Leaf Morphological and Biochemical Responses of Three Potato (Solanum tuberosum L.) Cultivars to Drought Stress and Aphid (Myzus persicae Sulzer) Infestation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions and Planting Materials
2.2. Aphid Culture
2.3. Experimental Design and Treatments
2.4. Determination of Aphid Performance
2.5. Aphid Water Content
2.6. Leaf Relative Water Content (RWC)
2.7. Relative Plant Biomass
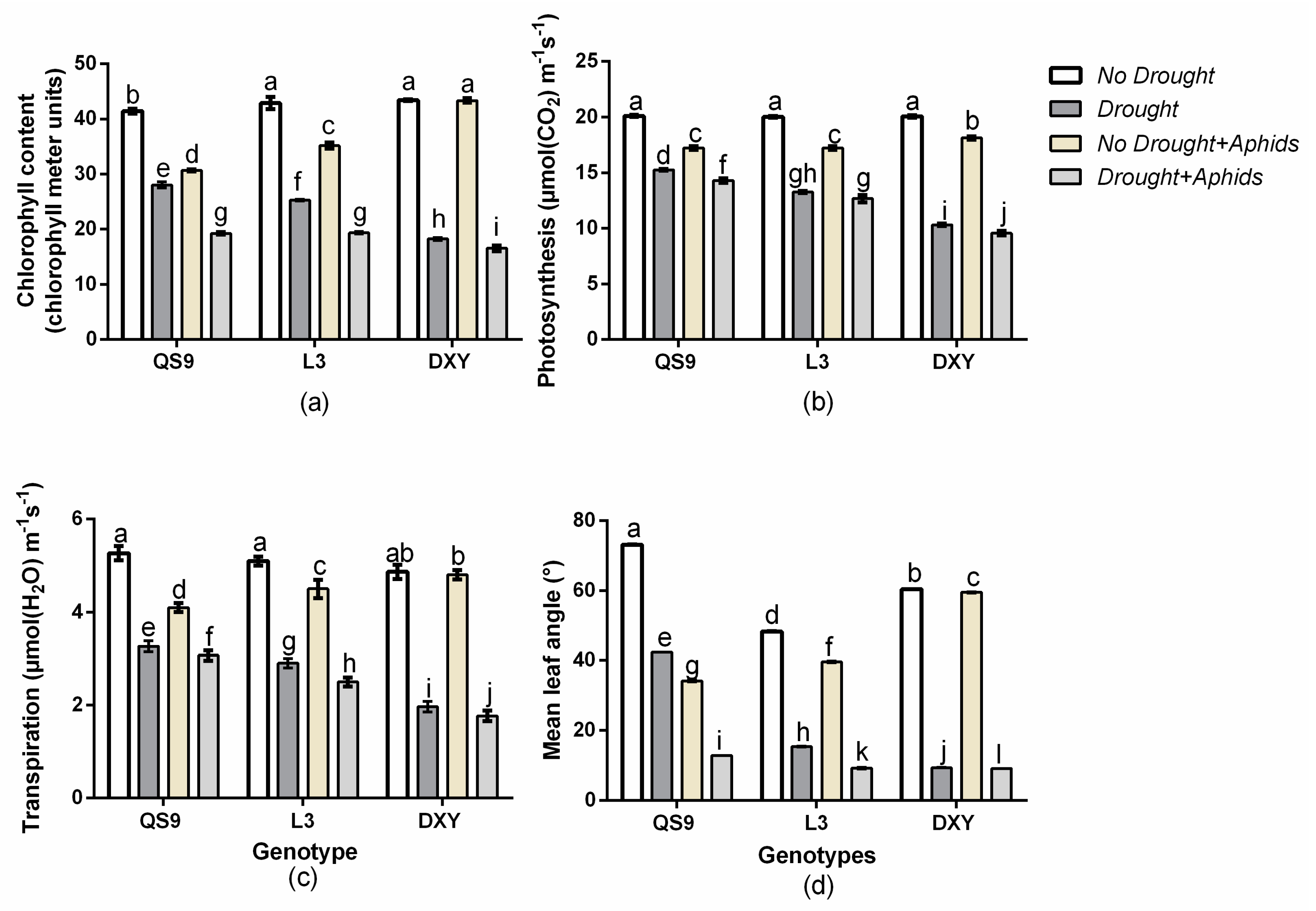
2.8. Chlorophyll Content, Net Photosynthesis, Transpiration Rate, and Leave Angle
2.9. Stomata and Leaf Hair Analysis
2.10. Determination of Proline (Pro) Content
2.11. Determination Hydrogen Peroxide (H2O2) Content
2.12. Determination of Malondialdehyde (MDA) Content
2.13. Determination of Antioxidant Enzyme Activities in Leaf Tissue
2.14. Statistical Analysis
3. Results
3.1. Aphid Performance
3.2. Aphid Water Content and Leaf Relative Water Content
3.3. Plant Growth Response to Drought Stress and Aphids Treatments
3.4. Correlation Analysis
3.5. Genotypic Variation of Physiological Response to Drought Stress and Aphids Treatments
3.6. Changes in Leaf Hair Density and Number of Potato Cultivars under Drought and Aphid Treatments
3.7. Changes in Leaf Stomata Density, Number, and Size of Potato Genotypes under Drought and Aphid Treatments
3.8. Effect of Drought Stress and Aphid Interaction on MDA, H2O2, and Proline Contents
3.9. Effect of Drought Stress and Aphid Interaction on Antioxidant Enzymes Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silva, A.X.; Jander, G.; Samaniego, H.; Ramsey, J.S.; Figueroa, C.C. Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) I: A transcriptomic survey. PLoS ONE 2012, 7, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huberty, A.; Denno, R. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Mewis, I.; Khan, M.A.; Glawischnig, E.; Schreiner, M.; Ulrichs, C. Water stress and aphid feeding differentially influence metabolite composition in Arabidopsis thaliana (L.). PLoS ONE 2012, 7, 4–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.; Ulrichs, C.; Mewis, I. Influence of water stress on the glucosinolate profile of Brassica oleracea var. italica and the performance of Brevicoryne brassicae and Myzus persicae. Entomol. Exp. Appl. 2010, 137, 229–236. [Google Scholar] [CrossRef]
- Simpson, K.L.S.; Jackson, G.E.; Grace, J. The response of aphids to plant water stress—The case of Myzus persicae and Brassica oleracea var. capitata. Entomol. Exp. Appl. 2012, 142, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Ehsanpour, A.A.; Razavizadeh, R. Effect of UV-C on drought tolerance of alfalfa (Medicago sativa) callus. Am. J. Biochem. Biotechnol. 2005, 1, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Ma, X.; Guo, H.; Sukiran, N.L.; Guo, B.; Assmann, S.M.; Ma, H. Flower development under drought stress: Morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell 2013, 25, 3785–3807. [Google Scholar] [CrossRef] [Green Version]
