Oviposition-Induced Volatiles Affect Electrophysiological and Behavioral Responses of Egg Parasitoids
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plants
2.2. Y-Tube Olfactometer Behavioral Experiments
2.3. Oviposition-Induced Volatiles
2.4. Headspace Collection and Identification
2.5. Gas Chromatography-Flame-Ionization-Electroantennographic Detection (GC-FID-EAD)
2.6. Statistical Analysis
3. Results
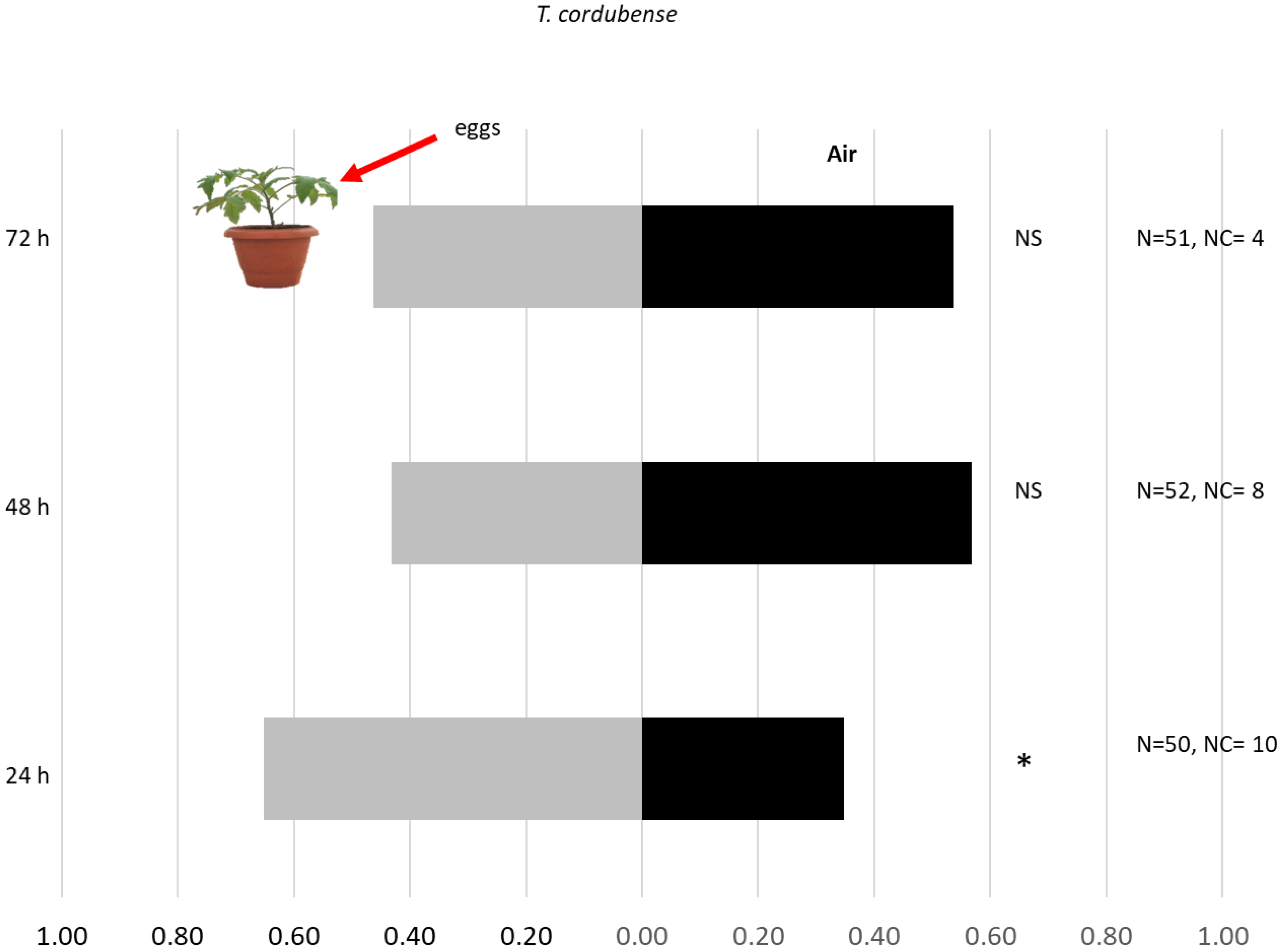
3.1. Response to Olfactometer
3.2. Headspace Volatiles
3.3. Identification of EAD Active Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the “cry for help”. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of Herbivore-Induced Plant Odors by Host-Seeking Parasitic Wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant Immunity to Insect Herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Heil, M. Indirect defence via tritrophic interactions. New Phytol. 2008, 178, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A. Induced plant defense: Evolution of induction and adaptive phenotypic plasticity. Inducible Plant Defenses Against Pathogens and Herbivores: Biochemistry, Ecology, and Agriculture; American Phytopathological Society Press: St. Paul, MN, USA, 1999; pp. 251–268. [Google Scholar]
- Agrawal, A.A. Current trends in the evolutionary ecology of plant defence. Funct. Ecol. 2011, 25, 420–432. [Google Scholar] [CrossRef]
- Hilker, M.; Fatouros, N.E. Plant responses to insect egg deposition. Annu. Rev. Entomol. 2015, 60, 493–515. [Google Scholar] [CrossRef] [PubMed]
- Colazza, S.; McElfresh, J.S.; Millar, J.G. Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid: Trissolcus basalis. J. Chem. Ecol. 2004, 30, 945–964. [Google Scholar] [CrossRef]
- Colazza, S.; Fucarino, A.; Peri, E.; Salerno, G.; Conti, E.; Bin, F. Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol. 2004, 207, 47–53. [Google Scholar] [CrossRef]
- Fatouros, N.E.; Bukovinszkine’Kiss, G.; Dicke, M.; Hilker, M. The response specificity of Trichogramma egg parasitoids towards infochemicals during host location. J. Insect Behav. 2007, 20, 53–65. [Google Scholar] [CrossRef]
- Salerno, G.; De Santis, F.; Iacovone, A.; Bin, F.; Conti, E. Short-range cues mediate parasitoid searching behavior on maize: The role of oviposition-induced plant synomones. Biol. Control 2013, 64, 247–254. [Google Scholar] [CrossRef]
