Effects of Variety and Grape Berry Condition of Vitis vinifera on Preference Behavior and Performance of Drosophila suzukii
Abstract
1. Introduction
2. Materials and Methods
2.1. Monitoring of Field Infestation
2.2. Insect Rearing Conditions and Colony Maintenance
2.3. Grape Varieties and Grape Condition for Laboratory Tests
2.4. Laboratory Dual-Choice Assays
2.5. Statistical Analysis
3. Results
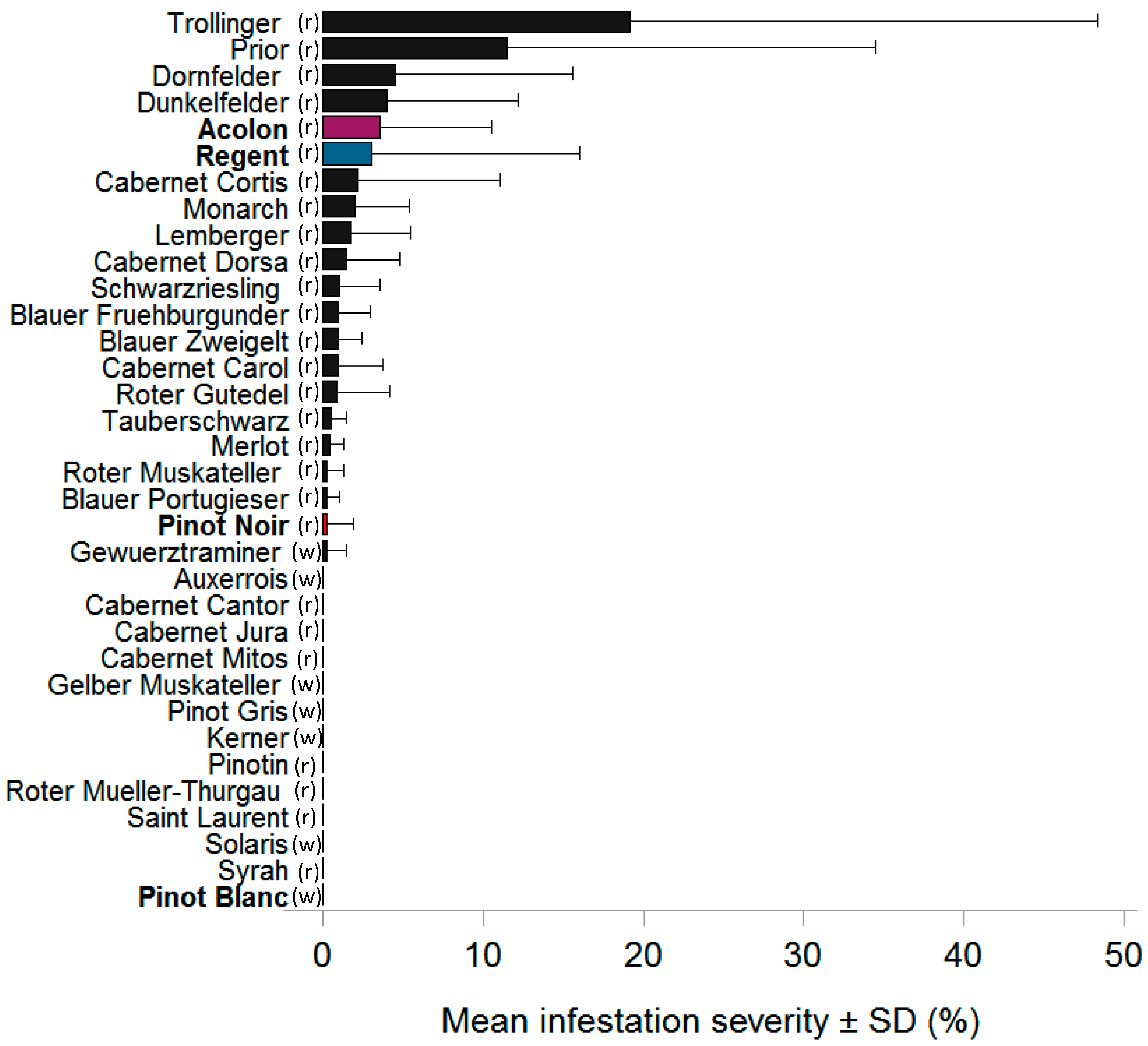
3.1. Field Infestation 2015–2018
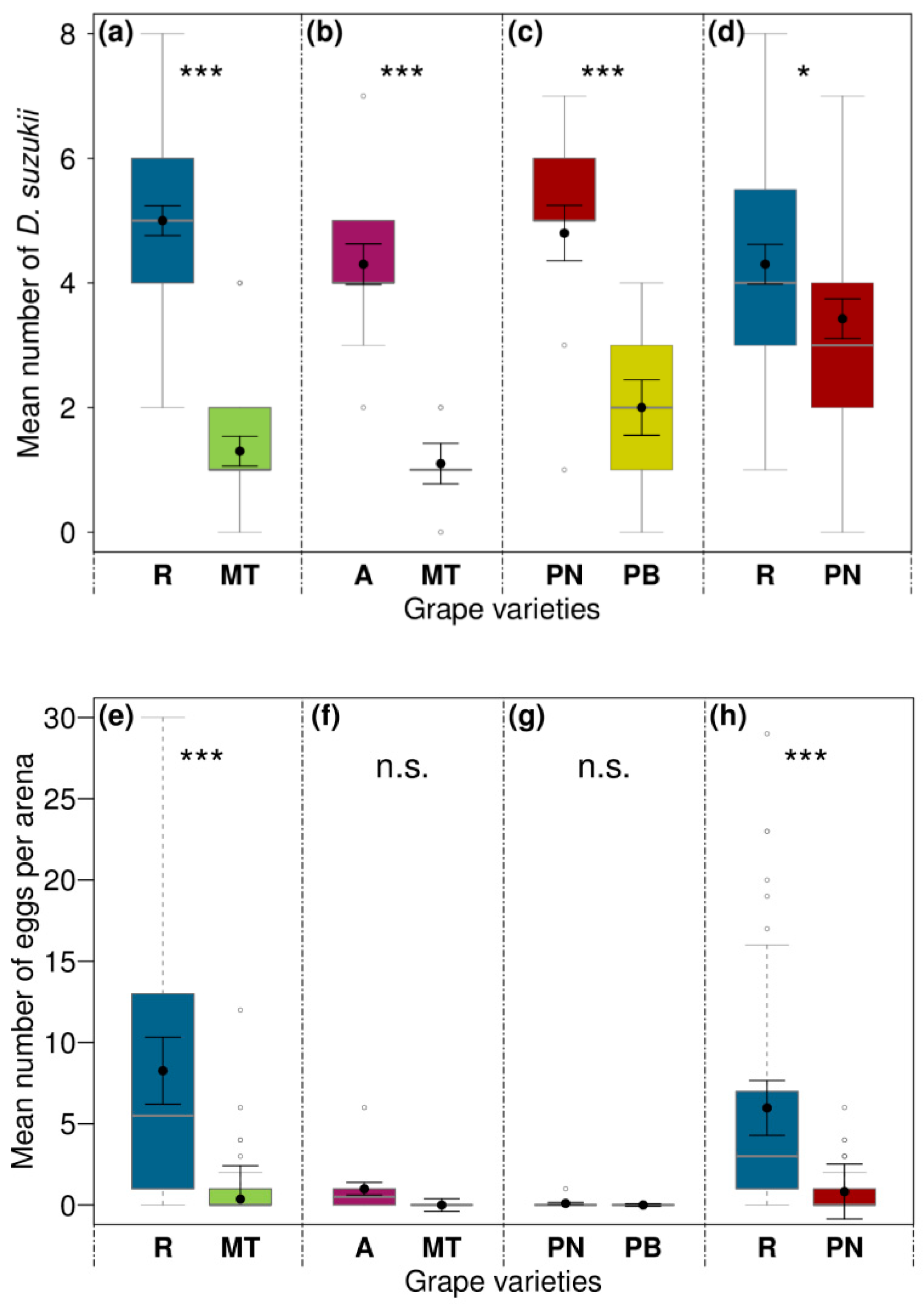
3.2. Influences of Grape Variety on Preference, Oviposition, and Emergence of Drosophila suzukii in the Laboratory
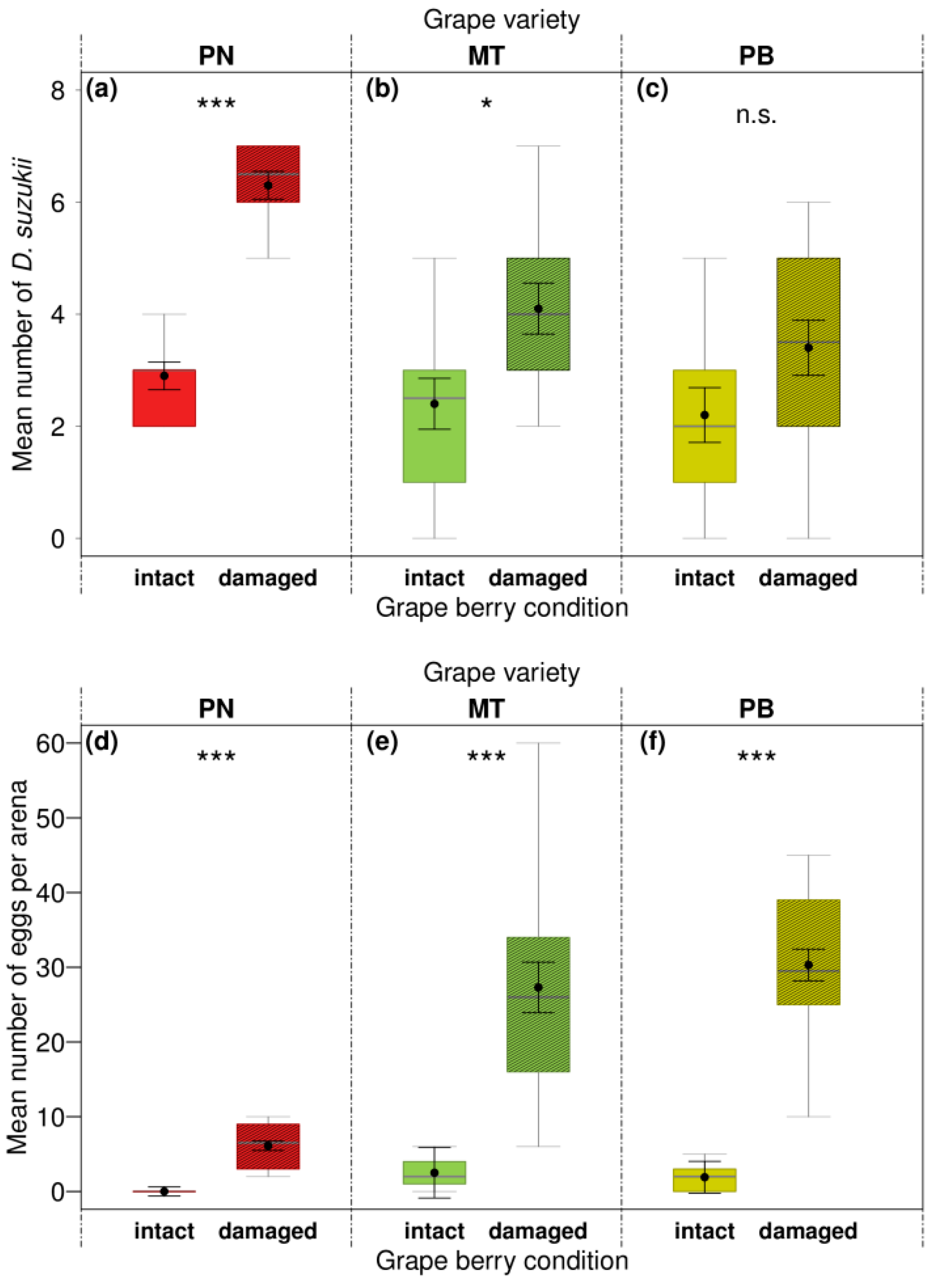
3.3. Influences of Berry Condition on Preference, Oviposition, and Emergence of Drosophila suzukii in the Laboratory
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, 1–7. [Google Scholar] [CrossRef]
- Pelton, E.; Gratton, C.; Guédot, C. Susceptibility of cold hardy grapes to Drosophila suzukii (Diptera: Drosophilidae). J. Appl. Entomol. 2017, 141, 644–652. [Google Scholar] [CrossRef]
- Poyet, M.; Le Roux, V.; Gibert, P.; Meirland, A.; Prevost, G.; Eslin, P.; Chabrerie, O. The wide potential trophic niche of the asiatic fruit fly Drosophila suzukii: The key of its invasion success in temperate Europe? PLoS ONE 2015, 10, e0142785. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, D.E.; Sisterson, M.S.; Walse, S.S. Quantifying host potentials: Indexing postharvest fresh fruits for spotted wing Drosophila, Drosophila suzukii. PLoS ONE 2013, 8, e61227. [Google Scholar] [CrossRef]
- Kenis, M.; Tonina, L.; Eschen, R.; van der Sluis, B.; Sancassani, M.; Mori, N.; Haye, T.; Helsen, H. Non-crop plants used as hosts by Drosophila suzukii in Europe. J. Pest Sci. 2016, 89, 735–748. [Google Scholar] [CrossRef]