- Turtola, S.; Rousi, M.; Pusenius, J.; Yamaji, K.; Heiska, S.; Tirkkonen, V.; Meier, B.; Julkunen-Tiitto, R. Clone-specific responses in leaf phenolics of willows exposed to enhanced UVB radiation and drought stress. Glob. Chang. Biol. 2005, 11, 1655–1663. [Google Scholar] [CrossRef]
- Mody, K.; Eichenberger, D.; Dorn, S. Stress magnitude matters: Different intensities of pulsed water stress produce non-monotonic resistance responses of host plants to insect herbivores. Ecol. Entomol. 2009, 34, 133–143. [Google Scholar] [CrossRef]
- Reddall, A.; Sadras, V.O.; Wilson, L.J.; Gregg, P.C. Physiological responses of cotton to two-spotted spider mite damage. Crop Sci. 2004, 44, 835–846. [Google Scholar] [CrossRef]
- Willmer, P. Microclimatic effects on insects at the plant surface. In Insects and the Plant Surface; Juniper, B.E., Southwood, T.R.E., Eds.; Edward Arnold: London, UK, 1986; Volume 16, pp. 65–80. [Google Scholar]
- Chu, C.-C.; Natwick, E.T.; Henneberry, T.J. Bemisia tabaci (Homoptera: Aleyrodidae) Biotype B Colonization on Okra- and Normal-Leaf Upland Cotton Strains and Cultivars. J. Econ. Entomol. 2002, 95, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.G.; Joshi, A.B.; Singh, M. Hairiness in relation to resistance to jassid (Amrasca devastans Distant) and other insect pests and quality characters in cotton (Gossypium spp.)—A review. Agric. Rev. 1982, 3, 1–8. [Google Scholar]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Guo, H.; Yuan, L.; Wei, J.; Zhang, W.; Ge, F. Plant stomatal closure improves aphid feeding under elevated CO2. Glob. Chang. Biol. 2015, 21, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, X.; Xiang, C.B. Stomatal density and bio-water saving. J. Integr. Plant Biol. 2007, 49, 1435–1444. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [Green Version]
- Maffei, M.E.; Mithofer, A.; Arimura, G.; Uchtenhagen, H.; Bossi, S.; Bertea, C.M.; Cucuzza, L.S.; Novero, M.; Volpe, V.; Quadro, S.; et al. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006, 140, 1022–1035. [Google Scholar] [CrossRef] [Green Version]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Dhandapani, R. Plant defence proteins during aphid infestation. Int. J. Appl. Biol. Pharm. Tech. 2011, 2, 52–57. [Google Scholar]
- Mao, H.; Yang, Y.H.; Guo, C.X.; Zhang, Q.W.; Liu, X.X. Changes in defensive enzyme expression in cotton leaves activity and defensive enzyme gene after feeding by Apolygus lucorum. Chin. J. Appl. Entomol. 2012, 49, 652–659. [Google Scholar]
- FAO. Food and Agricultural Organization of the United Nations Database; FAO: Rome, Italy, 2011. [Google Scholar]
- Visser, R.G.F.; Bachem, C.W.B.; de Boer, J.M.; Bryan, G.J.; Chakrabati, S.K.; Feingold, S.; Gromadka, R.; van Ham, R.C.H.J.; Huang, S.; Jacobs, J.M.E.; et al. Sequencing the potato genome: Outline and first results to come from the elucidation of the sequence of the world’s third most important food crop. Am. J. Potato Res. 2009, 86, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Watkinson, J.I.; Hendricks, L.; Sioson, A.A.; Heat, L.S.; Bohnert, H.J.; Grene, R. Tuber development phenotypes in adapted and acclimated, drought-stressed Solanum tuberosum ssp. andigena have distinct expression profiles of genes associated with carbon metabolism. Plant Physiol. Biochem. 2008, 46, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Liu, N.; Zhou, Q.; Ding, X.; Zhan, J.; Cheng, X.; Huang, J.; Lu, Y.; Yang, Y. Current status and management strategies for potato insect pests and diseases in China. Plant Prot. 2019, 45, 106–111. [Google Scholar] [CrossRef]
