Susceptibility of Various Developmental Stages of the Fall Armyworm, Spodoptera frugiperda, to Entomopathogenic Nematodes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Entomopathogenic Nematodes
2.2. Nematode Infection of S. frugiperda Larvae
2.2.1. Effect of Larval Developmental Stage and Exposure Time on EPN Virulence
2.2.2. Efficacy of EPNs against S. frugiperda Larvae in a Soil Column Assay
2.2.3. Efficacy of Selected EPNs against S. frugiperda Larvae in a Pot Assay
2.3. Nematode Infection of S. frugiperda Pupae
2.4. Assessment of EPN Reproduction Rate in Different Larval Stages of S. frugiperda
2.5. Statistical Analysis
3. Results
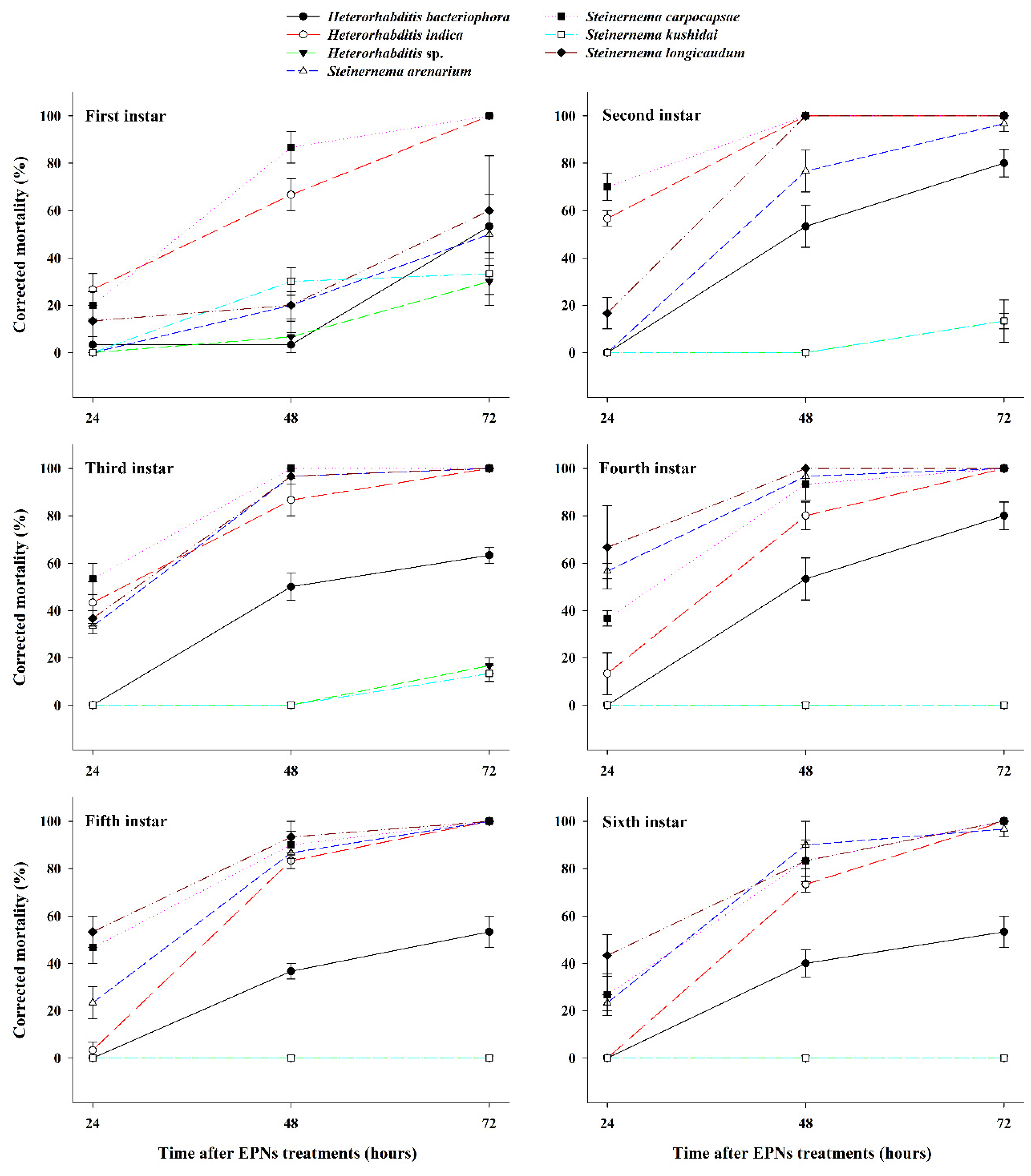
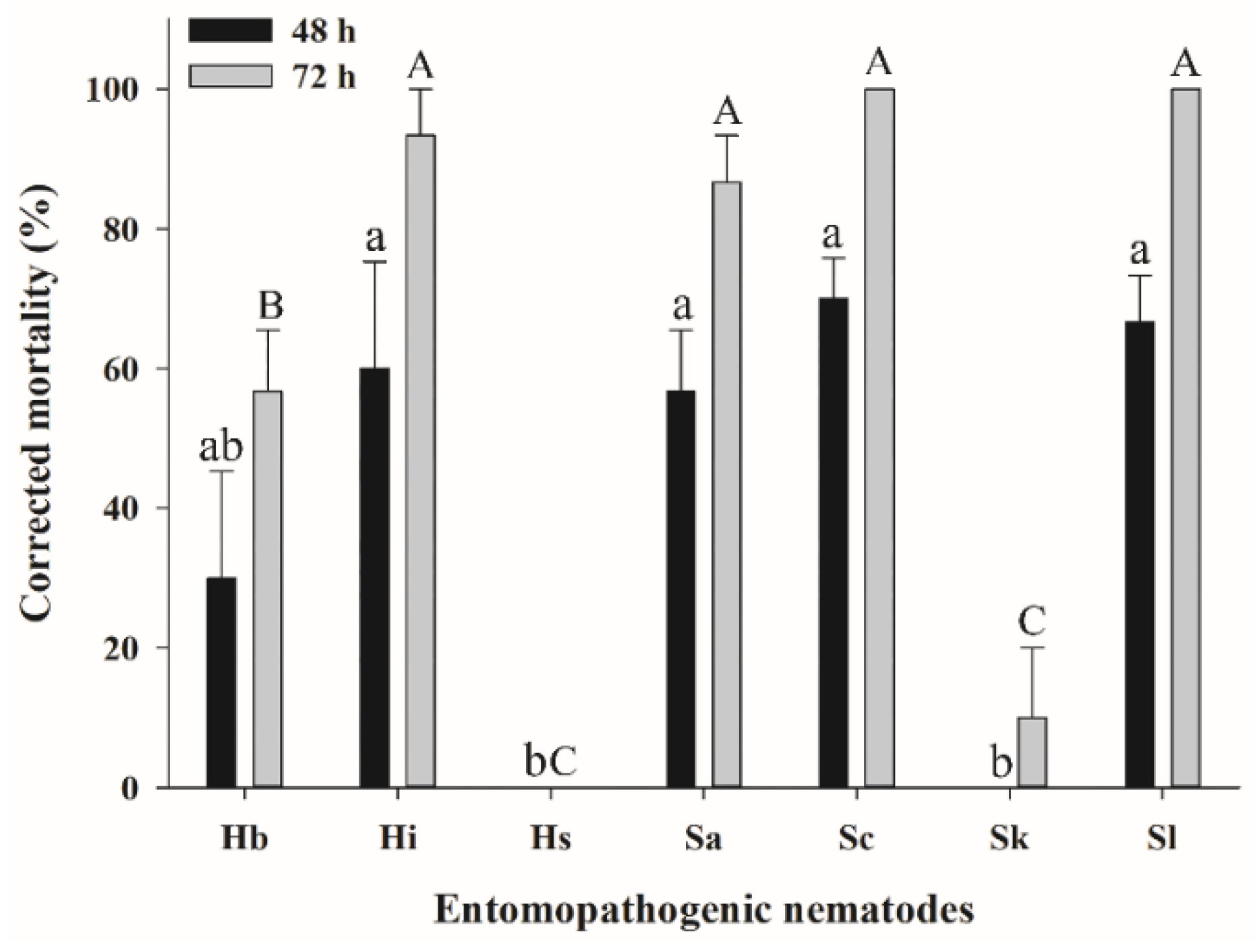
3.1. Effect of EPNs on the Mortality of Different S. frugiperda Larval Stages
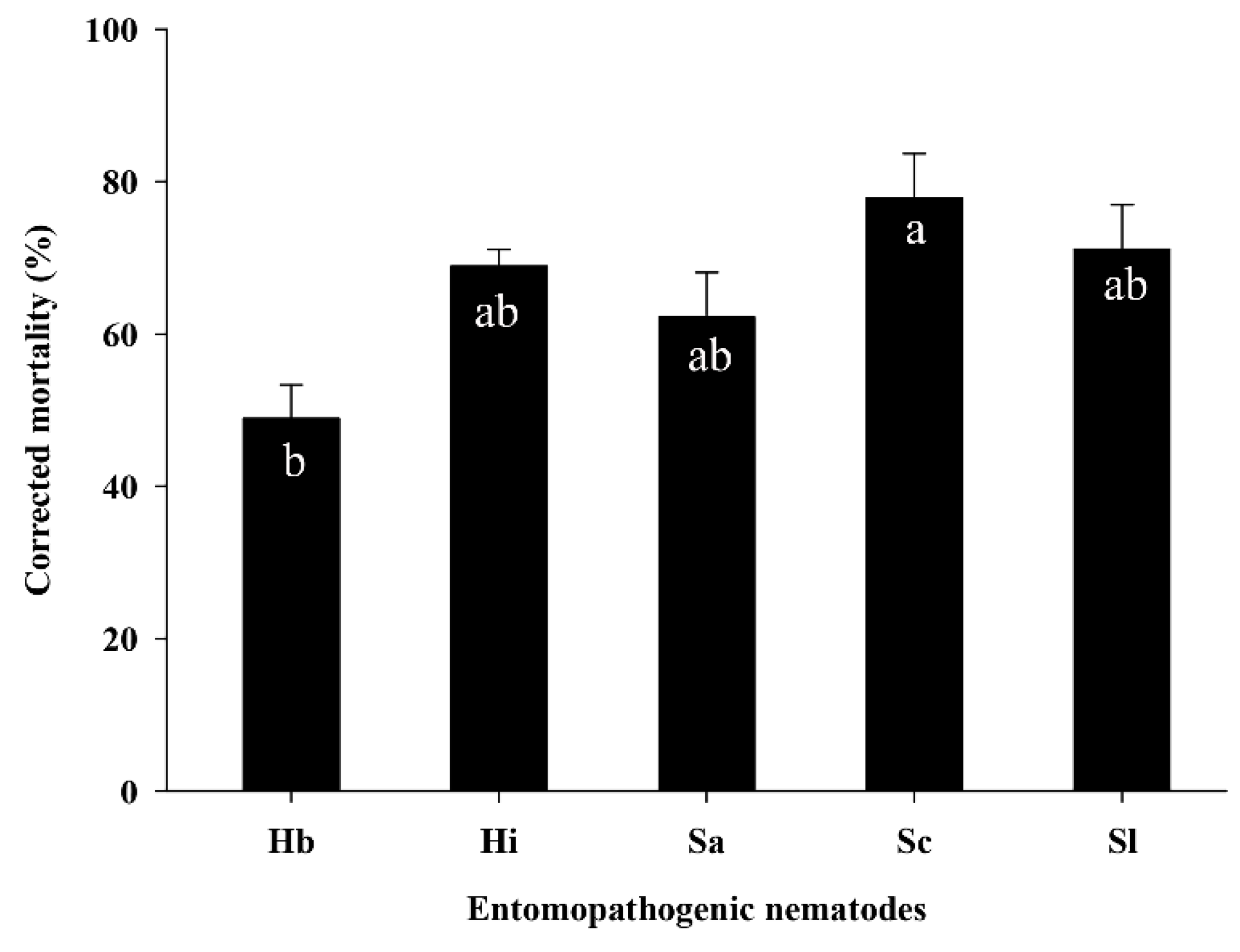
3.2. Efficacy of EPNs against S. frugiperda Larvae in the Soil Column Assay
3.3. Efficacy of EPNs against S. frugiperda Larvae in the Pot Assay
3.4. Effect of EPNs on the Adult Eclosion Rate of S. frugiperda Pupae
3.5. EPN Reproduction Rate in Different Larval Stages of S. frugiperda
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, D.P.; Guo, J.F.; Jiang, Y.Y.; Zhao, J.Z.; Sethi, A.; He, K.L.; Wang, Z.Y. Initial detections and spread of invasive Spodoptera frugiperda in China and comparisons with other noctuid larvae in cornfields using molecular techniques. Insect Sci. 2020, 27, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Seo, B.Y.; Lee, J.; Kim, H.; Song, J.H.; Lee, W. First report of the fall armyworm, Spodoptera frugiperda (Smith, 1797) (Lepidoptera, Noctuidae), a new migratory pest in Korea. Korean J. Appl. Entomol. 2020, 59, 73–78. [Google Scholar] [CrossRef]
- EPPO Spodoptera frugiperda (LAPHER). Available online: https://gd.eppo.int/taxon/LAPHFR/distribution (accessed on 12 October 2020).