- Lee, J.C.; Dalton, D.T.; Swoboda-Bhattarai, K.A.; Bruck, D.J.; Burrack, H.J.; Strik, B.C.; Woltz, J.M.; Walton, V.M. Characterization and manipulation of fruit susceptibility to Drosophila suzukii. J. Pest Sci. 2016, 89, 771–780. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In Focus: Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef]
- Burrack, H.J.; Fernandez, G.E.; Spivey, T.; Kraus, D.A. Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumara (Diptera: Drosophilidae), an invasive frugivore. Pest Manag. Sci. 2013, 69, 1173–1180. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef]
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectol. 2012, 65, 149–160. [Google Scholar]
- Alhmedi, A.; Clymans, R.; Van Kerckvoorde, V.; Bylemans, D.; Beliën, T. Preference and performance of Drosophila suzukii on Prunus species: A potential eco-friendly pest management tool. Crop Prot. 2019, 122, 35–41. [Google Scholar] [CrossRef]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Linder, C.; Martin, C.; Laboisse, S.; GChatelain, P.; Kerhli, P. Susceptibility of various grape cultivars to Drosophila suzukii and other vinegar flies. IOBC-WPRS Bull. 2014, 105, 219–224. [Google Scholar]
- Shrader, M.E.; Burrack, H.J.; Pfeiffer, D.G. Drosophila suzukii (Diptera: Drosophilidae) Oviposition and adult emergence in six wine grape varieties grown in Virginia. J. Econ. Entomol. 2019, 112, 139–148. [Google Scholar] [CrossRef]
- Ioriatti, C.; Walton, V.; Dalton, D.; Anfora, G.; Grassi, A.; Maistri, S.; Mazzoni, V. Drosophila suzukii (Diptera: Drosophilidae) and its potential impact to wine grapes during harvest in two cool climate wine grape production regions. J. Econ. Entomol. 2015, 108, 1148–1155. [Google Scholar] [CrossRef]
- Liu, S.; Gao, H.-H.; Zhai, Y.-F.; Chen, H.; Dang, H.-Y.; Qin, D.-Y.; Li, L.-L.; Li, Q.; Yu, Y. Oviposition suitability of Drosophila suzukii (Diptera: Drosophilidae) for nectarine varieties and its correlation with the physiological indexes. Insects 2019, 10, 221. [Google Scholar] [CrossRef]
