Behavioral Responses of the Invasive Fly Philornis downsi to Stimuli from Bacteria and Yeast in the Laboratory and the Field in the Galapagos Islands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Traps and Baits
2.2. Determination of Community Composition of Bacteria and Yeasts
2.3. Proboscis Extension Response Assay
2.4. Statistical Analysis
3. Results
3.1. Community Composition of Bacterial and Yeast Baits Used in Assays
3.2. Trapping
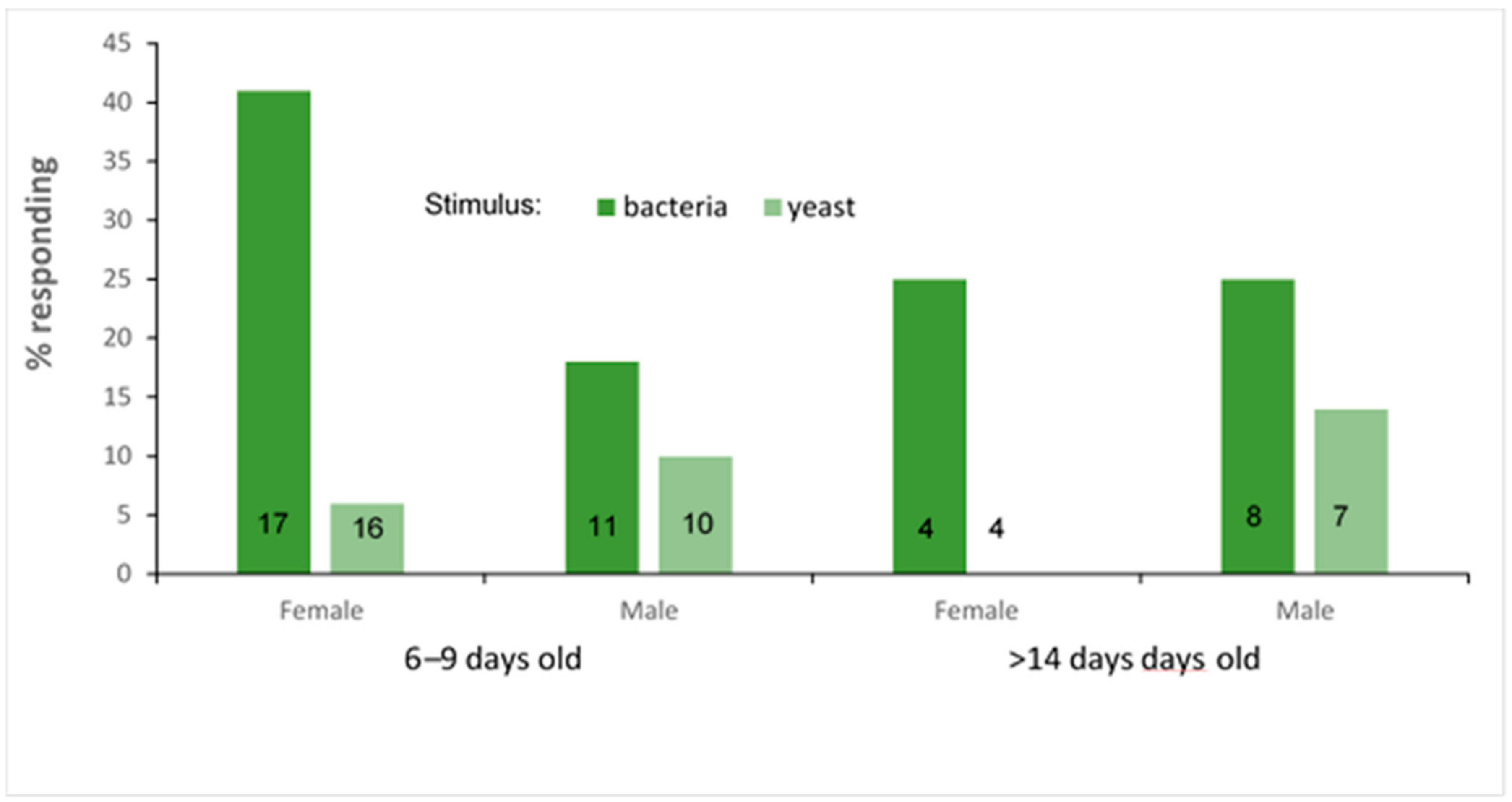
3.3. PER Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Causton, C.E.; Peck, S.B.; Sinclair, B.J.; Roque-Albedo, L.; Hodgson, C.J.; Landry, B. Alien insects: Threats and implications for the conservation of the Galapagos Islands. Ann. Entomol. Soc. Amer. 2006, 99, 121–143. [Google Scholar] [CrossRef]
- Fessl, B.; Heimpel, G.E.; Causton, C.E. Invasion of an Avian Nest Parasite, Philornis downsi, to the Galapagos Islands: Colonization History, Adaptations to Novel Ecosystems, and Conservation Challenges. In Disease Ecology: Galapagos Birds and Their Parasites; Parker, P.G., Ed.; Springer International Publishing: New York, NY, USA, 2018; pp. 213–266. [Google Scholar]
- Kleindorfer, S.; Dudaniec, R.Y. Host-parasite ecology, behavior and genetics: A review of the introduced fly parasite Philornis downsi and its Darwin’s finch hosts. BMC Zool. 2016, 1, 1. [Google Scholar] [CrossRef]
- Kleindorfer, S.; Sulloway, F.J. Naris deformation in Darwin’s finches: Experimental and historical evidence for a post-1960s arrival of the parasite Philornis downsi. Glob. Ecol. Conserv. 2016, 7, 122–131. [Google Scholar] [CrossRef]
- McNew, S.M.; Clayton, D.H. Alien invasion: Biology of philornis flies highlighting philornis downsi, an introduced Parasite of Galapagos birds. Ann. Rev. Entomol. 2018, 63, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Fessl, B.; Couri, M.S.; Tebbich, S. Philornis downsi Dodge & Aitken, new to the Galapagos Islands (Diptera, Muscidae). Stud. Dipterol. 2001, 8, 317–322. [Google Scholar]
- Dvorak, M.; Nemeth, E.; Wendelin, B.; Herrera, P.; Mosquera, D.; Anchundia, D.; Sevilla, C.; Tebbich, S.; Fessl, B. Conservation status of landbirds on Floreana: The smallest inhabited Galapagos Island. J. Field Ornithol. 2017, 88, 132–145. [Google Scholar] [CrossRef]
- Cimadom, A.; Ulloa, A.; Meidl, P.; Zottl, M.; Zottl, E.; Fessl, B.; Nemeth, E.; Dvorak, M.; Cunninghame, F.; Tebbich, S. Invasive parasites, habitat change and heavy rainfall reduce breeding success in Darwin’s Finches. PLoS ONE 2014, 9, e107518. [Google Scholar] [CrossRef]