- Fatouros, N.E.; Cusumano, A.; Danchin, E.G.J.; Colazza, S. Prospects of herbivore egg-killing plant defenses for sustainable crop protection. Ecol. Evol. 2016, 6, 6906–6918. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, C.; Cascone, P.; Cusumano, A.; Weldegergis, B.T.; Fatouros, N.E.; Guerrieri, E.; Dicke, M.; Gols, R. Volatile-mediated foraging behaviour of three parasitoid species under conditions of dual insect herbivore attack. Anim. Behav. 2016, 111, 197–206. [Google Scholar] [CrossRef]
- Cusumano, A.; Weldegergis, B.T.; Colazza, S.; Dicke, M.; Fatouros, N.E. Attraction of egg-killing parasitoids toward induced plant volatiles in a multi-herbivore context. Oecologia 2015, 179, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Tamiru, A.; Bruce, T.J.A.; Woodcock, C.M.; Caulfield, J.C.; Midega, C.A.O.; Ogol, C.K.P.O.; Mayon, P.; Birkett, M.A.; Pickett, J.A.; Khan, Z.R. Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol. Lett. 2011, 14, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Frati, F.; Cusumano, A.; Conti, E.; Colazza, S.; Peri, E.; Guarino, S.; Martorana, L.; Romani, R.; Salerno, G. Foraging behaviour of an egg parasitoid exploiting plant volatiles induced by pentatomids: The role of adaxial and abaxial leaf surfaces. PeerJ 2017, 5, e3326. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.-H.; Desneux, N. Ecology, Worldwide Spread, and Management of the Invasive South American Tomato Pinworm, Tuta absoluta: Past, Present, and Future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Ruescas, D.C.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Zappalà, L.; Biondi, A.; Alma, A.; Al-Jboory, I.J.; Arnò, J.; Bayram, A.; Chailleux, A.; El-Arnaouty, A.; Gerling, D.; Guenaoui, Y.; et al. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J. Pest Sci. 2013, 86, 635–647. [Google Scholar] [CrossRef]
- Urbaneja, A.; González-Cabrera, J.; Arnó, J.; Gabarra, R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag. Sci. 2012, 68, 1215–1222. [Google Scholar] [CrossRef]
- Oliveira, L.; Durão, A.C.; Fontes, J.; Roja, I.S.; Tavares, J. Potential of Trichogramma achaeae (Hymenoptera: Trichogrammatidae) in Biological Control of Tuta absoluta (Lepidoptera: Gelechiidae) in Azorean Greenhouse Tomato Crops. J. Econ. Entomol. 2017, 110, 2010–2015. [Google Scholar] [CrossRef]
- Cascone, P.; Carpenito, S.; Slotsbo, S.; Iodice, L.; Sørensen, J.G.; Holmstrup, M.; Guerrieri, E. Improving the efficiency of Trichogramma achaeae to control Tuta absoluta. BioControl 2015, 60, 761–771. [Google Scholar] [CrossRef]
