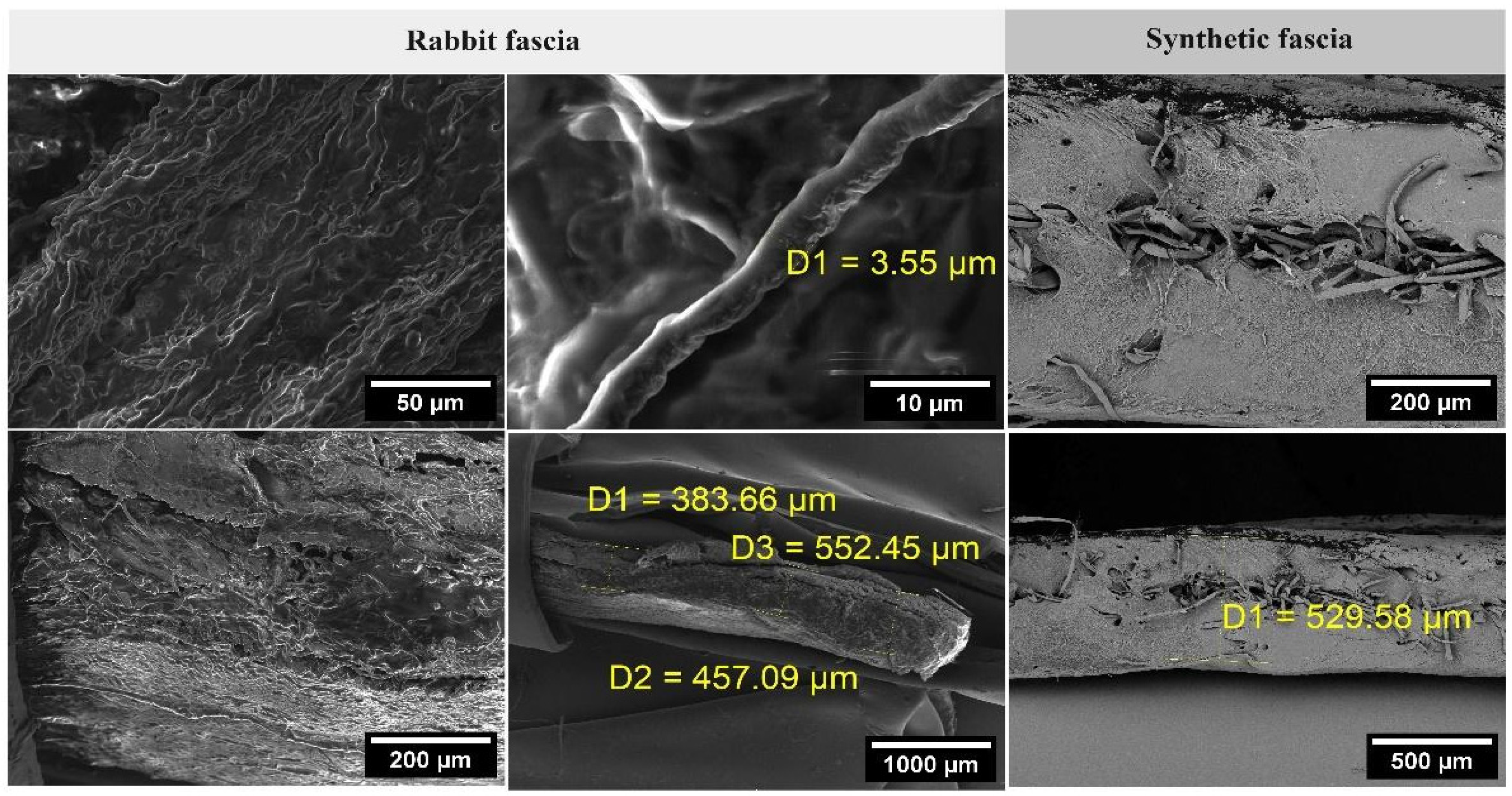
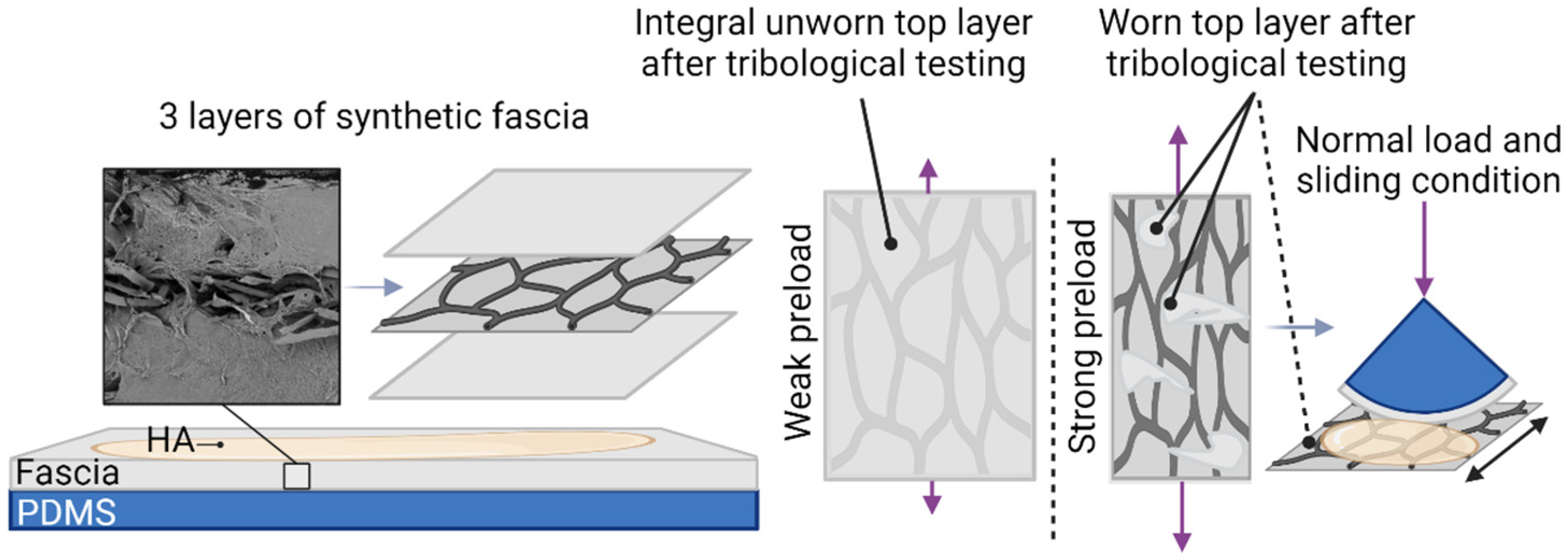
2.6. Preparation of Tribological Samples from Synthetic Fascia
2.7. Statistical Methods
3. Results
3.1. Testing of Preload Effect of Fascia on Friction
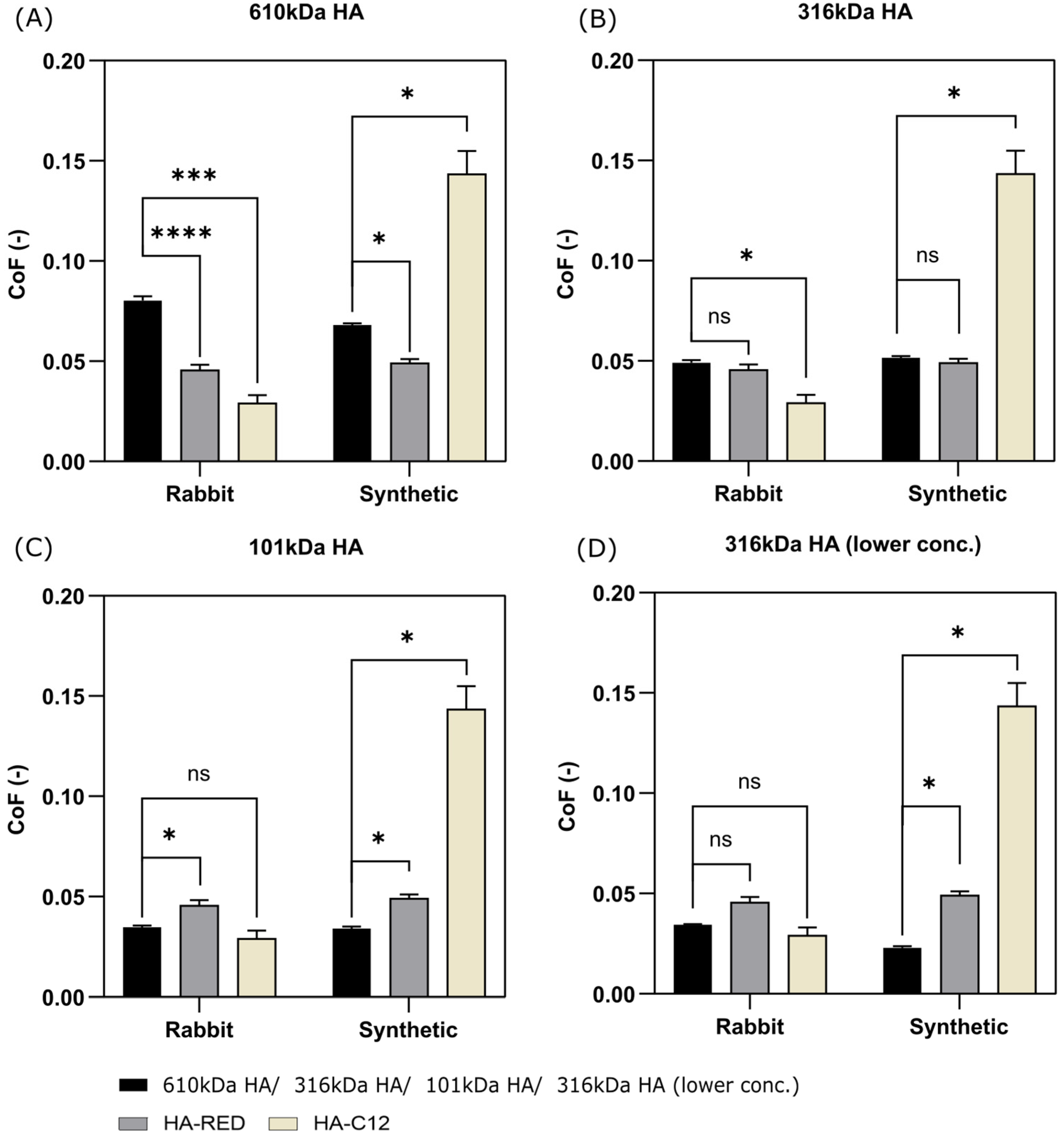
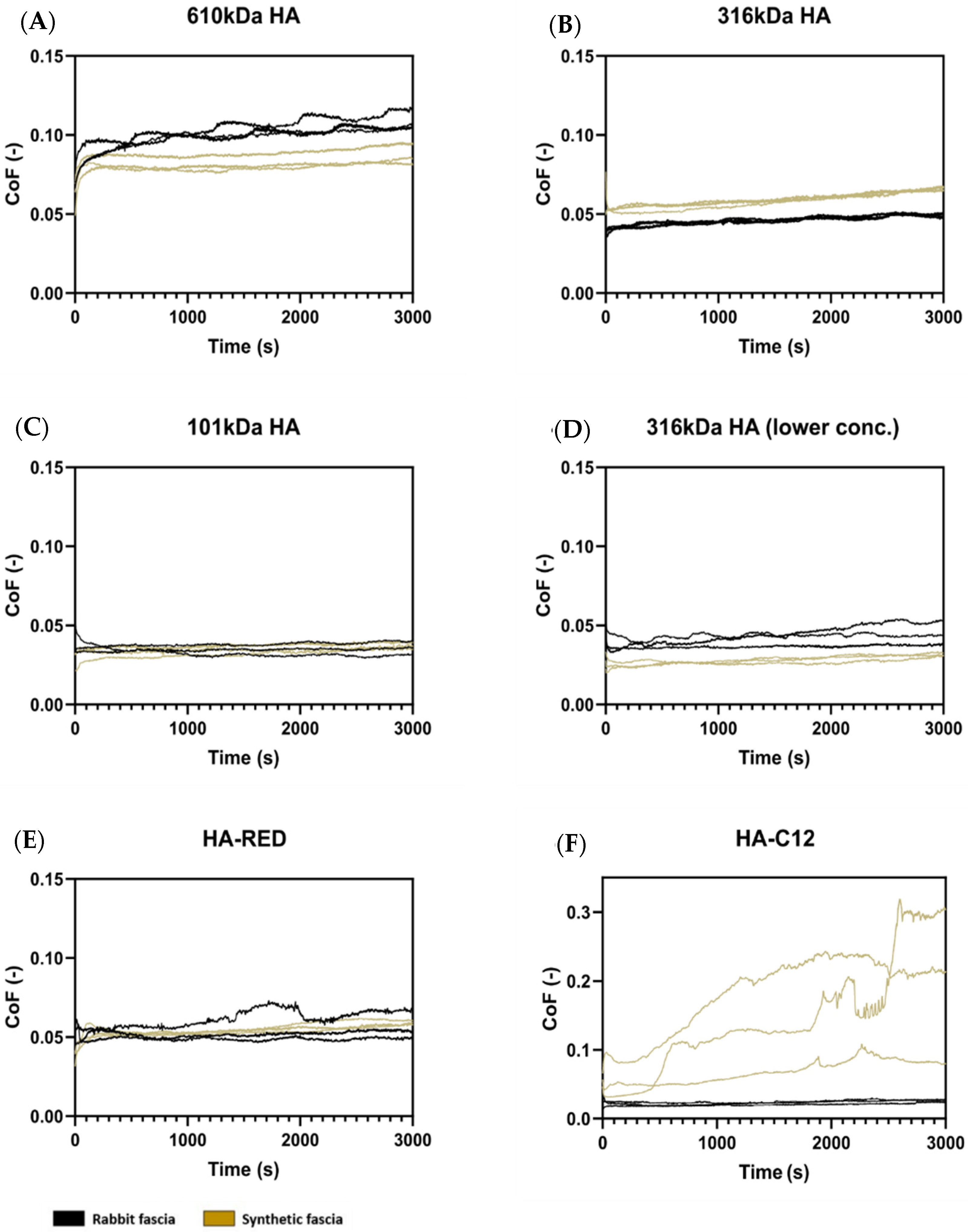
3.2. Short-Term and Long-Term Testing of Fascia Friction
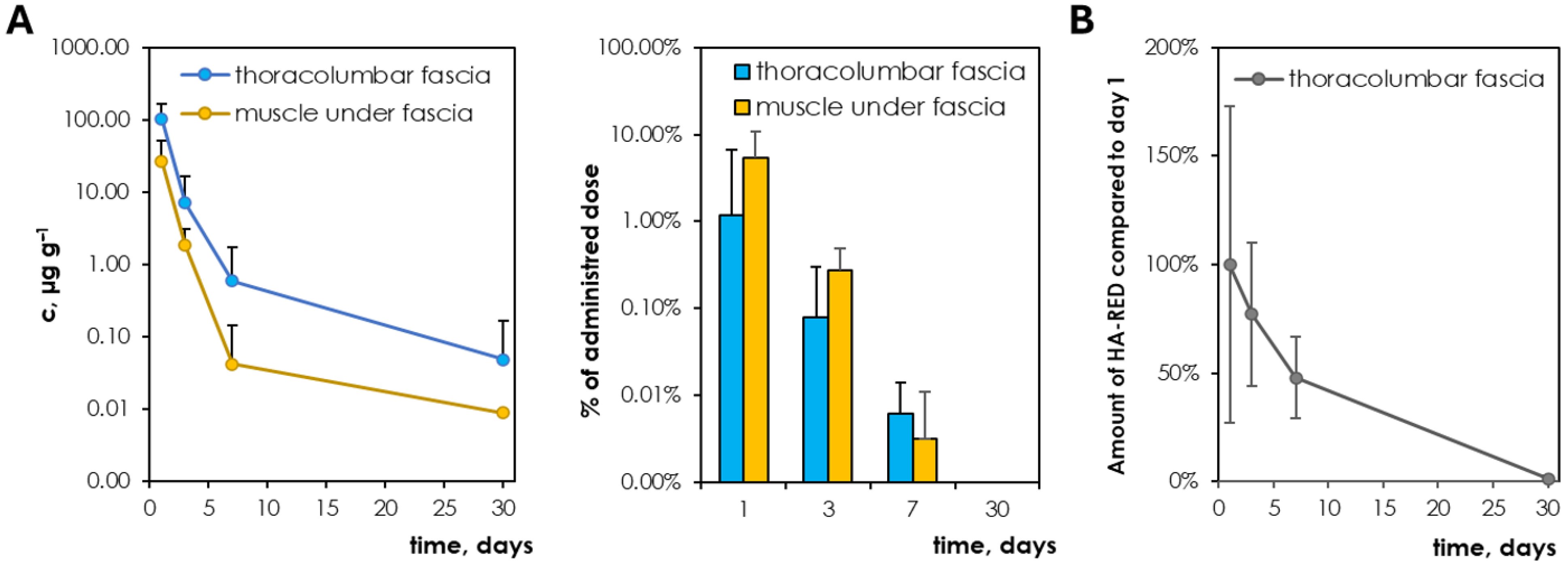
3.3. Pharmacokinetics of 316 kDa HA (Lower Conc.) and HA-RED After Application to the Fascia
4. Discussion
4.1. Influence of Preload of Fascia on Friction
4.2. Selection of Appropriate Properties of HA-Based Solution as a Viscosupplement of TLF Fascia Based on Friction Results
4.3. HA Degradation in Living Organisms and Viscosupplement Confrontation
5. Conclusions
- Tribological model—Optimization of fascia prestressing: The medium rabbit and synthetic fascia prestress ensures that the final friction results will be influenced by the effect of the lubricant without being affected by the degree of fascia tension;
- Lubricants: 316 kDa HA and HA-RED were identified as having the best and most stable tribological properties for fascia lubricants, driven by their MW and concentration;
- Pharmacokinetics of intrafascial HA-based viscosupplementation: Chemically modified HA (HA-RED) is eliminated within 30 days as well as native HA; however, it exhibits a longer residence time compared to native HA.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LBP | lower back pain |
| HA | hyaluronic acid |
| OA | osteoarthritis |
| SD | standard deviation |
| COF | coefficient of friction |
| PDMS | polydimethylsiloxane |
References