- Fessl, B.; Young, H.G.; Young, R.P.; Rodriguez-Matamoros, J.; Dvorak, M.; Tebbich, S.; Fa, J.E. How to save the rarest Darwin’s finch from extinction: The mangrove finch on Isabela Island. Phil. Trans. R. Soc. B 2010, 365, 1019–1030. [Google Scholar] [CrossRef]
- Causton, C.; Cunninghame, F.; Tapia, W. Management of the avian parasite Philornis downsi in the Galapagos Islands: A collaborative and strategic action plan. In Galapagos Report 2011–2012; Charles Darwin Foundation: Puerto Ayora, Ecuador, 2013; pp. 167–173. [Google Scholar]
- Cunninghame, F.; Fessl, B.; Sevilla, C.; Young, H.G.; La Greco, N. Long-term conservation management to save the critically endangered mangrove finch (Camarhynchus heliobates). In Galapagos Report 2015–2016; Charles Darwin Foundation: Puerto Ayora, Ecuador, 2017. [Google Scholar]
- Knutie, S.A.; McNew, S.M.; Bartlow, A.W.; Vargas, D.A.; Clayton, D.H. Darwin’s finches combat introduced nest parasites with fumigated cotton. Curr. Biol. 2014, 24, R355–R356. [Google Scholar] [CrossRef]
- Cha, D.H.; Mieles, A.E.; Lahuatte, P.F.; Cahuana, A.; Lincango, M.P.; Causton, C.E.; Tebbich, S.; Cimadom, A.; Teale, S.A. Identification and optimization of microbial attractants for Philornis downsi, an invasive fly parasitic on Galapagos birds. J. Chem. Ecol. 2016, 42, 1101–1111. [Google Scholar] [CrossRef]
- Bulgarella, M.; Quiroga, M.A.; Boulton, R.A.; Ramirez, I.E.; Moon, R.D.; Causton, C.E.; Heimpel, G.E. Life cycle and host specificity of the parasitoid Conura annulifera (Hymenoptera: Chalcididae), a potential biological control agent of Philornis downsi (Diptera: Muscidae) in the Galapagos Islands. Ann. Entomol. Soc. Amer. 2017, 110, 317–328. [Google Scholar] [CrossRef]
- Lahuatte, P.F.; Lincango, M.P.; Heimpel, G.E.; Causton, C.E. Rearing larvae of the Avian Nest Parasite, Philornis downsi (Diptera: Muscidae), on Chicken Blood-Based Diets. J. Insect Sci. 2016, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Ann. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Zindel, R.; Gottlieb, Y.; Aebi, A. Arthropod symbioses: A neglected parameter in pest- and disease-control programmes. J. Appl. Ecol. 2011, 48, 864–872. [Google Scholar] [CrossRef]
- Jurkevitch, E. Riding the Trojan horse: Combating pest insects with their own symbionts. Microb. Biotechnol. 2011, 4, 620–627. [Google Scholar] [CrossRef]
