The First Complete Mitochondrial Genomes of Two Sibling Species from Nitidulid Beetles Pests
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Extraction
2.2. Sequencing and Assembly
2.3. Gene Identification and Analyses
3. Results
3.1. Mt Genome Sequencing and Assembly
3.2. Mt Genome Organization and Nucleotide Composition
3.3. Protein Coding Genes
3.4. The tRNA and rRNA Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dobson, R.M. The species of Carpophilus stephens (Col. Nitidulidae) associated with stored products. Bull. Entomol. Res. 1954, 45, 389–402. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wu, Z. Beetles of Stored Products, 1st ed.; Science press: Beijing, China, 2016; pp. 269–280. [Google Scholar]
- Marini, F.; Mutinelli, F.; Montarsi, F.; Cline, A.R.; Gatti, E.; Audisio, P. First report in Italy of the dusky sap beetle, Carpophilus lugubris, a new potential pest for Europe. J. Pest Sci. 2013, 86, 157–160. [Google Scholar] [CrossRef]
- Brown, S.D.J.; Armstrong, K.F.; Cruickshank, R.H. Molecular phylogenetics of a South Pacific sap beetle species complex (Carpophilus spp., Coleoptera: Nitidulidae). Mol. Phylogenet. Evol. 2012, 64, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Valero, K.A.; Hannel, M.M.; Gill, R.F. Seasonal occurrence of postharvest dried fruit insects and their parasitoids in a culled fig warehouse. J. Econ. Entomol. 