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, T.V.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheumatol. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Casato, G.; Stecco, C.; Busin, R. Role of fasciae in nonspecific low back pain. Eur. J. Transl. Myol. 2019, 29, 8330. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Schleip, R.; Klingler, W.; Stecco, C. The Lumbodorsal Fascia as a Potential Source of Low Back Pain: A Narrative Review. Biomed Res. Int. 2017, 2017, 5349620. [Google Scholar] [CrossRef]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan-Naumann, A.C.; Bouffard, N.A.; Konofagou, E.A.; Lee, W.-N.; Triano, J.J.; Henry, S.M. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 622. [Google Scholar] [CrossRef]
- Matteini, P.; Dei, L.; Carretti, E.; Volpi, N.; Goti, A.; Pini, R. Structural Behavior of Highly Concentrated Hyaluronan. Biomacromolecules 2009, 10, 1516–1522. [Google Scholar] [CrossRef]
- Fede, C.; Angelini, A.; Stern, R.; Macchi, V.; Porzionato, A.; Ruggieri, P.; De Caro, R.; Stecco, C. Quantification of hyaluronan in human fasciae: Variations with function and anatomical site. J. Anat. 2018, 233, 552–556. [Google Scholar] [CrossRef]
- Stecco, A.; Meneghini, A.; Stern, R.; Stecco, C.; Imamura, M. Ultrasonography in myofascial neck pain: Randomized clinical trial for diagnosis and follow-up. Surg. Radiol. Anat. 2014, 36, 243–253. [Google Scholar] [CrossRef]
- Stecco, A.; Gesi, M.; Stecco, C.; Stern, R. Fascial Components of the Myofascial Pain Syndrome. Curr. Pain Headache Rep. 2013, 17, 352. [Google Scholar] [CrossRef]
- Stecco, A.; Stern, R.; Fantoni, I.; De Caro, R.; Stecco, C. Fascial Disorders: Implications for Treatment. Phys. Med. Rehabil. 2016, 8, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Schleip, R.; Yucesoy, C.A.; Banzer, W. Not merely a protective packing organ? A review of fascia and its force transmission capacity. J. Appl. Physiol. 