- Yuval, B.; Ben-Ami, E.; Behar, A.; Ben-Yosef, M.; Jurkevitch, E. The Mediterranean fruit fly and its bacteria—Potential for improving sterile insect technique operations. J. Appl Entomol. 2013, 137, 39–42. [Google Scholar] [CrossRef]
- Ben-Ami, E.; Yuval, B.; Jurkevitch, E. Manipulation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. ISME J. 2010, 4, 28–37. [Google Scholar]
- Gavriel, S.; Gazit, Y.; Yuval, B. Remating by female Mediterranean fruit flies (Ceratitis capitata, Diptera: Tephritidae): Temporal patterns and modulation by male condition. J. Insect Physiol. 2009, 55, 637–642. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- Leitao-Goncalves, R.; Carvalho-Santos, Z.; Francisco, A.P.; Fioreze, G.T.; Anjos, M.; Baltazar, C.; Elias, A.P.; Itskov, P.M.; Piper, M.D.W.; Ribeiro, C. Commensal bacteria and essential amino acids control food choice behavior and reproduction. PLoS Biol. 2017, 15, e2000862. [Google Scholar] [CrossRef]
- Wong, A.C.N.; Wang, Q.P.; Morimoto, J.; Senior, A.M.; Lihoreau, M.; Neely, G.G.; Simpson, S.J.; Ponton, F. Gut microbiota modifies olfactory-guided microbial preferences and foraging decisions in Drosophila. Curr. Biol. 2017, 27, 2397. [Google Scholar] [CrossRef] [PubMed]
- Uriel, Y.; Gries, R.; Tu, L.; Carroll, C.; Zhai, H.; Moore, M.; Gries, G. The fly factor phenomenon is mediated by interkingdom signaling between bacterial symbionts and their blow fly hosts. Insect Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Akami, M.; Andongma, A.A.; Chen, Z.Z.; Nan, J.; Khaeso, K.; Jurkevitch, E.; Niu, C.Y.; Yuval, B. Intestinal bacteria modulate the foraging behavior of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Broce, A.; Zurek, L. Role of bacteria in the oviposition behaviour and larval development of stable flies. Med. Vet. Entomol. 2006, 20, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Babor, D.; Duthie, B.; Babor, E.-M.; Moore, M.; Gries, G. Proliferating bacterial symbionts on house fly eggs affect oviposition behaviour of adult flies. Anim. Behav. 2007, 74, 81–92. [Google Scholar] [CrossRef]