- De Backer, L.; Megido, R.C.; Fauconnier, M.L.; Brostaux, Y.; Francis, F.; Verheggen, F. Tuta absoluta-induced plant volatiles: Attractiveness towards the generalist predator Macrolophus pygmaeus. Arthropod. Plant. Interact. 2015, 9, 465–476. [Google Scholar] [CrossRef]
- Silva, D.B.; Weldegergis, B.T.; Van Loon, J.J.A.; Bueno, V.H.P.; Van Loon, J.J.A.; Bueno, V.H.P. Qualitative and Quantitative Differences in Herbivore-Induced Plant Volatile Blends from Tomato Plants Infested by Either Tuta absoluta or Bemisia tabaci. J. Chem. Ecol. 2017, 43, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, E.; Balayannis, G.; Papanikolaou, N.E.; Michaelakis, A.N.; Milonas, P.G. Oviposition induced volatiles in tomato plants. Phytochem. Lett. 2015, 13, 262–266. [Google Scholar] [CrossRef]
- Silva, D.B.; Bueno, V.H.P.; Van Loon, J.J.A.; Peñaflor, M.F.G.V.; Bento, J.M.S.; Van Lenteren, J.C. Attraction of Three Mirid Predators to Tomato Infested by Both the Tomato Leaf Mining Moth Tuta absoluta and the Whitefly Bemisia Tab. J. Chem. Ecol. 2018, 44, 29–39. [Google Scholar] [CrossRef]
- Bodino, N.; Ferracini, C.; Tavella, L. Is host selection influenced by natal and adult experience in the parasitoid Necremnus tutae (Hymenoptera: Eulophidae)? Anim. Behav. 2016, 112, 221–228. [Google Scholar] [CrossRef]
- Gontijo, L.; Cascone, P.; Giorgini, M.; Michelozzi, M.; Rodrigues, H.S.; Spiezia, G.; Iodice, L.; Guerrieri, E. Relative importance of host and plant semiochemicals in the foraging behavior of Trichogramma achaeae, an egg parasitoid of Tuta absoluta. J. Pest Sci. 2019, 92, 1479–1488. [Google Scholar] [CrossRef]
- Milonas, P.G.; Martinou, A.F.; Kontodimas, D.C.; Karamaouna, F.; Konstantopoulou, M.A. Attraction of different Trichogramma species to Prays oleae sex pheromone. Ann. Entomol. Soc. Am. 2009, 102, 1145–1150. [Google Scholar] [CrossRef]
- Zakir, A.; Bengtsson, M.; Sadek, M.M.; Hansson, B.S.; Witzgall, P.; Anderson, P. Specific response to herbivore-induced de novo synthesized plant volatiles provides reliable information for host plant selection in a moth. J. Exp. Biol. 2013, 216, 3257–3263. [Google Scholar] [CrossRef]
- Anastasaki, E.; Drizou, F.; Milonas, P.G. Electrophysiological and Oviposition Responses of Tuta absoluta Females to Herbivore-Induced Volatiles in Tomato Plants. J. Chem. Ecol. 2018, 44, 288–298. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Pub Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Agresti, A. Categorical Data Analysis, 3rd ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Song, C.; Lai, W.C.; Reddy, K.M.; Wei, B. Temperature-programmed retention indices for GC and GC-MS of hydrocarbon fuels and simulated distillation GC of heavy oils. In Analytical Advances for Hydrocarbon Research; Springer: Boston, MA, USA, 2003; pp. 147–210. [Google Scholar]