- Olazcuaga, L.; Rode, N.O.; Foucaud, J.; Facon, B.; Ravigné, V.; Ausset, A.; Leménager, N.; Loiseau, A.; Gautier, M.; Estoup, A.; et al. Oviposition preference and larval performance of Drosophila suzukii (Diptera: Drosophilidae), spotted-wing drosophila: Effects of fruit identity and composition. Environ. Entomol. 2019. [Google Scholar] [CrossRef]
- Sward, G.F.H.; Glass, S.E.; Philips, C.R. The phenology of infestations and the impacts of different varieties of cold hardy red raspberries on Drosophila suzukii. Adv. Entomol. 2016, 4, 8. [Google Scholar] [CrossRef]
- Haye, T.; Girod, P.; Cuthbertson, A.G.S.; Wang, X.G.; Daane, K.M.; Hoelmer, K.A.; Baroffio, C.; Zhang, J.P.; Desneux, N. Current SWD IPM tactics and their practical implementation in fruit crops across different regions around the world. J. Pest Sci. 2016, 89, 643–651. [Google Scholar] [CrossRef]
- Lee, J.C.; Dreves, A.J.; Cave, A.M.; Kawai, S.; Isaacs, R.; Miller, J.C.; Van Timmeren, S.; Bruck, D.J. Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann. Entomol. Soc. Am. 2015, 108, 117–129. [Google Scholar] [CrossRef]
- Cini, A.; Anfora, G.; Escudero-Colomar, L.A.; Grassi, A.; Santosuosso, U.; Seljak, G.; Papini, A. Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J. Pest Sci. 2014, 87, 559–566. [Google Scholar] [CrossRef]
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The making of a pest: The evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. Biol. Sci. 2014, 281, 20132840. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa, T. Studies on Drosophila suzukii Mats; Yamanashi Agricultural Experiment Station: Kofu, Japan, 1939. [Google Scholar]
- Cloonan, K.; Abraham, J.; Angeli, S.; Syed, Z.; Rodriguez-Saona, C. Advances in the chemical ecology of the spotted wing Drosophila (Drosophila suzukii) and its applications. J. Chem. Ecol. 2018, 44, 922–939. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Cloonan, K.; Sanchez-Pedraza, F.; Zhou, Y.; Giusti, M.; Benrey, B. Differential susceptibility of wild and cultivated blueberries to an invasive frugivorous pest. J. Chem. Ecol. 2018, 45, 286–297. [Google Scholar] [CrossRef]