2018, 124, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, H.; Mabuchi, K.; Itokawa, T.; Okajima, Y.; Suzuki, T.; Hori, Y. Evaluation of the Lubricating Effect of Hyaluronic Acid on Contact Lenses Using a Pendulum-Type Friction Tester Under Mimicking Physiological Conditions. Eye Contact Lens 2022, 48, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Nešporová, K.; Matonohová, J.; Husby, J.; Toropitsyn, E.; Stupecká, L.D.; Husby, A.; Kleplová, T.S.; Streďanská, A.; Šimek, M.; Nečas, D.; et al. Injecting hyaluronan in the thoracolumbar fascia: A model study. Int. J. Biol. Macromol. 2023, 253, 126879. [Google Scholar] [CrossRef]
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical Modification of Hyaluronan and Their Biomedical Applications. Front. Chem. 2022, 10, 830671. [Google Scholar] [CrossRef]
- Griesser, J.; Hetényi, G.; Bernkop-Schnürch, A. Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry Behind to Product Developments—What Are the Capabilities? Polymers 2018, 10, 243. [Google Scholar] [CrossRef]
- Ikeda, J.; Zhao, C.; Sun, Y.-L.; An, K.-N.; Amadio, P.C. Carbodiimide-Derivatized Hyaluronic Acid Surface Modification of Lyophilized Flexor Tendon: A biomechanical study in a canine in vitro model. JBJS 2010, 92, 388–395. [Google Scholar] [CrossRef]
- Huerta-Ángeles, G.; Brandejsová, M.; Kopecká, K.; Ondreáš, F.; Medek, T.; Židek, O.; Kulhánek, J.; Vagnerová, H.; Velebný, V. Synthesis and Physicochemical Characterization of Undecylenic Acid Grafted to Hyaluronan for Encapsulation of Antioxidants and Chemical Crosslinking. Polymers 2020, 12, 35. [Google Scholar] [CrossRef]
- Huin-Amargier, C.; Marchal, P.; Payan, E.; Netter, P.; Dellacherie, E. New physically and chemically crosslinked hyaluronate (HA)-based hydrogels for cartilage repair. J. Biomed. Mater. Res. Part A 2006, 76A, 416–424. [Google Scholar] [CrossRef]
- Černohlávek, M.; Brandejsová, M.; Štěpán, P.; Vagnerová, H.; Hermannová, M.; Kopecká, K.; Kulhánek, J.; Nečas, D.; Vrbka, M.; Velebný, V.; et al. Insight into the Lubrication and Adhesion Properties of Hyaluronan for Ocular Drug Delivery. Biomolecules 2021, 11, 1431. [Google Scholar] [CrossRef]