- Maselou, D.A.; Anastasaki, E.; Milonas, P.G. The role of host plants, alternative food resources and herbivore induced volatiles in choice behavior of an omnivorous predator. Front. Ecol. Evol. 2019, 6, 241. [Google Scholar] [CrossRef]
- Fatouros, N.E.; Dicke, M.; Mumm, R.; Meiners, T.; Hilker, M. Foraging behavior of egg parasitoids exploiting chemical information. Behav. Ecol. 2008, 19, 677–689. [Google Scholar] [CrossRef]
- Tamiru, A.; Bruce, T.J.A.; Midega, C.A.O.; Woodcock, C.M.; Birkett, M.A.; Pickett, J.A.; Khan, Z.R. Oviposition Induced Volatile Emissions from African Smallholder Farmers’ Maize Varieties. J. Chem. Ecol. 2012, 38, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Midega, C.A.O.; Birkett, M.A.; Pickett, J.A.; Khan, Z.R. Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol. Lett. 2009, 6, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Raina, R.; Joseph, M.; Tungikar, V.B. Response of Trichogramma chilonis to infochemicals: An SEM and electrophysiological investigation. BioControl 2005, 50, 429–447. [Google Scholar] [CrossRef]
- Ángeles López, Y.I.; Martínez-Gallardo, N.A.; Ramírez-Romero, R.; López, M.G.; Sánchez-Hernández, C.; Délano-Frier, J.P. Cross-Kingdom Effects of Plant-Plant Signaling via Volatile Organic Compounds Emitted by Tomato (Solanum lycopersicum) Plants Infested by the Greenhouse Whitefly (Trialeurodes vaporariorum). J. Chem. Ecol. 2012, 38, 1376–1386. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Light, D.M. Tomato leaf volatile aroma components. J. Agric. Food Chem 1987, 35, 1039–1042. [Google Scholar] [CrossRef]
- Kant, M.R.; Ament, K.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 2004, 135, 483–495. [Google Scholar] [CrossRef]
- Dicke, M. Behavioural and community ecology of plants that cry for help. Plant. Cell Environ. 2009, 32, 654–665. [Google Scholar] [CrossRef]
- Du, W.M.; Xu, J.; Hou, Y.Y.; Lin, Y.; Zang, L.S.; Yang, X.; Zhang, J.J.; Ruan, C.C.; Desneux, N. Trichogramma parasitoids can distinguish between fertilized and unfertilized host eggs. J. Pest Sci. 2018, 91, 771–780. [Google Scholar] [CrossRef]
- Fatouros, N.E.; Lucas-Barbosa, D.; Weldegergis, B.T.; Pashalidou, F.G.; van Loon, J.J.A.; Dicke, M.; Harvey, J.A.; Gols, R.; Huigens, M.E. Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 2012, 7, e43607. [Google Scholar] [CrossRef] [PubMed]