- Rice, K.B.; Short, B.D.; Jones, S.K.; Leskey, T.C. Behavioral responses of Drosophila suzukii (Diptera: Drosophilidae) to visual stimuli under laboratory, semifield, and field conditions. Environ. Entomol. 2016, 45, 1480–1488. [Google Scholar] [CrossRef]
- Entling, W.; Anslinger, S.; Jarausch, B.; Michl, G.; Hoffmann, C. Berry skin resistance explains oviposition preferences of Drosophila suzukii at the level of grape cultivars and single berries. J. Pest Sci. 2019, 92, 477–484. [Google Scholar] [CrossRef]
- Baser, N.; Broutou, O.; Verrastro, V.; Porcelli, F.; Ioriatti, C.; Anfora, G.; Mazzoni, V.; Rossi Stacconi, M.V. Susceptibility of table grape varieties grown in south-eastern Italy to Drosophila suzukii. J. Appl. Entomol. 2017, 142, 465–472. [Google Scholar] [CrossRef]
- Young, Y.; Buckiewicz, N.; Long, T.A.F. Nutritional geometry and fitness consequences in Drosophila suzukii, the Spotted-Wing Drosophila. Ecol. Evol. 2018, 8, 2842–2851. [Google Scholar] [CrossRef]
- Takahara, B.; Takahashi, K.H. Associative learning of color and firmness of oviposition substrates in Drosophila suzukii. Entomol. Exp. Appl. 2017, 162, 13–18. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference–performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Jaenike, J. Host specialization in phytophagous insects. Annu. Rev. Ecol. Syst. 1990, 21, 243–273. [Google Scholar] [CrossRef]
- Briem, F.; Eben, A.; Gross, J.; Vogt, H. An invader supported by a parasite: Mistletoe berries as a host for food and reproduction of spotted wing drosophila in early spring. J. Pest Sci. 2016, 89, 749–759. [Google Scholar] [CrossRef]
- Stewart, T.J.; Wang, X.-G.; Molinar, A.; Daane, K.M. Factors limiting peach as a potential host for Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2014, 107, 1771–1779. [Google Scholar] [CrossRef]