- Streďanská, A.; Nečas, D.; Vrbka, M.; Suchánek, J.; Matonohová, J.; Toropitsyn, E.; Hartl, M.; Křupka, I.; Nešporová, K. Understanding frictional behavior in fascia tissues through tribological modeling and material substitution. J. Mech. Behav. Biomed. Mater. 2024, 155, 106566. [Google Scholar] [CrossRef] [PubMed]
- Buffa, R.; Klejch, T.; Hermannová, M.; Hejlová, L.; Svozil, V.; Vágnerová, H.; Škubalová, H.; Nešporová, K.; Velebný, V. Modified hyaluronic acid with enhanced resistance to degradation. Carbohydr. Polym. 2023, 320, 121241. [Google Scholar] [CrossRef] [PubMed]
- Šimek, M.; Nešporová, K.; Kocurková, A.; Foglová, T.; Ambrožová, G.; Velebný, V.; Kubala, L.; Hermannová, M. How the molecular weight affects the in vivo fate of exogenous hyaluronan delivered intravenously: A stable-isotope labelling strategy. Carbohydr. Polym. 2021, 263, 117927. [Google Scholar] [CrossRef] [PubMed]
- ISO 3696:1987; Water for Analytical Laboratory Use—Specification and Test Methods. International Organization for Standardization: Geneva, Switzerland, 1987.
- Šimek, M.; Turková, K.; Schwarzer, M.; Nešporová, K.; Kubala, L.; Hermannová, M.; Foglová, T.; Šafránková, B.; Šindelář, M.; Šrůtková, D.; et al. Molecular weight and gut microbiota determine the bioavailability of orally administered hyaluronic acid. Carbohydr. Polym. 2023, 313, 120880. [Google Scholar] [CrossRef]
- Schleip, R.; Klingler, W.; Lehmann-Horn, F. Active fascial contractility: Fascia may be able to contract in a smooth muscle-like manner and thereby influence musculoskeletal dynamics. Med. Hypotheses 2005, 65, 273–277. [Google Scholar] [CrossRef]
- Stecco, C.; Pavan, P.G.; Porzionato, A.; Macchi, V.; Lancerotto, L.; Carniel, E.L.; Natail, A.N.; De Caro, R. Mechanics of crural fascia: From anatomy to constitutive modelling. Surg. Radiol. Anat. 2009, 31, 523–529. [Google Scholar] [CrossRef]