- Clark, P.L.; Molina-Ochoa, J.; Martinelli, S.; Skoda, S.R.; Isenhour, D.J.; Lee, D.J.; Krumm, J.T.; Foster, J.E. Population variation of the fall armyworm, Spodoptera frugiperda, in the Western Hemisphere. J. Insect Sci. 2007, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagoshi, R.N. Can the amount of corn acreage predict fall armyworm (Lepidoptera: Noctuidae) infestation levels in nearby cotton? J. Econ. Entomol. 2009, 102, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks on pest management. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Sisay, B.; Simiyu, J.; Mendesil, E.; Likhayo, P.; Ayalew, G.; Mohamed, S.; Subramanian, S.; Tefera, T. Fall armyworm, Spodoptera frugiperda infestations in East Africa: Assessment of damage and parasitism. Insects 2019, 10, 195. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, R.A.; Omoto, C.; Field, L.M.; Williamson, M.S.; Bass, C. Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PLoS ONE 2013, 8, e62268. [Google Scholar] [CrossRef] [Green Version]
- Diez-Rodríguez, G.I.; Omoto, C. Inheritance of lambda-cyhalothrin resistance in Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2001, 30, 311–316. [Google Scholar] [CrossRef] [Green Version]
- do Nascimento, A.R.B.; Farias, J.R.; Bernardi, D.; Horikoshi, R.J.; Omoto, C. Genetic basis of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to the chitin synthesis inhibitor lufenuron. Pest Manag. Sci. 2016, 72, 810–815. [Google Scholar] [CrossRef]
- Blanco, C.A.; Portilla, M.; Jurat-Fuentes, J.L.; Sánchez, J.F.; Viteri, D.; Vega-Aquino, P.; Terán-Vargas, A.P.; Azuara-Domínguez, A.; López, J.D.; Arias, R.; et al. Susceptibility of isofamilies of Spodoptera frugiperda (Lepidoptera: Noctuidae) to Cry1Ac and Cry1Fa proteins of Bacillus thuringiensis. Southwest. Entomol. 2010, 35, 409–415. [Google Scholar] [CrossRef]
- Huang, F.; Qureshi, J.A.; Meagher, R.L.; Reisig, D.D.; Head, G.P.; Andow, D.A.; Ni, X.; Kerns, D.; Buntin, G.D.; Niu, Y.; et al. Cry1F resistance in fall armyworm Spodoptera frugiperda: Single gene versus pyramided Bt maize. PLoS ONE 2014, 9, e112958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhat, F.M.; Haggag, M.N.; Loutfy, N.M.; Osman, M.A.M.; Ahmed, M.T. Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemosphere 2015, 120, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, B.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall Armyworm in Africa: A guide for integrated pest management. In Handbook, 1st ed.; CDMX CIMMYT: Texcoco, Mexico, 2018; pp. 45–62. [Google Scholar]
- Akutse, K.S.; Kimemia, J.W.; Ekesi, S.; Khamis, F.M.; Ombura, O.L.; Subramanian, S. Ovicidal effects of entomopathogenic fungal isolates on the invasive fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Appl. Entomol. 2019, 143, 626–634. [Google Scholar] [CrossRef]
- Brixey, J. The potential for biological control to reduce Hylobius abietis damage. For. Comm. Res. Inf. Note 1997, 273, 1–6. [Google Scholar]
- Andalo, V.; Santos, V.; Moreira, G.F.; Moreira, C.C.; Junior, A.M. Evaluation of entomopathogenic nematodes under laboratory and greenhouses conditions for the control of Spodoptera frugiperda. Cienc. Rural 2010, 40, 1860–1866. [Google Scholar] [CrossRef] [Green Version]
- Giannasi, A.D.O.; Brambila, C.R.; Zart, M.; Guide, B.A.; Alves, V.S. Assessment of entomopathogenic nematodes in Agrotis ipsilon (Lepidoptera: Noctuidae) under laboratory and greenhouse conditions. Rev. Colomb. Entomol. 2018, 44, 25–31. [Google Scholar] [CrossRef]
