Effects of Urbanization on Plant–Pollinator Interactions in the Tropics: An Experimental Approach Using Exotic Plants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Site Selection
2.2. Experimental Exotic Plant Communities
2.3. Sampling
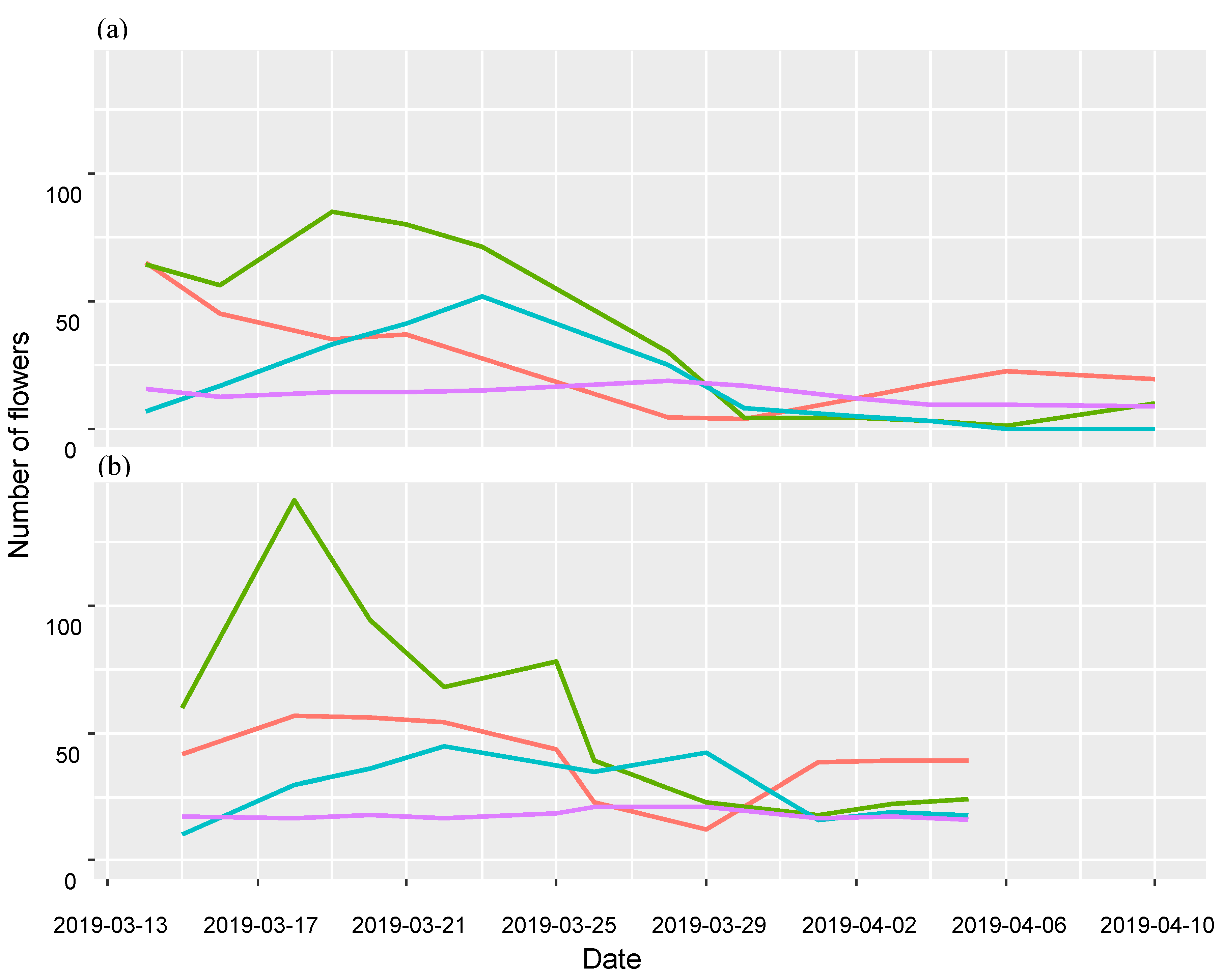
2.4. Data Analysis
2.5. Plant–Pollinator Networks
3. Results
3.1. Bee Visits in Relation with the Landscape Context
- The effect of urbanization on the number of visits realized by exotic wild bees could not be tested statistically simply because exotic wild bees realized no interaction in the natural context. In contrast, exotic wild bees realized 185 interactions in the urban context.