- Benetazzo, L.; Bizzego, A.; De Caro, R.; Frigo, G.; Guidolin, D.; Stecco, C. 3D reconstruction of the crural and thoracolumbar fasciae. Surg. Radiol. Anat. 2011, 33, 855–862. [Google Scholar] [CrossRef]
- Kumka, M.; Bonar, J. Fascia: A morphological description and classification system based on a literature review. J. Can. Chiropr. Assoc. 2012, 56, 179–191. [Google Scholar]
- Yahia, L.H.; Rhalmi, S.; Newman, N.; Isler, M. Sensory innervation of human thoracolumbar fascia. Acta Orthop. Scand. 2009, 63, 195–197. [Google Scholar] [CrossRef]
- Stecco, C.; Porzionato, A.; Lancerotto, L.; Stecco, A.; Macchi, V.; Ann Day, J.; De Caro, R. Histological study of the deep fasciae of the limbs. J. Bodyw. Mov. Ther. 2008, 12, 225–230. [Google Scholar] [CrossRef]
- Tiwari, A.; Dorogin, L.; Bennett, A.I.; Schulze, K.D.; Sawyer, W.G.; Tahir, M.; Heinrich, G.; Persson, B.N.J. The effect of surface roughness and viscoelasticity on rubber adhesion. Soft Matter 2017, 13, 3602–3621. [Google Scholar] [CrossRef] [PubMed]
- Ido, T.; Yamaguchi, T.; Shibata, K.; Matsuki, K.; Yumii, K.; Hokkirigawa, K. Sliding friction characteristics of styrene butadiene rubbers with varied surface roughness under water lubrication. Tribol. Int. 2019, 133, 230–235. [Google Scholar] [CrossRef]
- Anadere, I.; Chmiel, H.; Laschner, W. Viscoelasticity of “normal” and pathological synovial fluid. Biorheology 1979, 16, 179–184. [Google Scholar] [PubMed]
- Balazs, E.A. Viscosupplementation for treatment of osteoarthritis: From initial discovery to current status and results. Surg. Technol. 2004, 12, 278–289. [Google Scholar]
- Chang, D.P.; Abu-Lail, N.I.; Coles, J.M.; Guilak, F.; Jay, G.D.; Zauscher, S. Friction force microscopy of lubricin and hyaluronic acid between hydrophobic and hydrophilic surfaces. Soft Matter 2009, 5, 3438–3445. [Google Scholar] [CrossRef]