- Holle, S.G.; Burkness, E.C.; Cira, T.M.; Hutchison, W.D. Influence of previous fruit injury on susceptibility to spotted wing drosophila (Diptera: Drosophilidae) infestation in the midwestern united states. J. Entomol. Sci. 2017, 52, 207–215. [Google Scholar] [CrossRef]
- Grant, J.A.; Sial, A.A. Potential of muscadine grapes as a viable host of Drosophila suzukii (Diptera: Drosophilidae) in blueberry-producing regions of the southeastern United States. J. Econ. Entomol. 2016, 109, 1261–1266. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package Version 1.1-7. 2014. Available online: http://CRAN.R-project.org/package=lme4 (accessed on 11 November 2018).
- Zuur, R.A.F.; Ieno, E.N.; Walker, N.J.; Saviliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology; Springer: New York, NY, USA, 2009. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2000. [Google Scholar]
- Weißinger, L.; Schrieber, K.; Breuer, M.; Müller, C. Influences of blackberry margins on population dynamics of Drosophila suzukii and grape infestation in adjacent vineyards. J. Appl. Entomol. 2019, 143, 802–812. [Google Scholar] [CrossRef]
- Kehrli, P.; Linder, C.; Cahenzli, F.; Daniel, C. Susceptibility of various grape cultivars to Drosophila suzukii. Schweiz. Z. Obst.Weinbau 2017, 153, 10–12. [Google Scholar]
- Cahenzli, F.; Daniel, C. Susceptibility of Different Grape Varieties to Drosophila suzukii Oviposition; Forschungsinstitut für biologischen Landbau, FiBL: Frick, Schweiz, 2016. [Google Scholar]
- Diepenbrock, L.; Burrack, H. Variation of within-crop microhabitat use by Drosophila suzukii (Diptera: Drosophilidae) in blackberry. J. Appl. Entomol. 2016, 141, 1–7. [Google Scholar] [CrossRef]
- Kinjo, H.; Kunimi, Y.; Ban, T.; Nakai, M. Oviposition efficacy of Drosophila suzukii (Diptera: Drosophilidae) on different cultivars of blueberry. J. Econ. Entomol. 2013, 106, 1767–1771. [Google Scholar] [CrossRef]