- Viteri, D.M.; Linares, A.M.; Flores, L. Use of the entomopathogenic nematode Steinernema carpocapsae in combination with low-toxicity insecticides to control fall armyworm (Lepidoptera: Noctuidae) Larvae. Florida Entomol. 2018, 101, 327–329. [Google Scholar] [CrossRef] [Green Version]
- Bhat, A.H.; Chaubey, A.K.; Askary, T.H. Global distribution of entomopathogenic nematodes, Steinernema and Heterorhabditis. Egypt. J. Biol. Pest Control 2020, 30. [Google Scholar] [CrossRef] [Green Version]
- Hominick, W.M. Biogeography. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 115–144. [Google Scholar]
- Laznik, Ž.; Tóth, T.; Lakatos, T.; Vidrih, M.; Trdan, S. Control of the Colorado potato beetle (Leptinotarsa decemlineata [Say]) on potato under field conditions: A comparison of the efficacy of foliar application of two strains of Steinernema feltiae (Filipjev) and spraying with thiametoxam. J. Plant Dis. Prot. 2010, 117, 129–135. [Google Scholar] [CrossRef]
- Kepenekci, I.; Hazir, S.; Özdem, A. Evaluation of native entomopathogenic nematodes for the control of the European cherry fruit fly Rhagoletis cerasi L. (Diptera: Tephritidae) larvae in soil. Turkish J. Agric. For. 2015, 39, 74–79. [Google Scholar] [CrossRef]
- Oreste, M.; Baser, N.; Ibouh, K.; Verrastro, V.; Tarasco, E. Potential of entomopathogenic fungi and nematodes against Drosophila suzukiiin laboratory assays. Microb. Nematode Control Invertebr. Pests IOBC-WPRS Bull. 2017, 129, 77–78. [Google Scholar]
- Yuksel, E.; Taskesen, Y.E.; Erarslan, D.; Canhilal, R. Effectiveness of different entomopathogenic nematode species against the variegated cutworm, Peridroma saucia (Hubner) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2018, 28, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Acharya, R.; Yu, Y.-S.; Shim, J.-K.; Lee, K.-Y. Virulence of four entomopathogenic nematodes against the tobacco cutworm Spodoptera litura Fabricius. Biol. Control 2020, 150, 104348. [Google Scholar] [CrossRef]
- Kaya, H.K.; Gaugler, R. Entomopathogenic nematodes. Annu. Rev. Entomol. 1993, 181–206. [Google Scholar] [CrossRef]
- Gaugler, R. Entomopathogenic Nematology; CABI: New York, NY, USA, 2002. [Google Scholar]
- Dowds, B.C.; Peters, A. Virulence mechanism. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI International: Wallingford, UK, 2002; pp. 79–98. [Google Scholar]
- Griffin, C.T.; Boemare, N.E.; Lewis, E.E. Biology and behaviour. In Nematodes as Biocontrol Agents; Grewal, P.S., Ehlers, R.U., Shapiro-Ilan, D.I., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 47–64. [Google Scholar]
- Kaya, H.K.; Aguillera, M.M.; Alumai, A.; Choo, H.Y.; de la Torre, M.; Fodor, A.; Ganguly, S.; Hazir, S.; Lakatos, T.; Pye, A.; et al. Status of entomopathogenic nematodes and their symbiotic bacteria from selected countries or regions of the world. Biol. Control 2006, 38, 134–155. [Google Scholar] [CrossRef]
- Park, S.H.; Yu, Y.S.; Park, J.S.; Choo, H.Y.; Do Bae, S.; Nam, M.H. Biological control of tobacco cutworm, Spodoptera litura fabricius with entomopathogenic nematodes. Biotechnol. Bioprocess Eng. 2001, 6, 139–143. [Google Scholar] [CrossRef]
- Stock, S.P.; Kusakabe, A.; Orozco, R.A. Secondary metabolites produced by Heterorhabditis symbionts and their application in agriculture: What we know and what to do next. J. Nematol. 2017, 49, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Caroli, L.; Glazer, I.; Gaugler, R. Entomopathogenic nematode infectivity assay: Comparison of penetration rate into different hosts. Biocontrol Sci. Technol. 1996, 6, 227–234. [Google Scholar] [CrossRef]