- Greene, G.W.; Zappone, B.; Banquy, X.; Lee, D.W.; Söderman, O.; Topgaard, D.; Israelachvili, J.N. Hyaluronic acid–collagen network interactions during the dynamic compression and recovery of cartilage. Soft Matter 2012, 8, 9906–9914. [Google Scholar] [CrossRef]
- Jay, G.D.; Torres, J.R.; Warman, M.L.; Laderer, M.C.; Breuer, K.S. The role of lubricin in the mechanical behavior of synovial fluid. Proc. Natl. Acad. Sci. USA 2007, 104, 6194–6199. [Google Scholar] [CrossRef]
- Hilšer, P.; Suchánková, A.; Mendová, K.; Filipič, K.E.; Daniel, M.; Vrbka, M. A new insight into more effective viscosupplementation based on the synergy of hyaluronic acid and phospholipids for cartilage friction reduction. Biotribology 2021, 25, 100166. [Google Scholar] [CrossRef]
- Herzog, M.; Li, L.; Galla, H.-J.; Winter, R. Effect of hyaluronic acid on phospholipid model membranes. Colloids Surf. B Biointerfaces 2019, 173, 327–334. [Google Scholar] [CrossRef]
- Dėdinaitė, A.; Wieland, D.C.F.; Bełdowski, P.; Claesson, P.M. Biolubrication synergy: Hyaluronan—Phospholipid interactions at interfaces. Adv. Colloid Interface Sci. 2019, 274, 102050. [Google Scholar] [CrossRef]
- Rebenda, D.; Vrbka, M.; Čípek, P.; Toropitsyn, E.; Nečas, D.; Pravda, M.; Hartl, M. On the Dependence of Rheology of Hyaluronic Acid Solutions and Frictional Behavior of Articular Cartilage. Materials 2020, 13, 2659. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A. Viscoelastic properties of hyaluronic acid and biological lubrication. Univ. Mich. Med. Cent. J. 1968, 255–259. [Google Scholar]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- Han, M.; Russo, M.J.; Desroches, P.E.; Silva, S.M.; Quigley, A.F.; Kapsa, R.M.; Moulton, S.E.; Greene, G.W. Calcium ions have a detrimental impact on the boundary lubrication property of hyaluronic acid and lubricin (PRG-4) both alone and in combination. Colloids Surf. B Biointerfaces 2024, 234, 113741. [Google Scholar] [CrossRef]