| No | RI 1 | RIL 2 | Compound | Identification | Control | Hours after Oviposition | p Value | ||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||||||
| 1 | 800 | 800 A | octane | STD, MS, RI | 0.004 ± 0.004 | 0.003 ± 0.002 | nd | 0.003 ± 0.003 | 0.584 |
| 2 | 853 | 853 B | (Z)-3-hexen-1-ol | STD, MS, RI | nd 3 | nd | nd | 0.394 ± 0.381 | 0.097 |
| 3 | 858 | 858 B | p-xylene | MS, RI | 0.003 ± 0.001 | 0.008 ± 0.005 | 0.001 ± 0.001 | 0.008 ± 0.005 | 0.404 |
| 4 | 864 | 864 B | m-xylene | MS, RI | 0.004 ± 0.002 | 0.009 ± 0.004 | 0.002 ± 0.001 | 0.003 ± 0.003 | 0.404 |
| 5 | 887 | 890 B | o-xylene | MS, RI | nd | 0.003 ± 0.002 | nd | 0.006 ± 0.006 | 0.171 |
| 6 | 921 | 924 A | a-thujene | MS, RI | 0.001 ± 0.000 a,5 | 0.001 ± 0.000 a | nd a | 0.005 ± 0.002 b | 0.018 |
| 7 | 932 | 932 A | a-pinene | STD, MS, RI | 0.553 ± 0.042 | 0.541 ± 0.038 | 0.465 ± 0.024 | 0.727 ± 0.124 | 0.128 |
| 8 | 955 | Unk 1 4 | m/z:105, 120, 91 | 0.002 ± 0.002 | 0.004 ± 0.002 | 0.004 ± 0.002 | 0.005 ± 0.002 | 0.672 | |
| 9 | 958 | Unk 2 | m/z:105, 120, 106, 77 | 0.005 ± 0.003 | 0.002 ± 0.001 | nd | 0.010 ± 0.007 | 0.195 | |
| 10 | 970 | 970 C | verbenene | MS, RI | 0.381 ± 0.007a | 0.461 ± 0.055 a,b | 0.338 ± 0.022 a | 0.570 ± 0.052 b | 0.020 |
| 11 | 973 | 974 C | sabinene | STD, MS, RI | 0.016 ± 0.005 | 0.036 ± 0.012 | 0.011 ± 0.004 | 0.038 ± 0.013 | 0.199 |
| 12 | 978 | 980 A | β-pinene | STD, MS, RI | 0.001 ± 0.001 | 0.001 ± 0.001 | nd | 0.001 ± 0.0000 | 0.498 |
| 13 | 990 | 988 A | β-myrcene | STD, MS, RI | 0.153 ± 0.017 | 0.181 ± 0.040 | 0.103 ± 0.014 | 0.176 ± 0.016 | 0.091 |
| 14 | 1000 | 1001 A | 2-δ-carene | MS, RI | 2.731 ± 0.220 a,b | 2.435 ± 0.137 a | 2.190 ± 0.358 a | 3.883 ± 0.643 b | 0.032 |
| 15 | 1005 | 1002 A | α-phellandrene | STD, MS, RI | 0.462 ± 0.041 a,b | 0.446 ± 0.009 a | 0.380 ± 0.047 a | 0.700 ± 0.086 b | 0.010 |
| 16 | 1015 | 1014 A | α-terpinene | STD, MS, RI | 0.166 ± 0.021 a,b | 0.161 ± 0.020 a,b | 0.117 ± 0.027 a | 0.237 ± 0.027 b | 0.034 |
| 17 | 1024 | 1020 A | p-cymene | STD, MS, RI | 0.041 ± 0.017 | 0.042 ± 0.015 | 0.012 ± 0.002 | 0.025 ± 0.008 | 0.146 |
| 18 | 1029 | 1031 C | β-phellandrene | MS, RI | 7.556 ± 0.358 a | 8.333 ± 0.419 a,b | 6.995 ± 0.980 a | 11.402 ± 0.987 b | 0.011 |
| 19 | 1035 | 1032 E | benzyl alcohol | MS, RI | nd a | nd a | 0.002 ± 0.001 a,b | 0.089 ± 0.041 b | 0.011 |
| 20 | 1038 | 1037 A | (Z)-β-ocimene | MS, RI | 0.017 ± 0.003 | 0.020 ± 0.004 | 0.006 ± 0.002 | 0.025 ± 0.009 | 0.060 |