- Regarding honeybees, the landscape context had no effect on their number of interactions realized. Only the number of flowers of the experimental exotic plant communities had a significant positive effect on the number of interactions realized by honeybees (estimate = 2.60; p = 3.86 × 10−7 ***).
- None of the variables tested had a significant effect on the number of interactions realized by native bees.
3.2. Bee Visits within the Experimental Exotic Plant Communities
- Neither the landscape context nor the number of flowers of the experimental exotic plant communities had a significant effect on the number of visits received by the experimental exotic plant communities. However, the number of visits received by the experimental exotic plant communities was significantly lower in sites TTA2 (estimate = −1.70; p = 8.154 × 10−3 **) and TTA3 (estimate = −2.45; p = 8.57 × 10−4 ***), both in the natural area.
- Regarding the experimental exotic plant communities at the species level, the number of visits received by D. erecta was significantly higher in the urban context (estimate = 3.70; p = 3.75 × 10−7 ***; Figure 2). The number of visits received by D. erecta also significantly increased with its number of flowers (estimate = 0.89; p = 1.22 × 10−2 *). The landscape context had no effect on the number of visits received by Osteospermum sp. However, the number of visits received by Osteospermum sp. significantly increased with its number of flowers (estimate = 1.57; p = 2.49 × 10−4 ***) and significantly decreased with the number of flowers of the other species of the experimental exotic plant communities (estimate = −1.63; p = 1.2006 × 10−2 *). Finally, none of the variables tested influenced the number of visits received by either C. ugandense or C. hyssopifolia.
3.3. Plant–Pollinator Networks
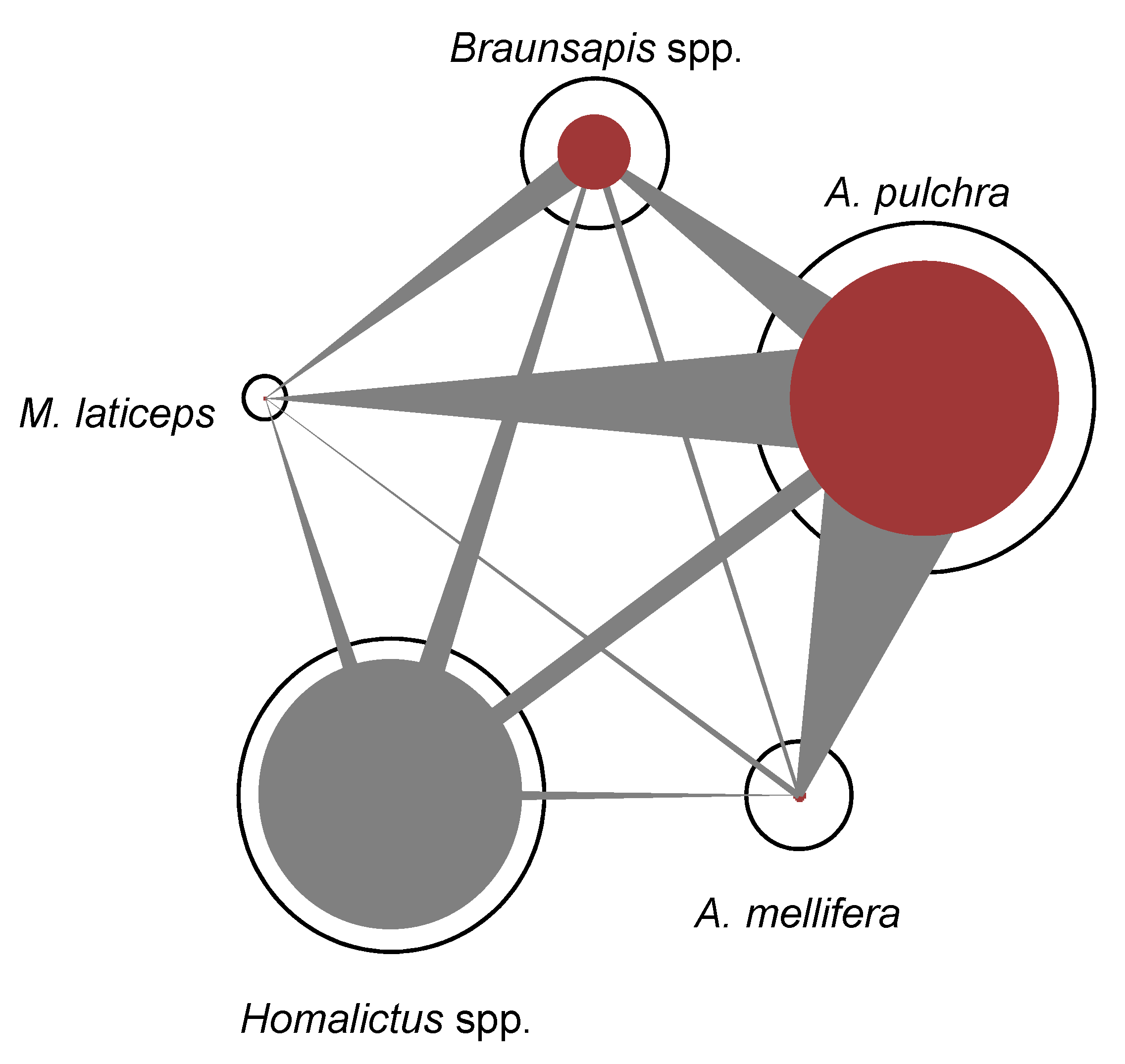
- Regarding the specialization index, in the urban context, the most specialized bee species was A. pulchra (d’ = 0.68), followed by Homalictus spp. (d’ = 0.63), Braunsapis spp. (d’ = 0.52), A. mellifera (d’ = 0.25), and M. laticeps (d’ = 0.13). Amegilla pulchra had the highest specialization index because it realized almost all of its interactions on D. erecta, making it the most visited plant species of the urban plant–pollinator network (Figure 3). In the natural context, the most specialized bee species was M. albomarginata (d’ = 0.70), followed by A. mellifera (d’ = 0.68), Homalictus spp. (d’ = 0.30), and A. sichelli (d’ = 0.12).
- Regarding Müller’s index, in the urban context (Figure 4), A. pulchra had the highest values as an acting bee compared to the other bee species (Table 1). Homalictus spp. had the highest intraspecific Müller’s index value (Table 1). In the natural context, Homalitcus spp. had both the highest Müller’s index values as an acting bee compared to the other bee species and the highest intraspecific Müller’s index value (Table 1).