- Lam, K.; Tsang, M.; Labrie, A.; Gries, R.; Gries, G. Semiochemical-Mediated oviposition avoidance by female house flies, Musca domestica, on animal feces colonized with harmful fungi. J. Chem. Ecol. 2010, 36, 141–147. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Crippen, T.L.; Tarone, A.M.; Singh, B.; Adams, K.; Rezenom, Y.H.; Benbow, M.E.; Flores, M.; Longnecker, M.; Pechal, J.L.; et al. Interkingdom responses of flies to bacteria mediated by fly physiology and bacterial quorum sensing. Anim. Behav. 2012, 84, 1449–1456. [Google Scholar] [CrossRef]
- Zheng, L.; Crippen, T.L.; Holmes, L.; Singh, B.; Pimsler, M.L.; Benbow, M.E.; Tarone, A.M.; Dowd, S.; Yu, Z.; Vanlaerhoven, S.L.; et al. Bacteria mediate oviposition by the black soldier fly, Hermetia illucens (L.), (Diptera: Stratiomyidae). Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Hoyt, C.P.; Osborne, G.O.; Mulcock, A.P. Production of an insect sex attractant by symbiotic bacteria. Nature 1971, 230, 472. [Google Scholar] [CrossRef]
- Madden, A.A.; Epps, M.J.; Fukami, T.; Irwin, R.E.; Sheppard, J.; Sorger, D.M.; Dunn, R.R. The ecology of insect–yeast relationships and its relevance to human industry. Proc. R. Soc. B 2018, 285, 20172733. [Google Scholar] [CrossRef]
- Robacker, D.C.; Martinez Adelaido, J.; Garcia Jose, A.; Bartelt Robert, J. Volatiles attractive to the Mexican fruit fly (Diptera: Tephritidae) from eleven bacteria taxa. Fla. Entomol. 1998, 81, 497–508. [Google Scholar] [CrossRef]
- Causton, C.E.; Moon, R.D.; Cimadom, A.; Boulton, R.A.; Cedeno, D.; Lincango, M.P.; Tebbich, S.; Ulloa, A. Population dynamics of an invasive bird parasite, Philornis downsi (Diptera: Muscidae), in the Galapagos Islands. PLoS ONE 2019, 14, e0224125. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Zaada, D.S.Y.; Dudaniec, R.Y.; Pasternak, Z.; Jurkevitch, E.; Smith, R.J.; Causton, C.E.; Lincango, M.P.; Tobe, S.S.; Mitchell, J.G.; et al. Host-specific associations affect the microbiome of Philornis downsi, an introduced parasite to the Galapagos Islands. Mol. Ecol. 2017, 26, 4644–4656. [Google Scholar] [CrossRef]
- Leroy, P.D.; Sabri, A.; Verheggen, F.J.; Francis, F.; Thonart, P.; Haubruge, E. The semiochemically mediated interactions between bacteria and insects. Chemoecology 2011, 21, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Wong, A.C.N.; Holmes, A.; Ponton, F.; Lihoreau, M.; Wilson, K.; Raubenheimer, D.; Simpson, S.J. Behavioral microbiomics: A multi-dimensional approach to microbial influence on behavior. Front. Microbio. 2015, 6, 1359. [Google Scholar] [CrossRef] [PubMed]
- Yuval, B.; Galun, R. Aspects of compound-conditioning to gustatory stimuli in the housefly Musca domestica L. J. Insect Physiol. 1987, 33, 159–165. [Google Scholar] [CrossRef]