2000, 93, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.N.; Wingfield, M.J.; Van Wyk, M.; Roux, J. Insect associates of Ceratocystis albifundus and patterns of association in a native savanna ecosystem in South Africa. Environ. Entomol. 2009, 38, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Ewing, C.P.; Cline, A.R. Key to adventive sap beetles (Coleoptera: Nitidulidae) in Hawaii, with notes on records and habits. Coleopts. Bull. 2005, 59, 167–183. [Google Scholar] [CrossRef]
- Bai, C.; Cao, Y.; Zhou, Y.; Wu, Y. Molecular diagnosis Carpophilus dimidiatu (Coleoptera: Nitidulidae) based on species-specific PCR. J. Stored Prod. Res. 2017, 74, 87–90. [Google Scholar] [CrossRef]
- Reales, N.; Rocamundi, N.; Marvaldi, A.E.; Fernández-Górgolas, M.C.; Stadler, T. Morphological and molecular identification of Carpophilus dimidiatus (Coleoptera: Nitidulidae) associated with stored walnut in Northwestern Argentina. J. Stored Prod. Res. 2018, 76, 37–42. [Google Scholar] [CrossRef]
- Connell, W.A. Carpophilus pilosellus motschulsky, new synonymy and distribution (Coleoptera: Nitidulidae). Coleopt. Bull. 1963, 17, 89–90. [Google Scholar]
- Wang, D.; Bai, X.; Zhou, Y.; Zhao, Y. Illustrated Book of Stored Grain Insects in China, 1st ed.; China Agricultural Science and Technology Press: Beijing, China, 2008; pp. 17–18. [Google Scholar]