4. Discussion
4.1. Exotic Wild Bees
4.2. Case of the Human Managed Honeybee
4.3. Recent Introductions of Exotic Bees
4.4. Native Bees
4.5. Experimental Exotic Plant Communities: A Potential Invasional Meltdown?
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Xiao, Y.; Li, X.; Cao, Y.; Dong, M. The diverse effects of habitat fragmentation on plant–pollinator interactions. Plant Ecol. 2016, 217, 857–868. [Google Scholar] [CrossRef]
- Traveset, A.; Castro-Urgal, R.; Rotllàn-Puig, X.; Lázaro, A. Effects of habitat loss on the plant-flower visitor network structure of a dune community. Oikos 2017, 127, 45–55. [Google Scholar] [CrossRef]
- Bates, A.J.; Sadler, J.P.; Fairbrass, A.J.; Falk, S.J.; Hale, J.D.; Matthews, T.J. Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS ONE 2011, 6, e23459. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.M.; Camilo, G.R.; Tonietto, R.K.; Ollerton, J.; Ahrné, K.; Arduser, M.; Ascher, J.S.; Baldock, K.C.R.; Fowler, R.; Frankie, G.; et al. The city as a refuge for insect pollinators. Conserv. Biol. 2017, 31, 24–29. [Google Scholar] [CrossRef]
- Geslin, B.; Gauzens, B.; Thébault, E.; Dajoz, I. Plant pollinator networks along a gradient of urbanisation. PLoS ONE 2013, 8, e63421. [Google Scholar] [CrossRef]
- Fortel, L.; Henry, M.; Guilbaud, L.; Guirao, A.L.; Kuhlmann, M.; Mouret, H.; Rollin, O.; Vaissière, B.E. Decreasing abundance, increasing diversity and changing structure of the wild bee community (Hymenoptera: Anthophila) along an urbanization gradient. PLoS ONE 2014, 9, e104679. [Google Scholar] [CrossRef]
- Baldock, K.C.R.; Goddard, M.A.; Hicks, D.M.; Kunin, W.E.; Mitschunas, N.; Morse, H.; Osgathorpe, L.M.; Potts, S.G.; Robertson, K.M.; Scott, A.V.; et al. A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat. Ecol. Evol. 2019, 3, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Ahrné, K.; Bengtsson, J.; Elmqvist, T. Bumble bees (Bombus spp) along a gradient of increasing urbanization. PLoS ONE 2009, 4, e5574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geslin, B.; Le Féon, V.; Folschweiller, M.; Flacher, F.; Carmignac, D.; Motard, E.; Perret, S.; Dajoz, I. The proportion of impervious surfaces at the landscape scale structures wild bee assemblages in a densely populated region. Ecol. Evol. 2016, 6, 6599–6615. [Google Scholar] [CrossRef]
- Pardee, G.L.; Philpott, S.M. Native plants are the bee’s knees: Local and landscape predictors of bee richness and abundance in backyard gardens. Urban Ecosyst. 2014, 17, 641–659. [Google Scholar] [CrossRef] [Green Version]
- Ropars, L.; Dajoz, I.; Geslin, B. La diversité des abeilles parisiennes. Osmia 2018, 7, 14–19. [Google Scholar] [CrossRef]
- Baldock, K.C.R.; Goddard, M.A.; Hicks, D.M.; Kunin, W.E.; Mitschunas, N.; Osgathorpe, L.M.; Potts, S.G.; Robertson, K.M.; Scott, A.V.; Stone, G.N.; et al. Where is the UK’s pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldock, K.C. Opportunities and threats for pollinator conservation in global towns and cities. Curr. Opin. Insect Sci. 2020, 38, 63–71. [Google Scholar] [CrossRef]
- Theodorou, P.; Radzevičiūtė, R.; Lentendu, G.; Kahnt, B.; Husemann, M.; Bleidorn, C.; Settele, J.; Schweiger, O.; Grosse, I.; Wubet, T.; et al. Urban areas as hotspots for bees and pollination but not a panacea for all insects. Nat. Commun. 2020, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Jamieson, M.A. The effects of urbanization on bee communities depends on floral resource availability and bee functional traits. PLoS ONE 2019, 14, e0225852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukase, J.; Simons, A.M. Increased pollinator activity in urban gardens with more native flora. Appl. Ecol. Environ. Res. 2016, 14, 297–310. [Google Scholar] [CrossRef]