- Das, S.; Banquy, X.; Zappone, B.; Greene, G.W.; Jay, G.D.; Israelachvili, J.N. Synergistic Interactions between Grafted Hyaluronic Acid and Lubricin Provide Enhanced Wear Protection and Lubrication. Biomacromolecules 2013, 14, 1669–1677. [Google Scholar] [CrossRef]
- Fraser, J.R.F.; Laurent, T.C.; Pertoft, H.; Baxter, E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem. J. 1981, 200, 415–424. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Schiller, J.; Mendichi, R. Hyaluronic Acid: Its Function and Degradation in in vivo Systems. Bioact. Nat. Prod. (Part N) 2008, 34, 789–882. [Google Scholar] [CrossRef]
- Reichenbach, S.; Blank, S.; Rutjes, A.W.; Shang, A.; King, E.A.; Dieppe, P.A.; Jüni, P.; Trelle, S. Hylan versus hyaluronic acid for osteoarthritis of the knee: A systematic review and meta-analysis. Arthritis Care Res. 2007, 57, 1410–1418. [Google Scholar] [CrossRef]
- Klejch, T.; Buffa, R.; Šimek, M.; Nešporová, K.; Exnerová, A.; Bednařík, J.; Brandejsová, M.; Vágnerová, H.; Fiala, F.; Velebný, V. Enzymatically stable unsaturated hyaluronan-derived oligosaccharides with selective cytostatic properties. Carbohydr. Polym. 2024, 336, 122129. [Google Scholar] [CrossRef]
- Larsen, N.E.; Dursema, H.D.; Pollak, C.T.; Skrabut, E.M. Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 457–462. [Google Scholar] [CrossRef]
- Trigkilidas, D.; Anand, A. The effectiveness of hyaluronic acid intra-articular injections in managing osteoarthritic knee pain. Ann. R. Coll. Surg. Engl. 2013, 95, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.; Basehore, B.M.; Goyal, A.; Zito, P.M. Hyaluronic Acid. Statpearls [Internet]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482440/ (accessed on 19 June 2024).