- Simões, N.; Rosa, J.S. Pathogenicity and host specificity of entomopathogenic nematodes. Biocontrol Sci. Technol. 1996, 6, 403–412. [Google Scholar] [CrossRef]
- Ramos-Rodríguez, O.; Campbell, J.F.; Ramaswamy, S.B. Pathogenicity of three species of entomopathogenic nematodes to some major stored-product insect pests. J. Stored Prod. Res. 2006, 42, 241–252. [Google Scholar] [CrossRef]
- Fuxa, J.R.; Richter, A.R.; Acudelo-Silva, F. Effect of host age and nematode strain on susceptibility of Spodoptera frugiperda to Steinernema feltiae. J. Nematol. 1988, 20, 91–95. [Google Scholar] [PubMed]
- Jackson, J.J.; Brooks, M.A. Parasitism of western corn rootworm larvae and pupae by Steinernema carpocapsae. J. Nematol. 1995, 27, 15–20. [Google Scholar] [PubMed]
- Acharya, R.; Hwang, H.-S.; Shim, J.-K.; Yu, Y.-S.; Lee, K.-Y. Control efficacy of fungus gnat, Bradysia impatiens, enhanced by a combination of entomopathogenic nematodes and predatory mites. Biol. Control 2019, 138, 104071. [Google Scholar] [CrossRef]
- Yan, X.; Shahid Arain, M.; Lin, Y.; Gu, X.; Zhang, L.; Li, J.; Han, R. Efficacy of entomopathogenic nematodes against the tobacco cutworm, Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 2020, 113, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.C.; Raetano, C.G.; Leite, L.G. Application technology for the entomopathogenic nematodes Heterorhabditis indica and Steinernema sp. (Rhabditida: Heterorhabditidae and Steinernematidae) to control Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in corn. Neotrop. Entomol. 2008, 37, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Caccia, M.G.; Del Valle, E.; Doucet, M.E.; Lax, P. Susceptibility of Spodoptera frugiperda and Helicoverpa gelotopoeon (Lepidoptera: Noctuidae) to the entomopathogenic nematode Steinernema diaprepesi (Rhabditida: Steinernematidae) under laboratory conditions. Chil. J. Agric. Res. 2014, 74, 123–126. [Google Scholar] [CrossRef]
- Platt, T.; Stokwe, N.F.; Malan, A.P. A review of the potential use of entomopathogenic nematodes to control above-ground insect pests in South Africa. S. Afr. J. Enol. Vitic. 2020, 41, 1–16. [Google Scholar] [CrossRef]
- Guo, W.; Yan, X.; Zhao, G.; Han, R. Efficacy of entomopathogenic Steinernema and Heterorhabditis nematodes against white grubs (Coleoptera: Scarabaeidae) in peanut fields. J. Econ. Entomol. 2013, 106, 1112–1117. [Google Scholar] [CrossRef]
- Kapranas, A.; Sbaiti, I.; Degen, T.; Turlings, T.C.J. Biological control of cabbage fly Delia radicum with entomopathogenic nematodes: Selecting the most effective nematode species and testing a novel application method. Biol. Control 2020, 144, 104212. [Google Scholar] [CrossRef]
- Öğretmen, A.; Yüksel, E.; Canhilal, R. Susceptibility of larvae of wireworms (Agriotes spp.) (Coleoptera: Elateridae) to some Turkish isolates of entomopathogenic nematodes under laboratory and field conditions. Biol. Control 2020, 149. [Google Scholar] [CrossRef]
- Mamiya, Y. Comparison of the infectivity of Steinernema kushidai and other nematodes for three different insects. Appl. Entomol. Zool. 1989, 24, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Ogura, N. Control of scarabaeid grubs with an entomogenous nematode, Steinernema kushidai. Japan Agric. Res. Q. 1993, 27, 49–54. [Google Scholar]