- Corbet, S.A.; Bee, J.; DasMahapatra, K.; Gale, S.; Gorringe, E.; La Ferla, B.; Moorhouse, T.; Trevail, A.; Van Bergen, Y.; Vorontsova, M. Native or exotic? Double or single? Evaluating plants for pollinator-friendly gardens. Ann. Bot. 2001, 87, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Wada, K.; Kato, Y.; Makino, S.; Okochi, I. Alien pollinator promotes invasive mutualism in an insular pollination system. Biol. Invasions 2010, 13, 957–967. [Google Scholar] [CrossRef]
- Hanley, M.E.; Awbi, A.J.; Franco, M. Going native? Flower use by bumblebees in English urban gardens. Ann. Bot. 2014, 113, 799–806. [Google Scholar] [CrossRef]
- Salisbury, A.; Armitage, J.; Bostock, H.; Perry, J.; Tatchell, M.; Thompson, K. EDITOR’S CHOICE: Enhancing gardens as habitats for flower-visiting aerial insects (pollinators): Should we plant native or exotic species? J. Appl. Ecol. 2015, 52, 1156–1164. [Google Scholar] [CrossRef]
- Williams, N.M.; Cariveau, D.; Winfree, R.; Kremen, C. Bees in disturbed habitats use, but do not prefer, alien plants. Basic Appl. Ecol. 2011, 12, 332–341. [Google Scholar] [CrossRef]
- Lowenstein, D.M.; Minor, E.S. Diversity in flowering plants and their characteristics: Integrating humans as a driver of urban floral resources. Urban Ecosyst. 2016, 19, 1735–1748. [Google Scholar] [CrossRef]
- Ropars, L.; Dajoz, I.; Fontaine, C.; Muratet, A.; Geslin, B. Wild pollinator activity negatively related to honey bee colony densities in urban context. PLoS ONE 2019, 14, e0222316. [Google Scholar] [CrossRef] [Green Version]
- Harrison, T.; Winfree, R. Urban drivers of plant-pollinator interactions. Funct. Ecol. 2015, 29, 879–888. [Google Scholar] [CrossRef]
- Matteson, K.C.; Langellotto, G.A. Small scale additions of native plants fail to increase beneficial insect richness in urban gardens. Insect Conserv. Divers. 2011, 4, 89–98. [Google Scholar] [CrossRef]
- Bartomeus, I.; Vilà, M.; Santamaría, L. Contrasting effects of invasive plants in plant–pollinator networks. Oecologia 2008, 155, 761–770. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Espíndola, A.; Aizen, M.A. Risks to pollinators and pollination from invasive alien species. Nat. Ecol. Evol. 2018, 2, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Kaiser-Bunbury, C.N.; Mougal, J.; Whittington, A.E.; Valentin, T.; Gabriel, R.; Olesen, J.M.; Blüthgen, N. Ecosystem restoration strengthens pollination network resilience and function. Nature 2017, 542, 223–227. [Google Scholar] [CrossRef]
- Morales, C.L.; Aizen, M.A. Does invasion of exotic plants promote invasion of exotic flower visitors? A case study from the temperate forests of the Southern Andes. Biol. Invasions 2002, 4, 87–100. [Google Scholar] [CrossRef]
- MacIvor, J.S.; Ruttan, A.; Salehi, B. Exotics on exotics: Pollen analysis of urban bees visiting Sedum on a green roof. Urban Ecosyst. 2014, 18, 419–430. [Google Scholar] [CrossRef]
- Geslin, B.; Gauzens, B.; Baude, M.; Dajoz, I.; Fontaine, C.; Henry, M.; Ropars, L.; Rollin, O.; Thébault, E.; Vereecken, N.J. Massively introduced managed species and their consequences for plant–pollinator interactions. In Advances in Ecological Research; Bohan, D.A., Dumbrell, A.J., Massol, F., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 57, pp. 147–199. [Google Scholar]
- Russo, L. Positive and negative impacts of non-native bee species around the world. Insects 2016, 7, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartomeus, I.; Fründ, J.; Williams, N.M.; Suarez, A.V.; Cassey, P.; Chapple, D.G.; Wong, B.B.; Griffin, A.S.; Guez, D.; Federspiel, I.; et al. Invasive plants as novel food resources, the pollinators’ perspective. In Biological Invasions and Animal Behaviour; Cambridge University Press: Cambridge, UK, 2016; pp. 119–132. [Google Scholar]
- Traveset, A.; Richardson, D.M. Mutualistic interactions and biological invasions. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Aslan, C.E.; Zavaleta, E.S.; Tershy, B.; Croll, D. Mutualism disruption threatens global plant biodiversity: A systematic review. PLoS ONE 2013, 8, e66993. [Google Scholar] [CrossRef] [Green Version]