| 21 | 1049 | 1044 A | (E)-β-ocimene | STD, MS, RI | 0.073 ± 0.004 b | 0.048 ± 0.010 a | 0.047 ± 0.006 a | 0.083 ± 0.017 b | 0.047 |
| 22 | 1059 | 1054 A | γ-terpinene | STD, MS, RI | 0.025 ± 0.001 a | 0.021 ± 0.002 a | 0.029 ± 0.006 a,b | 0.037 ± 0.003 b | 0.035 |
| 23 | 1085 | 1086 A | terpinolene | STD, MS, RI | 0.032 ± 0.004 a,b | 0.039 ± 0.010 a,b | 0.026 ± 0.004 a | 0.049 ± 0.004 b | 0.029 |
| 24 | 1108 | 1108 C | nonanal | STD, MS, RI | 0.027 ± 0.015 | 0.095 ± 0.042 | 0.036 ± 0.016 | 0.025 ± 0.009 | 0.336 |
| 25 | 1115 | Terpene 1 | m/z:93, 136, 121, 91, 79 | 0.008 ± 0.002 a,b | 0.004 ± 0.002 a | 0.010 ± 0.001 a,b | 0.017 ± 0.003 b | 0.011 | |
| 26 | 1122 | 1118 A | cis-p-menth-2-en-1-ol | MS, RI | nd | nd | 0.001 ± 0.001 | 0.002 ± 0.001 | 0.061 |
| 27 | 1124 | 1119 A | trans-p-mentha-2,8-dien-1-ol | MS, RI | 0.001 ± 0.000 | nd | 0.001 ± 0.001 | 0.015 ± 0.014 | 0.102 |
| 28 | 1133 | 1133 A | cis-p-mentha-2,8-dien-1-ol | MS, RI | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.006 ± 0.002 | 0.177 |
| 29 | 1141 | 1141 A | camphor | STD, MS, RI | 0.007 ± 0.005 | nd | 0.007 ± 0.005 | 0.001 ± 0.000 | 0.357 |
| 30 | 1173 | Unk 3 | m/z:109,79,91 | 0.005 ± 0.004 | nd | 0.001 ± 0.001 | 0.007 ± 0.004 | 0.107 | |
| 31 | 1175 | 1177 A | (E)-isocitral | MS, RI | 0.004 ± 0.001 | 0.007 ± 0.004 | 0.003 ± 0.002 | 0.019 ± 0.006 | 0.211 |
| 32 | 1185 | 1184 A | dill ether | MS, RI | 0.008 ± 0.002 | 0.007 ± 0.005 | 0.008 ± 0.002 | 0.014 ± 0.003 | 0.151 |
| 33 | 1195 | 1195 C | methyl salicylate | STD, MS, RI | nda | nda | 0.002 ± 0.001a | 0.025 ± 0.009 b | 0.001 |
| 34 | 1200 | 1200 A | dodecane | STD, MS, RI | 0.022 ± 0.010 | 0.039 ± 0.018 | 0.020 ± 0.007 | 0.031 ± 0.017 | 0.902 |
| 35 | 1208 | 1208 C | decanal | STD, MS, RI | 0.014 ± 0.007 | 0.032 ± 0.013 | 0.019 ± 0.008 | 0.015 ± 0.007 | 0.417 |
| 36 | 1231 | 1232 A | (Z)-3-hexenyl-2-methyl butanoate | STD, MS, RI | nd a | nd a | 0.004 ± 0.002 b | 0.003 ± 0.002 a,b | 0.017 |
| 37 | 1237 | 1234 A | ascaridole | 0.003 ± 0.001 b | nd a | 0.001 ± 0.000 a,b | 0.003 ± 0.001 b | 0.023 | |
| 38 | 1247 | 1244 A | car-3-en-2-one | MS, RI | 0.001 ± 0.001 | nd | nd | 0.001 ± 0.000 | 0.095 |
| 39 | 1300 | 1300 A | tridecane | STD, MS, RI | 0.022 ± 0.010 | 0.011 ± 0.006 | 0.015 ± 0.008 | 0.007 ± 0.002 | 0.478 |
| 40 | 1304 | Unk 4 | m/z:97, 54, 69 | 0.003 ± 0.001 | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.004 ± 0.002 | 0.112 | |
| 41 | 1333 | 1335 A | δ-elemene | MS, RI | 0.132 ± 0.016 | 0.173 ± 0.027 | 0.111 ± 0.020 | 0.195 ± 0.044 | 0.448 |