- Jackson, M.H. Galapagos, a Natural History; University of Calgary Press: Calgary, AB, Canada, 1994. [Google Scholar]
- Grant, P.R. Ecology and Evolution of Darwin’s Finches; Princeton University Press: Princeton, NJ, USA, 1986. [Google Scholar]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. Msystems 2016, 1. [Google Scholar] [CrossRef] [Green Version]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aq. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Naqib, A.; Poggi, S.; Wang, W.; Hyde, M.; Kunstman, K.; Green, S.J. Making and Sequencing Heavily Multiplexed, High-Throughput 16S Ribosomal RNA Gene Amplicon Libraries Using a Flexible, Two-Stage PCR Protocol. In Gene Expression Analysis: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2018; Volume 1783, pp. 149–169. [Google Scholar]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Gloeckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Gloeckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Knutie, S.A.; Chaves, J.A.; Gotanda, K.M. Human activitity can influence the gut microbiota of Darwin’s finches in the Galapagos islands. Mol. Ecol. 2019, 28, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Simcock, N.K.; Gray, H.E.; Wright, G.A. Single amino acids in sucrose rewards modulate feeding and associative learning in the honeybee. J. Insect Physiol. 2014, 69, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, P.W.; Nieh, J.C. Salt preferences of honey bee water foragers. J. Exper. Biol. 2016, 219, 790–796. [Google Scholar] [CrossRef] [Green Version]
- Mustard, J.A.; Akyol, E.; Robles, K.D.; Ozturk, C.; Kaftanoglu, O. Influence of sugar experience during development on gustatory sensitivity of the honey bee. J. Insect Physiol. 2019, 116, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Arien, Y.; Dag, A.; Zarchin, S.; Masci, T.; Shafir, S. Omega-3 deficiency impairs honey bee learning. Proc. Natl. Acad. Sci. USA 2015, 112, 15761–15766. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.L.; Chen, X.Y.; Zeng, X.N. Classical olfactory conditioning in the oriental Fruit Fly, Bactrocera dorsalis. PLoS ONE 2015, 10, e0122155. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.L.; Chen, H.L.; Chen, X.Y.; Cui, R.K.; Guerrero, A.; Zeng, X.N. Factors influencing aversive learning in the oriental fruit fly, Bactrocera dorsalis. J. Comp. Physiol. A 2017, 203, 57–65. [Google Scholar] [CrossRef]