- Groom, S.V.C.; Schwarz, M.P. Bees in the Southwest Pacific: Origins, diversity and conservation. Apidologie 2011, 42, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Bernardello, G.; Anderson, G.J.; Stuessy, T.F.; Crawford, D.J. A survey of floral traits, breeding systems, floral visitors, and pollination systems of the angiosperms of the Juan Fernández Islands (Chile). Bot. Rev. 2001, 67, 255–308. [Google Scholar] [CrossRef]
- Rathcke, B.J. Pollination and predation limit fruit set in a shrub, Bourreria succulenta (Boraginaceae), after hurricanes on San Salvador Island, Bahamas. Biotropica 2001, 33, 330–338. [Google Scholar] [CrossRef]
- Olesen, J.M.; Eskildsen, L.I.; Venkatasamy, S. Invasion of pollination networks on oceanic islands: Importance of invader complexes and endemic super generalists. Divers. Distrib. 2002, 8, 181–192. [Google Scholar] [CrossRef]
- Anderson, S.H. The relative importance of birds and insects as pollinators of the New Zealand flora. N. Z. J. Ecol. 2003, 27, 83–94. [Google Scholar]
- Dupont, Y.L.; Hansen, D.M.; Valido, A.; Olesen, J.M. Impact of introduced honey bees on native pollination interactions of the endemic Echium wildpretii (Boraginaceae) on Tenerife, Canary Islands. Biol. Conserv. 2004, 118, 301–311. [Google Scholar] [CrossRef]
- Kaiser-Bunbury, C.N.; Traveset, A.; Hansen, D.M. Conservation and restoration of plant–animal mutualisms on oceanic islands. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 131–143. [Google Scholar] [CrossRef]
- Crichton, A.; Francis, N.; Doherty, S.; Tuiwawa, M.; Hayes, S.; Stevens, M.I.; Schwarz, M.P. Low endemic bee diversity and very wide host range in lowland Fiji: Support for the pollinator super-generalist hypothesis in island biogeography. Pac. Conserv. Biol. 2018, 25, 135–142. [Google Scholar] [CrossRef]
- Pauly, A.; Munzinger, J. Contribution à la connaissance des Hymenoptera Apoidea de Nouvelle-Calédonie et de leurs relations avec la flore butinée. Ann. Société Entomol. Fr. 2003, 39, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Donovan, B.J.; Munzinger, J.; Pauly, A.; McPherson, G. Flower-visiting records of the native bees of New Caledonia. Ann. Mo. Bot. Gard. 2013, 99, 19–43. [Google Scholar] [CrossRef]
- Pauly, A.; Walker, K.; Munzinger, J.; Donovan, B. Endémisme insulaire et cleptoparasitisme chez les Lasioglossum Curtis 1833 (Hymenoptera: Apoidea: Halictidae) de Nouvelle-Calédonie. Ann. Société Entomol. Fr. 2013, 49, 127–153. [Google Scholar] [CrossRef]
- Pauly, A.; Donovan, B.; Muzinger, J. Les Austronomia Michener, 1965 de Nouvelle-Calédonie et de l’archipel du Vanuatu (Hymenoptera: Apoidea: Halictidae: Nomiinae). Belg. J. Entomol. 2013, 11, 1–29. [Google Scholar]
- Pauly, A.; Donovan, B.; Munzinger, J. Les abeilles du genre Homalictus Cockerell, 1919 en Nouvelle-Calédonie (Hymenoptera: Apoidea: Halictidae). Belg. J. Entomol. 2015, 34, 1–30. [Google Scholar]
- Météo France. Available online: http://www.meteofrance.com/climat/outremer/nouvelle-caledonie/nouvellecal/normales (accessed on 12 May 2020).
- Isnard, S.; L’Huillier, L.; Rigault, F.; Jaffré, T. How did the ultramafic soils shape the flora of the New Caledonian hotspot? Plant Soil 2016, 403, 53–76. [Google Scholar] [CrossRef]
- Morat, P.; Jaffré, T.; Tronchet, F.; Munzinger, J.; Pillon, Y.; Veillon, J.-M.; Chalopin, M.; Birnbaum, P.; Rigault, F.; Dagostini, G.; et al. Le référentiel taxonomique Florical et les caractéristiques de la flore vasculaire indigène de la Nouvelle-Calédonie. Adansonia 2012, 34, 179–221. [Google Scholar] [CrossRef] [Green Version]
- Institut de la Statistique et des Études Économiques Nouvelle-Calédonie. Available online: https://www.isee.nc/population/recensement/structure-de-la-population-et-evolutions (accessed on 12 May 2020).