- Toon, A.; Daglish, G.J.; Ridley, A.W.; Emery, R.N.; Holloway, J.C.; Walter, G.H. Significant population structure in Australian Cryptolestes ferrugineus and interpreting the potential spread of phosphine resistance. J. Stored Prod. Res. 2018, 77, 219–224. [Google Scholar] [CrossRef]
- Ndiaye, M.R.; Sembène, M. Genetic structure and phylogeographic evolution of the West African populations of Sitophilus zeamais (Coleoptera, Curculionidae). J. Stored Prod. Res. 2018, 77, 135–143. [Google Scholar] [CrossRef]
- Devi, S.R.; Thomas, A.; Rebijith, K.B.; Ramamurthy, V.V. Biology, morphology and molecular characterization of Sitophilus oryzae and S. zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 2017, 73, 135–141. [Google Scholar] [CrossRef]
- Wolstenholme, D.R. Animal mitochondrial DNA: Structure and evolution. Int. Rev. Cytol. 1992, 141, 173–216. [Google Scholar] [PubMed]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef]
- Karagozlu, M.Z.; An, H.; Park, S.H.; Shin, S.E.; Kim, C. Comparative analyses of the three complete mitochondrial genomes from forensic important beetle genus Dermestes with phylogenetic relationships. Gene 2019, 706, 146–153. [Google Scholar] [CrossRef]
- Jiang, F.; Pan, X.; Li, X.; Yu, Y.; Zhang, J.; Jiang, H.; Dou, L.; Zhu, S. The first complete mitochondrial genome of Dacus longicornis (Diptera:Tephritidae) using next-generation sequencing and mitochondrial genome phylogeny of dacini tribe. Sci. Rep. 2016, 6, 36426. [Google Scholar] [CrossRef]