- Rosenzweig, C.J.; Elliott, D.; Deryng, A.C.; Ruane, C.; Müller, A.; Arneth, J.; Jones, W. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef] [Green Version]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on food legume production. PLoS ONE 2015, 10, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Jaganathan, D.; Thudi, M.; Kale, S.; Azam, S.; Roorkiwal, M.; Gaur, P.M.; Kishor, P.B.; Nguyen, H.; Sutton, T.; Varshney, R.K. Genotyping-by-sequencing based intra-specific genetic map refines a ‘QTL-hotspot’ region for drought tolerance in chickpea. Mol. Genet. Genom. 2015, 290, 559–571. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, J.; Yang, Y.; Tang, H.; Lu, H.; Fan, M.; Shi, Y.; Dong, D.; Wang, G.; Wang, W.; et al. Status of major diseases and insect pests of potato and pesticides usage in China. Sci. Agric. Sin. 2019, 2, 2800–2808. [Google Scholar] [CrossRef]
- Deblonde, P.M.K.; Ledent, J.F. Effects of moderate drought conditions on green leaf number, stem height, leaf length and tuber yield of potato cultivars. Eur. J. Agron. 2001, 14, 31–41. [Google Scholar] [CrossRef]
- Lahlou, O.; Ledent, J.F. Root mass and depth, stolons and roots formed on stolons in four cultivars of potato under water stress. Eur. J. Agron. 2005, 22, 159–173. [Google Scholar] [CrossRef]
- Alva, A.K.; Moore, A.D.; Collins, H.P. Impact of deficit irrigation on tuber yield and quality of potato cultivars. J. Crop Improv. 2012, 26, 211–227. [Google Scholar] [CrossRef]
- Hale, B.K.; Bale, J.S.; Pritchard, J.; Masters, G.J. Effects of host plant drought stress on the performance of the bird cherry-oat aphid, Rhopalosiphum padi (L.): A mechanistic analysis. Ecol. Entomol. 2003, 28, 666–677. [Google Scholar] [CrossRef]
- Agele, S.O.; Ofuya, T.I.; James, P.O. Effects of watering regimes on aphid infestation and performance of selected varieties of cowpea (Vigna unguiculata L. Walp) in a humid rainforest zone of Nigeria. Crop Prot. 2006, 25, 73–78. [Google Scholar] [CrossRef]
- Gould, N.; Thorpe, M.R.; Minchin, P.E.H.; Pritchard, J.; White, P.J. Solute is imported to elongating root cells of barley as a pressure driven-flow of solution. Funct. Plant Biol. 2004, 31, 391–397. [Google Scholar] [CrossRef]
- Barry, A.; Berry, R.E.; Tamaki, G. Host preference of the green peach aphid, Myzus persicae (Hemiptera: Aphididae). Environ. Entomol. 1982, 11, 824–827. [Google Scholar]
- Hoy, C.W.; Boiteau, G.; Alyokhin, A.; Dively, G.; Alvarez, J.M. Managing insects and mites. In Potato Health Management, 2nd ed.; Johnson, D.A., Ed.; Plant Health Management Series; American Phytopathological Society: St Paul, MN, USA, 2008; pp. 133–147. [Google Scholar]