- Wulff, A.S.; Hollingsworth, P.M.; Ahrends, A.; Jaffré, T.; Veillon, J.-M.; L’Huillier, L.; Fogliani, B. Conservation priorities in a biodiversity hotspot: Analysis of narrow endemic plant species in New Caledonia. PLoS ONE 2013, 8, e73371. [Google Scholar] [CrossRef] [Green Version]
- Leijs, R.; Batley, M.; Hogendoorn, K. The genus Amegilla (Hymenoptera, Apidae, Anthophorini) in Australia: A revision of the subgenera Notomegilla and Zonamegilla. ZooKeys 2017, 653, 79–140. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A language and environment for statistical computing. 2018. Available online: https://www.r-project.org/ (accessed on 13 February 2012).
- R Studio Team. Integrated Development for R; PBC: Boston, MA, USA, 2016. [Google Scholar]
- Dormann, C.F.; Gruber, B.; Fruend, J. Introducing the bipartite package: Analysing ecological networks. R News 2008, 8, 8–11. [Google Scholar]
- Müller, C.B.; Adriaanse, I.C.T.; Belshaw, R.; Godfray, H.C.J. The structure of an aphid–parasitoid community. J. Anim. Ecol. 1999, 68, 346–370. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Biesmeijer, J.C.; Benadi, G.; Fründ, J.; Stang, M.; Bartomeus, I.; Kaiser-Bunbury, C.N.; Baude, M.; Gomes, S.I.F.; Merckx, V.; et al. The potential for indirect effects between co-flowering plants via shared pollinators depends on resource abundance, accessibility and relatedness. Ecol. Lett. 2014, 17, 1389–1399. [Google Scholar] [CrossRef] [Green Version]
- Bellard, C.; Leroy, B.; Thuiller, W.; Rysman, J.-F.; Courchamp, F. Major drivers of invasion risks throughout the world. Ecosphere 2016, 7, 01241. [Google Scholar] [CrossRef] [Green Version]
- Matteson, K.C.; Ascher, J.S.; Langellotto, G.A. Bee richness and abundance in New York City urban gardens. Ann. Entomol. Soc. Am. 2008, 101, 140–150. [Google Scholar] [CrossRef]
- Normandin, É.; Vereecken, N.J.; Buddle, C.M.; Fournier, V. Taxonomic and functional trait diversity of wild bees in different urban settings. PeerJ 2017, 5, e3051. [Google Scholar] [CrossRef] [Green Version]
- Lamaignere, H. L’apiculture en Nouvelle-Calédonie. Ph.D. Thesis, University Paul-Sabatier, Toulouse, France, 2001. [Google Scholar]
- Mallinger, R.E.; Gaines-Day, H.R.; Gratton, C. Do managed bees have negative effects on wild bees? A systematic review of the literature. PLoS ONE 2017, 12, e0189268. [Google Scholar] [CrossRef] [Green Version]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef]
- Henry, M.; Rodet, G. Controlling the impact of the managed honeybee on wild bees in protected areas. Sci. Rep. 2018, 8, 9308. [Google Scholar] [CrossRef] [Green Version]
- Ropars, L.; Affre, L.; Schurr, L.; Flacher, F.; Genoud, D.; Mutillod, C.; Geslin, B. Land cover composition, local plant community composition and honeybee colony density affect wild bee species assemblages in a Mediterranean biodiversity hot-spot. Acta Oecologica 2020, 104, 103546. [Google Scholar] [CrossRef]
- Aizen, M.A.; Morales, C.L.; Vázquez, D.P.; Garibaldi, L.A.; Sáez, A.; Harder, L.D. When mutualism goes bad: Density-dependent impacts of introduced bees on plant reproduction. New Phytol. 2014, 204, 322–328. [Google Scholar] [CrossRef]
- Willmer, P.G.; Cunnold, H.; Ballantyne, G. Insights from measuring pollen deposition: Quantifying the pre-eminence of bees as flower visitors and effective pollinators. Arthropod Plant Interact. 2017, 97, 141–425. [Google Scholar] [CrossRef] [Green Version]