- Song, F.; Li, H.; Jiang, P.; Zhou, X.; Liu, J.; Sun, C.; Vogler, A.P.; Cai, W. Capturing the phylogeny of Holometabola with mitochondrial genome data and Bayesian Site-Heterogeneous Mixture Models. Genome Biol. Evol. 2016, 8, 1411–1426. [Google Scholar] [CrossRef]
- Feng, S.; Yang, Q.; Li, H.; Song, F.; Stejskal, V.; Opit, G.P.; Cai, W.; Li, Z.; Shao, R. The highly divergent mitochondrial genomes indicate that the booklouse, Liposcelis bostrychophila (Psocoptera: Liposcelididae) is a cryptic species. G3-Genes Genome Genet. 2018, 8, 1039–1047. [Google Scholar] [CrossRef]
- Lohse, M.; Bolger, A.M.; Nagel, A.; Fernie, A.R.; Lunn, J.E.; Stitt, M.; Usadel, B. RobiNA: A user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res. 2012, 40, W622–W627. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Kambhampati, S.; Smith, P.T. PCR primers for the amplification of four insect mitochondrial gene fragments. Insect Mol. Biol. 1995, 4, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Bernt, M.; Dnonath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canbäck, B. ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Grant, J.R.; Stothard, P. The CGView Server: A comparative genomics tool for circular genomes. Nucleic Acids Res. 2008, 36, W181–W184. [Google Scholar] [CrossRef]
- Sun, T.Y.; Li, L.; Xin, T.; Wang, Y.; Xia, B. The complete mitochondrial genome of Cryptolestes ferrugineus (stephens) (Coleoptera: Laemophloeidae). Mitochondr. DNA Part A 2015, 27, 3676–3677. [Google Scholar] [CrossRef]