- Elsensohn, J.; Loeb, G. Non-crop host sampling yields insights into small-scale population dynamics of Drosophila suzukii (Matsumura). Insects 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Maiguashca, F.; Ferguson, H.; Bahder, B.; Brooks, T.; O’Neal, S.; Walsh, D. SWD Ovipositing on Grapes in Laboratory: Partial Maggot Survival Inconclusive; Washington State University Extension, Spotted Wing Drosophila Grape Update: Prosser, WA, USA, 2010. [Google Scholar]
- Bellutti, N.; Gallmetzer, A.; Innerebner, G.; Schmidt, S.; Zelger, R.; Koschier, E.H. Dietary yeast affects preference and performance in Drosophila suzukii. J. Pest Sci. 2018, 91, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Friberg, M.; Posledovich, D.; Wiklund, C. Decoupling of female host plant preference and offspring performance in relative specialist and generalist butterflies. Oecologia 2015, 178, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.P. Can mechanism help explain insect host choice? J. Evol. Biol. 2012, 25, 244–251. [Google Scholar] [CrossRef]
- Steffan, S.A.; Lee, J.C.; Singleton, M.E.; Vilaire, A.; Walsh, D.B.; Lavine, L.S.; Patten, K. Susceptibility of cranberries to Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2013, 106, 2424–2427. [Google Scholar] [CrossRef] [PubMed]

| Test | Grape Varieties (V) | Combination Tested | Replicates (Arenas) | Year | Brix | ||
|---|---|---|---|---|---|---|---|
| V1 | V2 | V1 | V2 | ||||
| 1 | Red vs. white | Regent | Müller-Thurgau | 10 | 2016 | 20.5° | 19.8° |
| 10 | 2017 | 20.5° | 18.4° | ||||
| 10 | 2017 | 19.8° | 17.7° | ||||
| 2 | Acolon | Müller-Thurgau | 10 | 2016 | 18.8° | 19.8° | |
| 3 | Pinot Noir | Pinot Blanc | 10 | 2016 | 23.8° | 22.4° | |
| 4 | Red vs. red | Regent | Pinot Noir | 10 | 2016 | 21.4° | 24° |
| 10 | 2016 | 20.9° | 22.8° | ||||
| 10 | 2017 | 22.4° | 20.2° | ||||
| 10 | 2017 | 21.2° | 23.5° | ||||
| Berry condition | |||||||
| 5 | Pinot Noir | intact | injured | 10 | 2016 | 24° | |
| 6 | Müller-Thurgau | intact | injured | 10 | 2017 | 17.7° | |