- Stecco, C.; Fede, C.; Macchi, V.; Porzionato, A.; Petrelli, L.; Biz, C.; Stern, R.; De Caro, R. The fasciacytes: A new cell devoted to fascial gliding regulation. Clin. Anat. 2018, 31, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Vertuani, S.; Panozzo, G.; Pecorelli, A.; Valacchi, G.; Manfredini, S. Novel Artificial Tears Containing Cross-Linked Hyaluronic Acid: An In Vitro Re-Epithelialization Study. Molecules 2017, 22, 2104. [Google Scholar] [CrossRef]
- Owen, S.C.; Kuo, J.-W.; Prestwich, G.D. 2.14 Hyaluronic Acid. Compr. Biomater. II 2017, 2, 306–331. [Google Scholar] [CrossRef]

| Designation | Molecular Weight (kDa) | Degree of Substitution | Concentration (mg/mL) | Viscosity (mPa·s, 37 °C) |
|---|---|---|---|---|
| 610 kDa HA | 610 | - | 20 | 1191 |
| 316 kDa HA | 316 | - | 20 | 202 |
| 101 kDa HA | 101 | - | 20 | 27 |
| 316 kDa HA (lower conc.) | 316 | - | 10 | 59 |
| HA-RED | 275 | 18% | 20 | 122 |
| HA-C12 | 318 | 9.1% | 3 | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streďanská, A.; Šimek, M.; Matonohová, J.; Nečas, D.; Vrbka, M.; Suchánek, J.; Pavliňáková, V.; Vojtová, L.; Hartl, M.; Křupka, I.; et al. Optimizing Hyaluronan-Based Lubricants for Treating Thoracolumbar Fascia Pathologies: Insights from Tribological and Pharmacokinetic Studies. Lubricants 2025, 13, 184. https://doi.org/10.3390/lubricants13040184
Streďanská A, Šimek M, Matonohová J, Nečas D, Vrbka M, Suchánek J, Pavliňáková V, Vojtová L, Hartl M, Křupka I, et al. Optimizing Hyaluronan-Based Lubricants for Treating Thoracolumbar Fascia Pathologies: Insights from Tribological and Pharmacokinetic Studies. Lubricants. 2025; 13(4):184. https://doi.org/10.3390/lubricants13040184
Chicago/Turabian StyleStreďanská, Alexandra, Matěj Šimek, Jana Matonohová, David Nečas, Martin Vrbka, Jakub Suchánek, Veronika Pavliňáková, Lucy Vojtová, Martin Hartl, Ivan Křupka, and et al. 2025. "Optimizing Hyaluronan-Based Lubricants for Treating Thoracolumbar Fascia Pathologies: Insights from Tribological and Pharmacokinetic Studies" Lubricants 13, no. 4: 184. https://doi.org/10.3390/lubricants13040184
APA StyleStreďanská, A., Šimek, M., Matonohová, J., Nečas, D., Vrbka, M., Suchánek, J., Pavliňáková, V., Vojtová, L., Hartl, M., Křupka, I., & Nešporová, K. (2025). Optimizing Hyaluronan-Based Lubricants for Treating Thoracolumbar Fascia Pathologies: Insights from Tribological and Pharmacokinetic Studies. Lubricants, 13(4), 184. https://doi.org/10.3390/lubricants13040184