- Friedrich, M.; Muqim, N. Sequence and phylogenetic analysis of the complete mitochondrial genome of the flour beetle Tribolium castanaeum. Mol. Phylogenet. Evol. 2003, 26, 502–512. [Google Scholar] [CrossRef]
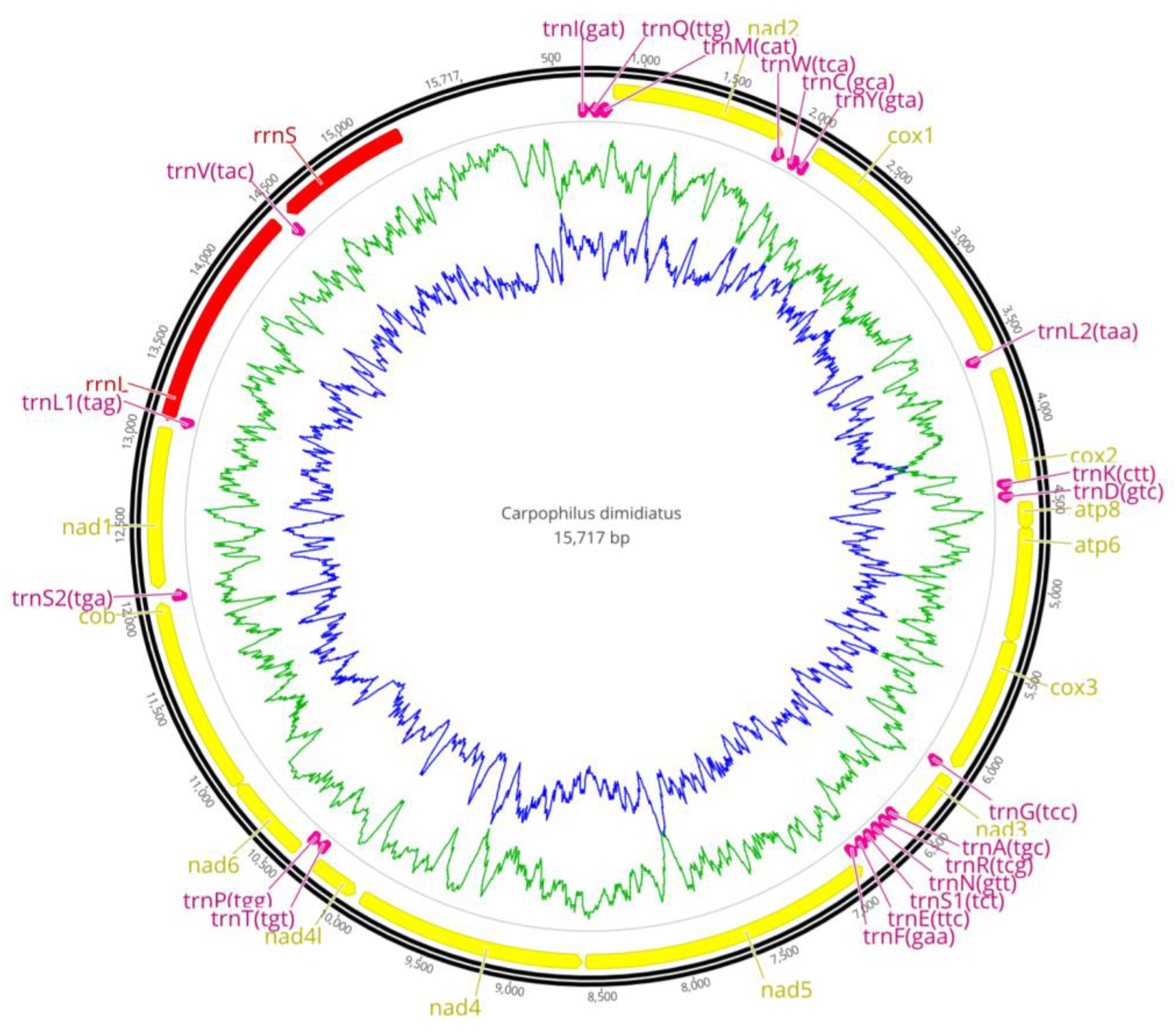

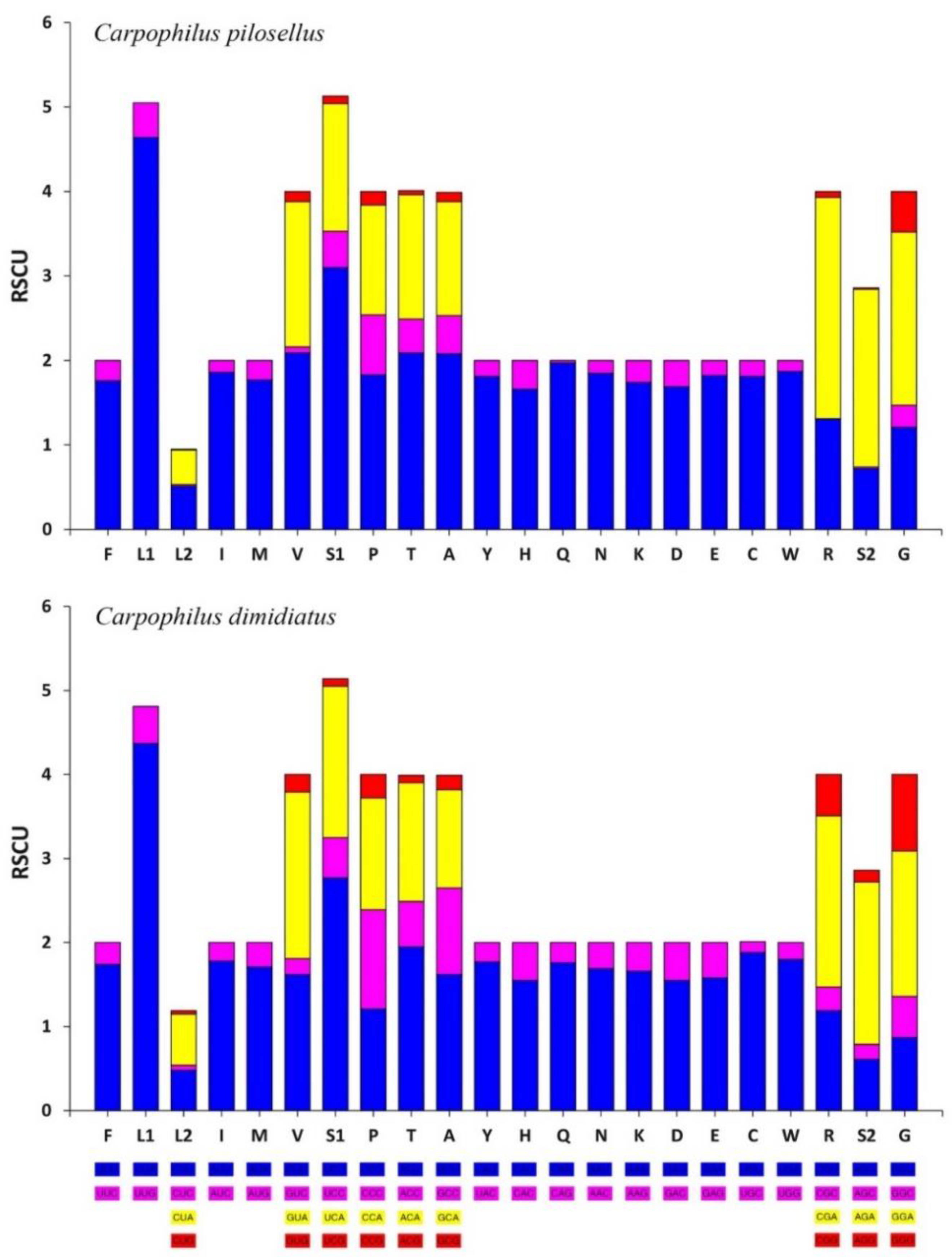
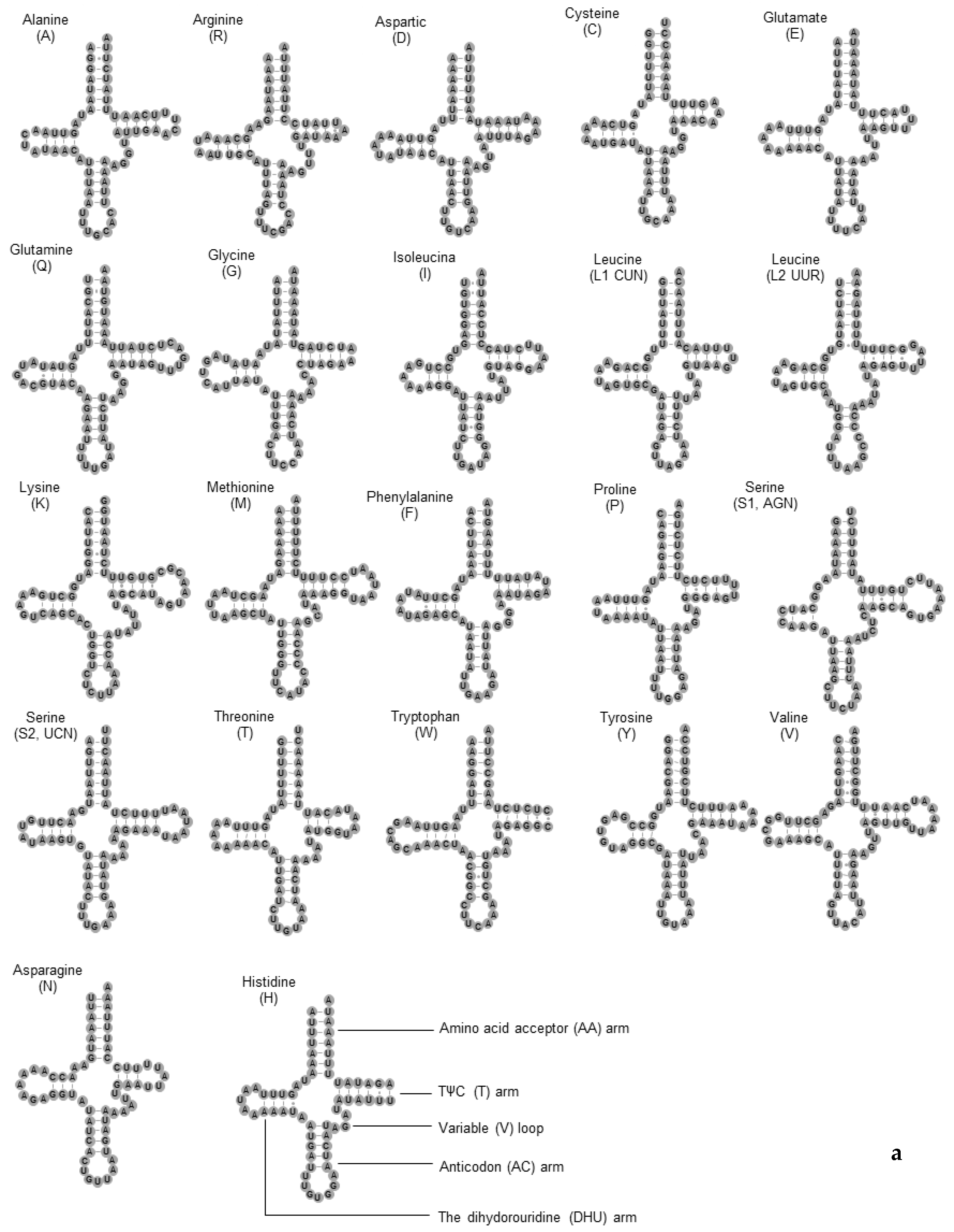
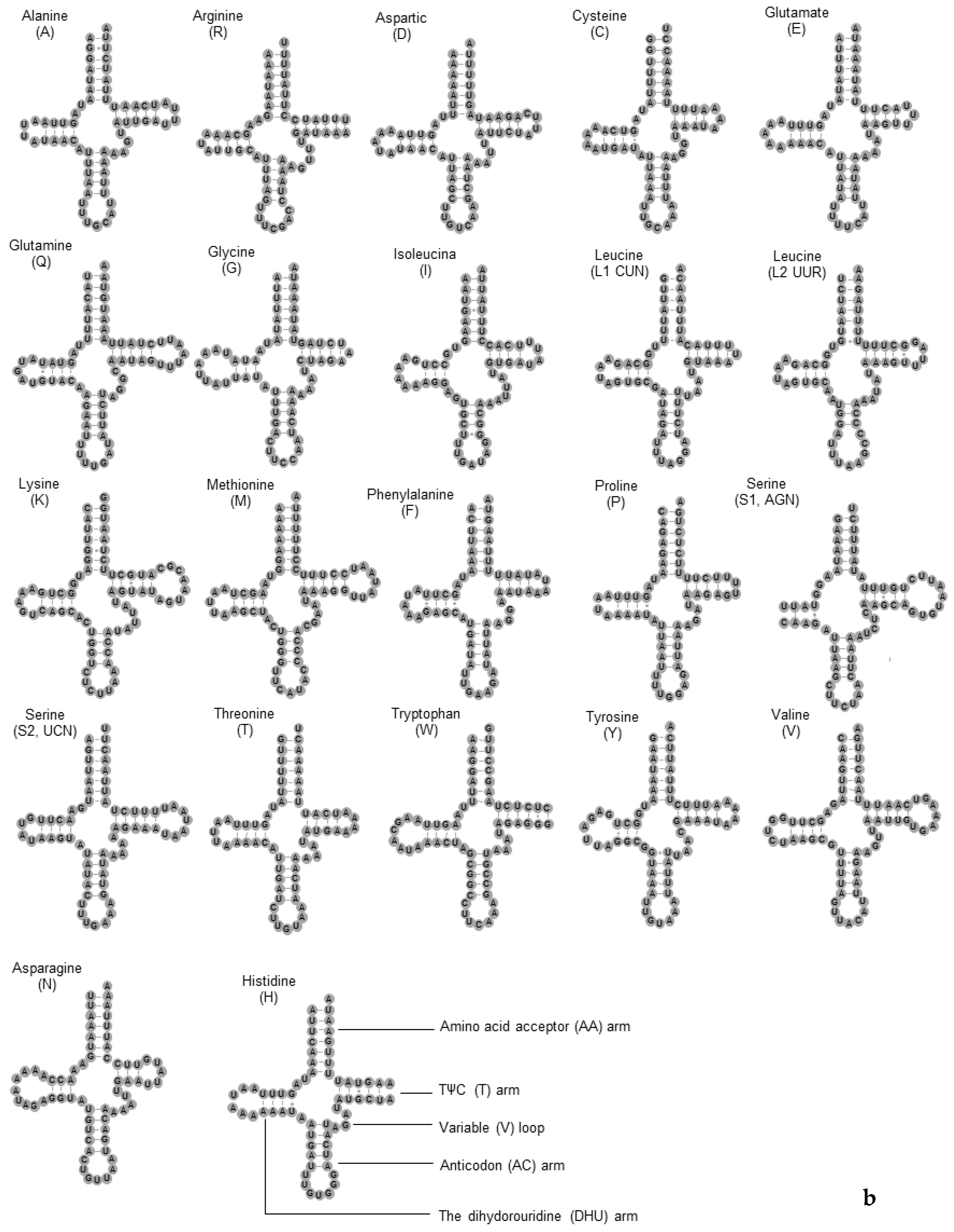
| Gene | Strand | Position | Length (bp) | Anti/Start Codon | Stop Codon | Intergenic Sequence (bp) |
|---|---|---|---|---|---|---|
| rrnS | N | 14505-15293/14565-15352 | 789/788 | 0 | ||
| trnV (tac) | N | 14436-14504/14496-14564 | 69 | 0 | ||
| rrnL | N | 13144-14418/13205-14472 | 1275/1268 | 15/23 | ||
| trnL1 (tag) | N | 13085-13146/13146-13207 | 62 | 1 | ||
| nad1 | N | 12133-13065/12195-13142 | 933/948 | ATA/ATG | TAG | 19/3 |
| trnS2 (tga) | J | 12048-12115/12110-12177 | 68 | 17 | ||
| cob | J | 10910-12049/10969-12111 | 1140/1143 | ATG | TAG | −2 |
| nad6 | J | 10404-10910/10463-10969 | 507 | ATA | TAA | −1 |
| trnP (tgg) | N | 10337-10399/10396-10458 | 63 | 4 | ||
| trnT (tgt) | J | 10273-10336/10332-10395 | 64 | 0 | ||
| nad4l | N | 9989-10270/10048-10329 | 282 | ATG | TAA | 2 |
| nad4 | N | 8669-9980/8716-10051 | 1312/1336 | ATT/ATA | T | 8/−4 |
| trnH (cac) | N | 8608-8671/8666-8730 | 64/65 | −3/−15 | ||
| nad5 | N | 6880-8592/6938-8665 | 1713/1728 | ATT | TAA | 17/0 |