- Thomas, S.; Dogimont, C.; Boissot, N. Association between Aphis gossypii genotype and phenotype on melon accessions. Arthrop. Plant Interact. 2012, 6, 93–101. [Google Scholar] [CrossRef]
- Guo, H.; Sun, Y.; Peng, X.; Wang, Q.; Harris, M.; Ge, F. Up-regulation of abscisic acid signaling pathway facilitates aphid xylem absorption and osmoregulation under droughtstress. J. Exp. Bot. 2016, 67, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Talbot, M.J.; White, R.G. Cell surface and cell outline imaging in plant tissues using the backscattered electron detector in a variable pressure scanning electron microscope. Plant Methods 2013, 9, 36–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sanchez-Diaz, M. Water stress induced changes in concentrations of proline and totalsoluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 2013, 22, 959–973. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Su, X.; Xia, Z. Grafting improves drought tolerance by regulating antioxidant enzyme activities and stressresponsive gene expression in tobacco. Environ. Exp. Bot. 2014, 107, 173–179. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Koricheva, J.; Larsson, S. Insect performance on experimentally stressed woody plants: A meta-analysis. Annu. Rev. Entomol. 1998, 43, 195–216. [Google Scholar] [CrossRef] [Green Version]
- Staley, J.T.; Mortimer, S.R.; Masters, G.J.; Morecroft, M.D.; Brown, V.K.; Taylor, M.E. Drought stress differentially affects leaf mining species. Ecol. Entomol. 2006, 31, 460–469. [Google Scholar] [CrossRef]
- Stone, A.C.; Gehring, C.A.; Whitham, T.G. Drought negatively affects communities on a foundation tree: Growth rings predict diversity. Oecologia 2010, 164, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, T.; Fernandes, G.W.; Vasconcellos-Neto, J. Size does matter: Variation in herbivory between and within plants and the plant vigor hypothesis. Oikos 2008, 117, 1121–1130. [Google Scholar] [CrossRef]
- White, T.C.R. Plant vigour versus plant stress: A false dichotomy. Oikos 2009, 118, 807–808. [Google Scholar] [CrossRef]
- Karley, A.J.; Douglas, A.E.; Parker, W.E. Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. J. Exp. Bot. 2002, 205, 3009–3018. [Google Scholar]
- Bale, J.S.; Ponder, K.L.; Pritchard, J. Coping with Stress. In Aphids as Crop Pests; Van Emden, H.F., Harrington, R., Eds.; CAB International: Wallingford, UK, 2007; Volume 287, p. 309. [Google Scholar]
- Wearing, C.H. Responses of Myzus persicae and Brevicoryne brassicae to leaf age and water stress in Brussels sprouts grown in pots. Entomol. Exp. Appl. 1972, 15, 61–80. [Google Scholar] [CrossRef]
- Johnson, S.N.; Staley, J.T.; Mcleod, F.A.L.; Hartley, S.E. Plant-mediated effects of soil invertebrates and summer drought on above-ground multitrophic interactions. J. Ecol. 2011, 99, 57–65. [Google Scholar] [CrossRef]
- Bethke, J.A.; Redak, R.A.; Schuch, U.K. Melon aphid performance on chrysanthemum as mediated by cultivar, and differential levels of fertilization and irrigation. Entomol. Exp. Appl. 1998, 88, 41–47. [Google Scholar] [CrossRef]