| 7 | Pinot Blanc | intact | injured | 10 | 2017 | 21.2° | |
| Year | Grape Varieties | |||||
|---|---|---|---|---|---|---|
| Acolon | Regent | Pinot Noir | Müller-Thurgau | Pinot Blanc | ||
| 2015 | Severity (%) | 0 | 1.58 ± 5.47 | 0.44 ± 1.68 | 0.30 ± 0.96 | 0 |
| First oviposition (cw) | - | 36 | 36 | 38 | - | |
| 2016 | Severity (%) | 3.57 ± 6.96 | 3.01 ± 13.03 | 0.29 ± 1.61 | N/A | 0 |
| First oviposition (cw) | 33 | 34 | 36 | N/A | - | |
| 2017 | Severity (%) | 2.60 ± 7.23 | 2.13 ± 6.82 | 1.23 ± 6.87 | 0 | N/A |
| First oviposition (cw) | 33 | 33 | 36 | - | N/A | |
| 2018 | Severity (%) | 0.04 ± 0.25 | 0.06 ± 0.56 | 0.03 ± 0.37 | N/A | N/A |
| First oviposition (cw) | 32 | 36 | 36 | N/A | N/A | |
| Combination Tested | Regent vs. Müller-Thurgau | Acolon vs. Müller-Thurgau | Pinot Noir vs. Pinot Blanc | Regent vs. Pinot Noir | |||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed effects | Df | Χ2 | p | Χ2 | p | Χ2 | p | Χ2 | p |
| Location preference | |||||||||
| Grape variety | 1 | 66.06 | <0.001 | 26.14 | <0.001 | 13.76 | <0.001 | 4.62 | 0.032 |
| Random effects | Var | Obs | Var | Obs | Var | Obs | Var | Obs | |
| ID | 0 | 30 | 0 | 10 | 0 | 10 | 0 | 40 | |
| Year: date | 0 | 3 | 0.09 | 4 | |||||
| Year | 0 | 2 | 0 | 2 | |||||
| Residuals | 1.71 | 60 | 0.95 | 20 | 1.98 | 20 | 3.22 | 80 | |
| Oviposition preference | |||||||||
| Grape variety | 1 | 16.95 | <0.001 | 3.08 | 0.079 | 1.08 | 0.298 | 18.63 | <0.001 |
| Random effects | Var | Obs | Var | Obs | Var | Obs | Var | Obs | |
| ID | 0 | 30 | 0 | 10 | 0 | 10 | 0 | 40 | |
| Year: date | 0 | 3 | 0 | 4 | |||||
| Year | 5.19 | 2 | 4.43 | 2 | |||||
| Residuals | 47.56 | 60 | 1.5 | 20 | 0 | 20 | 25.20 | 80 | |
| Test | Grape Variety | Berry Condition | Emergence Rate (%) | W | p |
|---|---|---|---|---|---|
| 1 | Regent | intact | 8.7 ± 5.0 | 231 | 0.305 |
| Müller-Thurgau | intact | 7.9 ± 4.4 | |||
| 4 | Regent | intact | 19.5 ± 7.3 | 288 | 0.003 |
| Pinot Noir | intact | 1.7 ± 1.6 | |||