| trnF (gaa) | N | 6832-6896/6891-6954 | 65/64 | −17 | ||
| trnE (ttc) | J | 6771-6833/6830-6892 | 63 | −2 | ||
| trnS1 (tct) | J | 6704-6770/6763-6829 | 67 | 0 | ||
| trnN (gtt) | J | 6640-6703/6697-6762 | 64/66 | 0 | ||
| trnR (tcg) | J | 6577-6639/6635-6697 | 63 | 0/−1 | ||
| trnA (tgc) | J | 6513-6577/6571-6635 | 65 | −1 | ||
| nad3 | J | 6161-6514/6225-6572 | 354/348 | ATT | TAG | −2 |
| trnG (tcc) | J | 6097-6160/6155-6218 | 64 | 0/6 | ||
| cox3 | J | 5310-6096/5368-6154 | 787 | ATG | T | 0 |
| atp6 | J | 4642-5310/4703-5368 | 669/666 | ATG/ATA | TAA | −1 |
| atp8 | J | 4493-4648/4551-4706 | 156 | ATC | TAG | −7/−4 |
| trnD (gtc) | J | 4427-4492/4485-4550 | 66 | 0 | ||
| trnK (ctt) | J | 4352-4422/4410-4480 | 71 | 4 | ||
| cox2 | J | 3664-4386/3722-4409 | 723/688 | ATC/ATT | TAA/T | −35 |
| trnL2 (taa) | J | 3599-3663/3657-3721 | 65 | 0 | ||
| cox1 | J | 2059-3603/2117-3661 | 1545 | ATT | TAA | 3/−5 |
| trnY (gta) | N | 2001-2066/2059-2124 | 66 | −8 | ||
| trnC (gca) | N | 1939-2000/1997-2058 | 62 | 0 | ||
| trnW (tca) | J | 1848-1914/1821-1887 | 67 | 24/109 | ||
| nad2 | J | 837-1844/828-1817 | 1008/990 | ATT | TAA | 3 |
| trnM (cat) | J | 768-836/741-809 | 69 | 0/18 | ||
| trnQ (ttg) | N | 696-764/672-740 | 69 | 3/0 | ||
| trnI (gat) | J | 634-698/611-674 | 65/64 | −3 |
| Feature | A + T% | AT-Skew | GC-Skew | Similarity | |||
|---|---|---|---|---|---|---|---|
| C. dimidiatus | C. pilosellus | C. dimidiatus | C. pilosellus | C. dimidiatus | C. pilosellus | ||