- Sieuwerts, S.; De Bok, F.A.; Hugenholtz, J.; van Hylckama Vlieg, J.E. Unraveling microbial interactions in food fermentations: From classical to genomics approaches. Appl. Environ. Microbiol. 2008, 74, 4997–5007. [Google Scholar] [CrossRef] [Green Version]

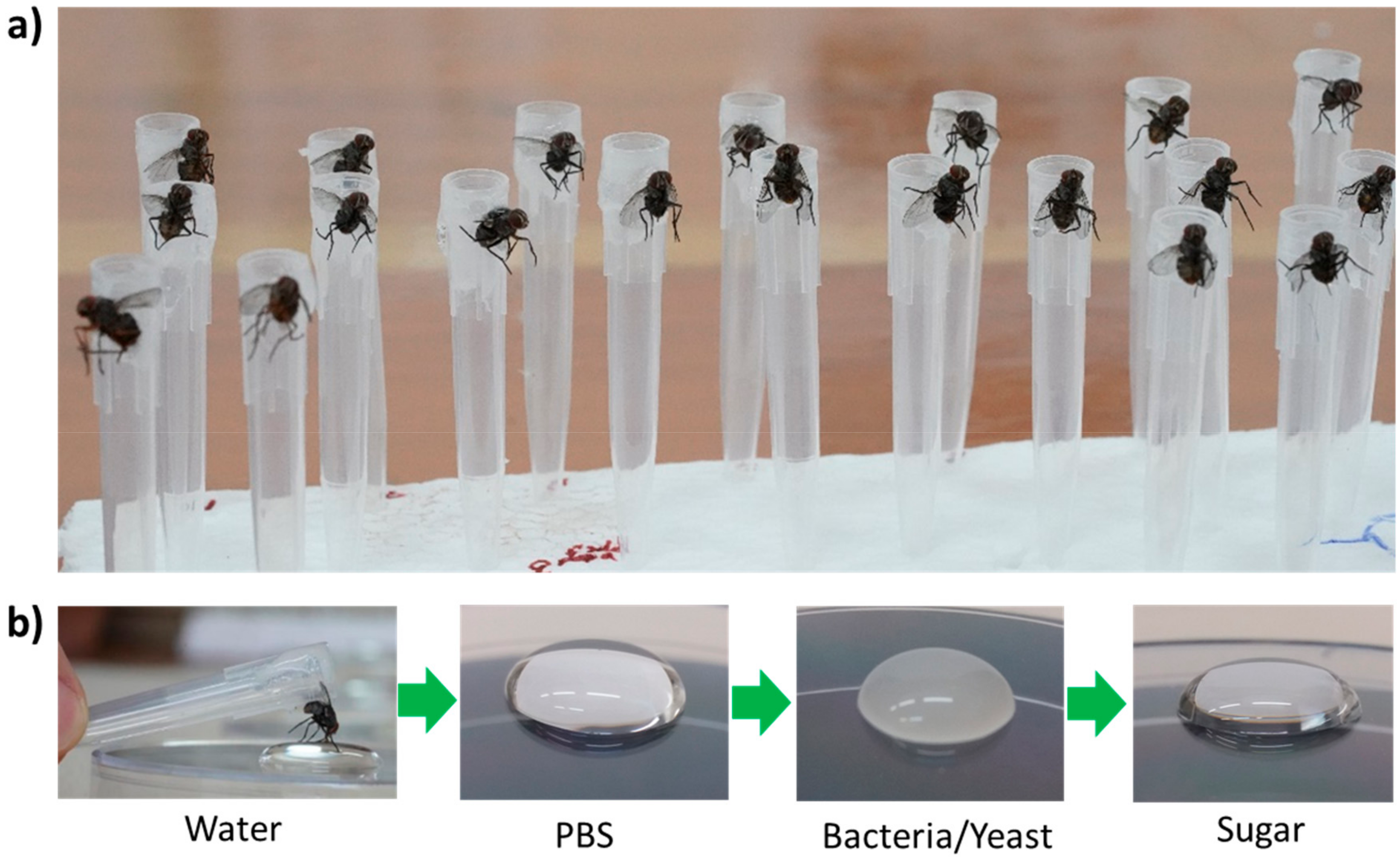

| Location | Number of Trap-Nights | Bait/Medium | No. of Philornis | Mean Number (SD) of Other Flies/Trap | Other Insects Trapped |
|---|---|---|---|---|---|
| El Barranco | 20 | bacteria/LB | 1 (female) | 4.6 (5.2) | 1 ant, 3 wasps |
| El Barranco | 8 | finch feces/LB | 1 (female) | 5.2 (2.7) | 8 ants |
| El Barranco | 3 | control/LB | 0 | 5.6 (5.1) | 1 wasp |
| El Barranco | 12 | bacteria/TA | 0 | 14.6 (16.2) | 2 ants, 3 wasps |
| El Barranco | 3 | control/TA | 0 | 15.6 (16.8) | 1 wasp |
| El Barranco | 8 | bacteria/BH | 0 | 3.3 (1.9) | 3 ants, 21 wasps, 3 moths |
| El Barranco | 2 | control/BH | 0 | 1 (1.4) | 2 wasps |
| El Barranco | 8 | yeasts/YPD | 0 | 0.3 (0.7) | 1 ant, 1 wasp |
| El Barranco | 2 | control/YPD | 0 | 1.5 (2.1) | 1 ant 1 wasp |
| Los Gemelos | 16 | bacteria/LB | 0 | 1.8 (1.6) | 0 |
| Los Gemelos | 4 | bacteria/LB | 0 | 2 (2.1) | 0 |
| Los Gemelos | 16 | bacteria/TA | 0 | 2.8 (2.5) | 1 moth |
| Los Gemelos | 8 | fermented papaya/TA | 0 | 1.8 (1.5) | 1 cockroach |
| Los Gemelos | 2 | control/TA | 0 | 7.5 (4.9) | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuval, B.; Lahuatte, P.; Jose, P.A.; Causton, C.E.; Jurkevitch, E.; Kouloussis, N.; Ben-Yosef, M. Behavioral Responses of the Invasive Fly Philornis downsi to Stimuli from Bacteria and Yeast in the Laboratory and the Field in the Galapagos Islands. Insects 2019, 10, 431. https://doi.org/10.3390/insects10120431
Yuval B, Lahuatte P, Jose PA, Causton CE, Jurkevitch E, Kouloussis N, Ben-Yosef M. Behavioral Responses of the Invasive Fly Philornis downsi to Stimuli from Bacteria and Yeast in the Laboratory and the Field in the Galapagos Islands. Insects. 2019; 10(12):431. https://doi.org/10.3390/insects10120431
Chicago/Turabian StyleYuval, Boaz, Paola Lahuatte, Polpass Arul Jose, Charlotte E. Causton, Edouard Jurkevitch, Nikos Kouloussis, and Michael Ben-Yosef. 2019. "Behavioral Responses of the Invasive Fly Philornis downsi to Stimuli from Bacteria and Yeast in the Laboratory and the Field in the Galapagos Islands" Insects 10, no. 12: 431. https://doi.org/10.3390/insects10120431