| 6 | Müller-Thurgau | intact | 1.7 ± 1.7 | 9 | 0.001 |
| damaged | 26.1 ± 5.5 | ||||
| 7 | Pinot Blanc | intact | 5.0 ± 5.0 | 6.5 | <0.001 |
| damaged | 35.6 ± 4.3 |
| Combination Tested | Intact vs. Damaged Berries | ||||||
|---|---|---|---|---|---|---|---|
| Pinot Noir | Müller-Thurgau | Pinot Blanc | |||||
| Fixed effects | Df | Χ2 | p | Χ2 | p | Χ2 | p |
| Location preference | |||||||
| Berry condition | 1 | 36.67 | <0.001 | 6.00 | 0.014 | 2.80 | 0.095 |
| Random effects | Var | Obs | Var | Obs | Var | Obs | |
| ID | 0 | 10 | 0 | 10 | 0 | 10 | |
| Residuals | 0.55 | 20 | 2.07 | 20 | 2.4 | 20 | |
| Oviposition preference | |||||||
| Berry condition | 1 | 24.59 | <0.001 | 17.17 | <0.001 | 34.02 | <0.001 |
| Random effects | Var | Obs | Var | Obs | Var | Obs | |
| ID | 0 | 10 | 12.55 | 10 | 3.03 | 10 | |
| Residuals | 3.85 | 20 | 101.78 | 20 | 42.12 | 20 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weißinger, L.; Samuel, N.; Breuer, M.; Müller, C. Effects of Variety and Grape Berry Condition of Vitis vinifera on Preference Behavior and Performance of Drosophila suzukii. Insects 2019, 10, 432. https://doi.org/10.3390/insects10120432
Weißinger L, Samuel N, Breuer M, Müller C. Effects of Variety and Grape Berry Condition of Vitis vinifera on Preference Behavior and Performance of Drosophila suzukii. Insects. 2019; 10(12):432. https://doi.org/10.3390/insects10120432
Chicago/Turabian StyleWeißinger, Lisa, Niklas Samuel, Michael Breuer, and Caroline Müller. 2019. "Effects of Variety and Grape Berry Condition of Vitis vinifera on Preference Behavior and Performance of Drosophila suzukii" Insects 10, no. 12: 432. https://doi.org/10.3390/insects10120432
APA StyleWeißinger, L., Samuel, N., Breuer, M., & Müller, C. (2019). Effects of Variety and Grape Berry Condition of Vitis vinifera on Preference Behavior and Performance of Drosophila suzukii. Insects, 10(12), 432. https://doi.org/10.3390/insects10120432