| 42 | 1349 | Ester 1 | m/z: 71, 83 | 0.001 ± 0.001 | nd | nd | 0.010 ± 0.007 | 0.100 | |
| 43 | 1355 | Unk 4 | m/z:57, 71, 85 | 0.001 ± 0.001 | nd | nd | 0.017 ± 0.015 | 0.100 | |
| 44 | 1370 | Ester 2 | m/z:71, 89, 56 | 0.001 ± 0.001 | 0.001 ± 0.001 | nd | 0.002 ± 0.002 | 0.265 | |
| 45 | 1374 | 1374 A | α-copaene | MS, RI | 0.013 ± 0.007 | 0.002 ± 0.002 | 0.001 ± 0.001 | 0.010 ± 0.002 | 0.053 |
| 46 | 1387 | 1389 A | β-elemene | STD, MS, RI | 0.016 ± 0.002 | 0.034 ± 0.012 | 0.014 ± 0.003 | 0.038 ± 0.012 | 0.126 |
| 47 | 1400 | 1400 A | tetradecane | STD, MS, RI | 0.059 ± 0.028 | 0.061 ± 0.028 | 0.034 ± 0.017 | 0.033 ± 0.010 | 0.763 |
| 48 | 1417 | 1417 A | β-caryophyllene | STD, MS, RI | 0.367 ± 0.028 | 0.341 ± 0.033 | 0.263 ± 0.045 | 0.517 ± 0.115 | 0.164 |
| 49 | 1427 | 1432 D | γ-elemene | MS, RI | 0.005 ± 0.002 | 0.002 ± 0.002 | 0.002 ± 0.000 | 0.007 ± 0.002 | 0.056 |
| 50 | 1439 | 1442 A | guaidiene-6,9 | MS, RI | 0.010 ± 0.001 | 0.020 ± 0.006 | 0.008 ± 0.002 | 0.014 ± 0.004 | 0.179 |
| 51 | 1447 | 1448 A | muurola-3,5-diene | MS, RI | 0.005 ± 0.002 | 0.001 ± 0.001 | nd | 0.003 ± 0.001 | 0.085 |
| 52 | 1459 | 1459 D | α-humulene | STD, MS, RI | 0.077 ± 0.008 | 0.075 ± 0.006 | 0.050 ± 0.008 | 0.103 ± 0.023 | 0.114 |
| 53 | 1481 | 1484 A | germacrene D | MS, RI | 0.011 ± 0.000 | 0.009 ± 0.003 | 0.010 ± 0.003 | 0.016 ± 0.003 | 0.281 |
| 54 | 1495 | 1500 A | α-muurolene | MS, RI | 0.006 ± 0.002 b | 0.001 ± 0.001 a | 0.001 ± 0.001 a | 0.005 ± 0.001 b | 0.008 |
| 55 | 1500 | 1500 A | pentadecane | STD, MS, RI | 0.011 ± 0.006 | 0.018 ± 0.006 | 0.009 ± 0.003 | 0.014 ± 0.005 | 0.207 |
| 56 | 1504 | 1508 Α | germacrene Α | MS, RI | 0.001 ± 0.001 b | nd a | nd a | 0.004 ± 0.003 b | 0.020 |
| 57 | 1524 | Terpene 2 | m/z:121, 93, 91, 105, 161 | 0.001 ± 0.000 a | 0.001 ± 0.000 a | nd a | 0.003 ± 0.001 b | 0.003 | |
| 58 | 1552 | Unk 5 | m/z:55, 83, 69 | 0.005 ± 0.002 b | 0.001 ± 0.001 a | 0.001 ± 0.000a | 0.005 ± 0.002 b | 0.007 | |
| 59 | 1557 | 1559 A | germacrene B | MS, RI | 0.008 ± 0.002 b | 0.016 ± 0.005 b | 0.002 ± 0.001a | 0.008 ± 0.002 b | 0.018 |
| 60 | 1562 | 1561 A | nerolidol | STD, MS, RI | 0.007 ± 0.003 c | 0.001 ± 0.001 b | nd a,b | 0.008 ± 0.002 c | 0.002 |
| 61 | 1574 | 1573 C | (Ε-Ε)-TMTT | MS, RI | 0.016 ± 0.004 | 0.014 ± 0.007 | 0.003 ± 0.001 | 0.004 ± 0.001 | 0.152 |
| 62 | 1581 | 1582 A | caryophyllene oxide | STD, MS, RI | 0.008 ± 0.002 | 0.003 ± 0.002 | 0.006 ± 0.001 | 0.013 ± 0.005 | 0.229 |