| Whole mt genome | 75.2 | 77.2 | 0.037 | 0.026 | −0.202 | −0.175 | 86.7 |
| PCGs | 74.3 | 76.6 | 0.039 | 0.023 | −0.191 | −0.149 | 86.6 |
| tRNA | 74.9 | 76.3 | 0.031 | 0.020 | −0.092 | −0.089 | 91.6 |
| tRNA genes | 75.0 | 78.5 | 0.091 | 0.070 | −0.373 | −0.361 | 89.6 |
| rrnL gene | 74.9 | 75.4 | 0.036 | 0.053 | −0.331 | −0.360 | 91.1 |
| rrnS gene | 75.2 | 77.2 | 0.037 | 0.026 | −0.202 | −0.175 | 86.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Lan, Y.; Xia, L.; Cui, M.; Sun, W.; Dong, Z.; Cao, Y. The First Complete Mitochondrial Genomes of Two Sibling Species from Nitidulid Beetles Pests. Insects 2020, 11, 24. https://doi.org/10.3390/insects11010024
Wu Y, Lan Y, Xia L, Cui M, Sun W, Dong Z, Cao Y. The First Complete Mitochondrial Genomes of Two Sibling Species from Nitidulid Beetles Pests. Insects. 2020; 11(1):24. https://doi.org/10.3390/insects11010024
Chicago/Turabian StyleWu, Yi, Yangming Lan, Liyuan Xia, Miao Cui, Weiwei Sun, Zhen Dong, and Yang Cao. 2020. "The First Complete Mitochondrial Genomes of Two Sibling Species from Nitidulid Beetles Pests" Insects 11, no. 1: 24. https://doi.org/10.3390/insects11010024
APA StyleWu, Y., Lan, Y., Xia, L., Cui, M., Sun, W., Dong, Z., & Cao, Y. (2020). The First Complete Mitochondrial Genomes of Two Sibling Species from Nitidulid Beetles Pests. Insects, 11(1), 24. https://doi.org/10.3390/insects11010024