- Park, M.G.; Raguso, R.A.; Losey, J.E.; Danforth, B.N. Per-visit pollinator performance and regional importance of wild Bombus and Andrena (Melandrena) compared to the managed honey bee in New York apple orchards. Apidologie 2016, 47, 145–160. [Google Scholar] [CrossRef] [Green Version]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Groom, S.V.C.; Ngo, H.T.; Rehan, S.M.; Skelton, P.; Stevens, M.I.; Schwarz, M.P. Multiple recent introductions of apid bees into Pacific archipelagos signify potentially large consequences for both agriculture and indigenous ecosystems. Biol. Invasions 2014, 16, 2293–2302. [Google Scholar] [CrossRef]
- Groom, S.V.C.; Stevens, M.I.; Ramage, T.; Schwarz, M.P. Origins and implications of apid bees (Hymentopera: Apidae) in French Polynesia. Entomol. Sci. 2017, 20, 65–75. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B: Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Groom, S.V.C.; Hayes, S.E.; Ngo, H.T.; Stevens, M.I.; Schwarz, M.P. Recipe for disruption: Multiple recent arrivals of megachilid bees in Pacific archipelagos. J. Insect Conserv. 2014, 18, 613–622. [Google Scholar] [CrossRef]
- Groutsch, J.K.; Miller, N.C.; Tuiwawa, M.; Hayes, S.; Stevens, M.I.; Schwarz, M.P. Not all exotic pollinator introductions are bad: An introduced buzz-pollinating bee Amegilla pulchra (Hymenoptera: Apidae) in Fiji indicates little potential for enhancing the spread of weeds. Austral. Entomol. 2018, 58, 533–539. [Google Scholar] [CrossRef]


| (a) | |||||
| Braunsapisspp. | A.pulchra | A. mellifera | Homalictusspp. | M. laticeps | |
| Braunsapis spp. | 0.503 | 0.293 | 0.032 | 0.143 | 0.029 |
| A. pulchra | 0.054 | 0.787 | 0.075 | 0.076 | 0.008 |
| A. mellifera | 0.061 | 0.767 | 0.104 | 0.055 | 0.013 |
| Homalictus spp. | 0.033 | 0.095 | 0.007 | 0.863 | 0.002 |
| M. laticeps | 0.314 | 0.489 | 0.078 | 0.095 | 0.024 |
| (b) | |||||
| A. mellifera | Homalictusspp. | M. albomarginata | A. sichelli | ||
| A. mellifera | 0.695 | 0.305 | 0 | 0 | |
| Homalictus spp. | 0.098 | 0.844 | 0.001 | 0.056 | |
| M. albomarginata | 0 | 0.856 | 0.144 | 0 | |
| A. sichelli | 0 | 0.933 | 0 | 0.066 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakardjian, M.; Geslin, B.; Mitran, V.; Franquet, E.; Jourdan, H. Effects of Urbanization on Plant–Pollinator Interactions in the Tropics: An Experimental Approach Using Exotic Plants. Insects 2020, 11, 773. https://doi.org/10.3390/insects11110773
Zakardjian M, Geslin B, Mitran V, Franquet E, Jourdan H. Effects of Urbanization on Plant–Pollinator Interactions in the Tropics: An Experimental Approach Using Exotic Plants. Insects. 2020; 11(11):773. https://doi.org/10.3390/insects11110773
Chicago/Turabian StyleZakardjian, Marie, Benoît Geslin, Valentin Mitran, Evelyne Franquet, and Hervé Jourdan. 2020. "Effects of Urbanization on Plant–Pollinator Interactions in the Tropics: An Experimental Approach Using Exotic Plants" Insects 11, no. 11: 773. https://doi.org/10.3390/insects11110773
APA StyleZakardjian, M., Geslin, B., Mitran, V., Franquet, E., & Jourdan, H. (2020). Effects of Urbanization on Plant–Pollinator Interactions in the Tropics: An Experimental Approach Using Exotic Plants. Insects, 11(11), 773. https://doi.org/10.3390/insects11110773