- King, C.; Jacob, H.S.; Berlandier, F. The influence of water deficiency on the relationship between canola (Brassica napus L.), and two aphid species (Hemiptera: Aphididae), Lipaphis erysimi (Kaltenbach) and Brevicoryne brassicae (L.). Aust. J. Agric. Res. 2006, 57, 439–445. [Google Scholar] [CrossRef]
- Wang, Z.L.; Huang, B.R. Physiological recovery of Kentucky bluegrass from simultaneous drought and heat stress. Crop Sci. 2004, 44, 1729–1736. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, Y.Q.; Song, L.L.; Jacobs, D.F.; Mei, L.; Liu, P.; Jin, S.H. Physiological response to drought stress in Camptotheca acuminata seedlings from two provenances. Front. Plant Sci. 2015, 6, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrage, S.W. The micro-climate at the leaf surface. In Biology of Leaf Surface Organisms; Preece, T.F., Dickinson, C.H., Eds.; Academic Press: London, UK, 1971; pp. 91–101. [Google Scholar]
- Casson, S.A.; Gray, J.E. Influence of environmental factors on stomatal development. New Phytol. 2008, 178, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Ünyayar, S.; ÇEKİÇ, F.Ö. Changes in antioxidative enzymes of young and mature leaves of tomato seedlings under drought stress. Turk. J. Biol. 2005, 29, 211–216. [Google Scholar]







| RWC | AWC | ADR | CS | SR | HA | LHD | PB | SS | |
|---|---|---|---|---|---|---|---|---|---|
| RWC | 1 | 0.206 | 0.217 | 0.359 | 0.545 * | 0.588 * | 0.599 ** | 0.886 ** | 0.596 ** |
| AWC | 1 | 0.151 | 0.23 | 0.317 | 0.248 | −0.433 | 0.184 | −0.442 | |
| ADR | 1 | 0.78 | −0.496 | 0.464 | −0.529 | 0.429 | −0.433 | ||
| CS | 1 | 0.765 ** | 0.812 ** | −0.231 | 0.171 | −0.560 * | |||
| SR | 1 | 0.983 ** | −0.448 | 0.514 * | 0.495 * | ||||
| HA | 1 | −0.468 * | 0.513 * | 0.527 * | |||||
| LHD | 1 | 0.752 ** | 0.475 ** | ||||||
| PB | 1 | 0.541 ** | |||||||
| SS | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quandahor, P.; Lin, C.; Gou, Y.; A. Coulter, J.; Liu, C. Leaf Morphological and Biochemical Responses of Three Potato (Solanum tuberosum L.) Cultivars to Drought Stress and Aphid (Myzus persicae Sulzer) Infestation. Insects 2019, 10, 435. https://doi.org/10.3390/insects10120435
Quandahor P, Lin C, Gou Y, A. Coulter J, Liu C. Leaf Morphological and Biochemical Responses of Three Potato (Solanum tuberosum L.) Cultivars to Drought Stress and Aphid (Myzus persicae Sulzer) Infestation. Insects. 2019; 10(12):435. https://doi.org/10.3390/insects10120435
Chicago/Turabian StyleQuandahor, Peter, Chunyan Lin, Yuping Gou, Jeffrey A. Coulter, and Changzhong Liu. 2019. "Leaf Morphological and Biochemical Responses of Three Potato (Solanum tuberosum L.) Cultivars to Drought Stress and Aphid (Myzus persicae Sulzer) Infestation" Insects 10, no. 12: 435. https://doi.org/10.3390/insects10120435
APA StyleQuandahor, P., Lin, C., Gou, Y., A. Coulter, J., & Liu, C. (2019). Leaf Morphological and Biochemical Responses of Three Potato (Solanum tuberosum L.) Cultivars to Drought Stress and Aphid (Myzus persicae Sulzer) Infestation. Insects, 10(12), 435. https://doi.org/10.3390/insects10120435