| 63 | 1598 | Terpene 3 | m/z:93, 80, 121, 149 | nd a | nd a | nd a | 0.017 ± 0.015 b | 0.003 | |
| 64 | 1600 | 1600 A | hexadecane | STD, MS, RI | 0.278 ± 0.145 | 0.136 ± 0.055 | 0.117 ± 0.056 | 0.146 ± 0.068 | 0.831 |
| 65 | 1608 | 1608 A | Humulene epoxide II | MS, RI | 0.004 ± 0.004 | nd | nd | 0.044 ± 0.031 | 0.222 |
| 66 | 1621 | Terpene 4 | m/z: 81, 161, 105, 119, 93 | nd | nd | 0.016 ± 0.011 | 0.080 ± 0.068 | 0.195 | |
| 67 | 1630 | 1630 A | muurola-4,10 (14)-dien-1b-ol | MS, RI | 0.004 ± 0.001 | 0.002 ± 0.001 | nd | 0.011 ± 0.006 | 0.078 |
| 68 | 1641 | 1639 A | Allo-aromadendrene epoxide | MS, RI | nd | nd | nd | 0.081 ± 0.080 | 0.222 |
| Total | 13.40 ± 0.43 a | 13.90 ± 0.41 a | 11.51 ± 1.35 a | 20.08 ± 1.91 b | 0.005 | ||||
| No. | Compound | VIP Value |
|---|---|---|
| 1 | α-phellandrene | 1.97 |
| 2 | 2-δ-carene | 1.92 |
| 3 | β-phellandrene | 1.88 |
| 4 | benzyl alcohol | 1.84 |
| 5 | verbenene | 1.75 |
| 6 | α-terpinene | 1.70 |
| 7 | β-caryophyllene | 1.54 |
| 8 | β-myrcene | 1.52 |
| 9 | δ-elemene | 1.42 |
| 10 | nonanal | 1.40 |
| 11 | α-pinene | 1.39 |
| 12 | p-cymene | 1.29 |
| 13 | (E)-β-ocimene | 1.28 |
| 14 | allo-aromadendrene epoxide | 1.26 |
| 15 | γ-terpinene | 1.24 |
| 16 | α-humulene | 1.23 |
| 17 | germacrene B | 1.22 |
| 18 | (E)-isocitral | 1.18 |
| 19 | terpinolene | 1.14 |
| 20 | muurola-4,10 (14)-dien-1b-ol | 1.14 |
| 21 | β-elemene | 1.13 |
| 22 | sabinene | 1.13 |
| 23 | unknown 5 | 1.10 |
| 24 | p-xylene | 1.09 |
| 25 | terpene 1 | 1.07 |
| 26 | hydrocarbon 1 | 1.06 |
| 27 | camphor | 1.03 |
| 28 | unknown 2 | 1.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milonas, P.G.; Anastasaki, E.; Partsinevelos, G. Oviposition-Induced Volatiles Affect Electrophysiological and Behavioral Responses of Egg Parasitoids. Insects 2019, 10, 437. https://doi.org/10.3390/insects10120437
Milonas PG, Anastasaki E, Partsinevelos G. Oviposition-Induced Volatiles Affect Electrophysiological and Behavioral Responses of Egg Parasitoids. Insects. 2019; 10(12):437. https://doi.org/10.3390/insects10120437
Chicago/Turabian StyleMilonas, Panagiotis G, Eirini Anastasaki, and Georgios Partsinevelos. 2019. "Oviposition-Induced Volatiles Affect Electrophysiological and Behavioral Responses of Egg Parasitoids" Insects 10, no. 12: 437. https://doi.org/10.3390/insects10120437
APA StyleMilonas, P. G., Anastasaki, E., & Partsinevelos, G. (2019). Oviposition-Induced Volatiles Affect Electrophysiological and Behavioral Responses of Egg Parasitoids. Insects, 10(12), 437. https://doi.org/10.3390/insects10120437