- Woodring, J.L.; Kaya, H.K. Steinernematid and heterorhabditid nematodes: A handbook of biology and techniques. South Coop. Ser. Bull. 1988, 331. [Google Scholar]
- White, G.F. A method for obtaining infective nematodes larvae from cultures. Science 1927, 66, 302. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. Base SAS 9.4 Procedures Guide, Statistical Procedures, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Abbott, W.S. The valueof the dry substitutes for liquid lime. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Littell, R.C.; Stroup, W.W.; Freund, R.J. SAS for Linear Models; SAS Publishing: Cary, NC, USA, 2002. [Google Scholar]
- Patil, J.; Vijayakumar, R.; Linga, V.; Sivakumar, G. Susceptibility of Oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae) larvae and pupae to native entomopathogenic nematodes. J. Appl. Entomol. 2020, 1–8. [Google Scholar] [CrossRef]
- Kaya, H.K.; Hara, A.H. Susceptibility of various species of lepidopterous pupae to the entomogenous nematode. Neoaplectana carpocapsae. J. Nematol. 1981, 13, 291–294. [Google Scholar]
- Kondo, E.; Ishibashi, N. Infection efficacyof Steinernema feltiae (DD-136) to thecommon cutworm, Spodoptera litura (Lepidoptera: Noctuidae), on the soil. Appl. Entomol. Zool. 1986, 21, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, K.; Gopalakrishnan, C. Effect of entomogenous nemamode, Steinernema feltiae (Rhabditida: Steinernematldae) to the pre-pupa, pupa and adult of Spodoptera litura (Noctuidae: Lepidoptera). Indian J. Nematol. 1987, 17, 273–276. [Google Scholar]
- Gulcu, B.; Ulug, D.; Hazir, C.; Karagoz, M.; Hazir, S. Biological control potential of native entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) against Spodoptera cilium (Lepidoptera: Noctuidae) in turfgrass. Biocontrol Sci. Technol. 2014, 24, 965–970. [Google Scholar] [CrossRef]
- Campbell, J.F.; Lewis, E.; Yoder, F.; Gaugler, R. Entomopathogenic nematode (Heterorhabditidae and Steinernematidae) spatial distribution in turfgrass. Parasitology 1996, 113, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Moyle, P.L.; Kaya, H.K. Dispersal and infectivityof the entomogenous nematode, Neoaplectana carpocapsae Weiser (Rhabditida: Steinernematidae), in sand. J. Nematol. 1981, 13, 295–300. [Google Scholar]
- Pathak, M.D.; Khan, J.R. Insect Pests of Rice; International Rice Research Institute (IRRI): Los banios, Philippines; International Centre of Insect Physiology and Ecology (ICIPE): Nairobi, Kenya, 1994; p. 89. [Google Scholar]
- El-Borai, F.E.; Stuart, R.J.; Campos-Herrera, R.; Pathak, E.; Duncan, L.W. Entomopathogenic nematodes, root weevil larvae, and dynamic interactions among soil texture, plant growth, herbivory, and predation. J. Invertebr. Pathol. 2012, 109, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Toledo, J.; Williams, T.; Pérez, C.; Liedo, P.; Valle, J.F.; Ibarra, J.E. Abiotic factors affecting the infectivity of Steinernema carpocapsae (Rhabditida: Steinernematidae) on larvae of Anastrepha obliqua (Diptera: Tephritidae). Biocontrol Sci. Technol. 2009, 19, 887–898. [Google Scholar] [CrossRef]
- Toepfer, S.; Kurtz, B.; Kuhlmann, U. Influence of soil on the efficacy of entomopathogenic nematodes in reducing Diabrotica virgifera virgifera in maize. J. Pest Sci. 2010, 83, 257–264. [Google Scholar] [CrossRef] [Green Version]

| Source | DF | F Value | p |
|---|---|---|---|
| Larval stage (L) | 5 | 20.29 | 0.0001 |
| Post-treatment time (T) | 2 | 579.6 | 0.0001 |
| EPN species (S) | 6 | 391.1 | 0.0001 |
| L × T | 10 | 3.3 | 0.0017 |
| L × S | 30 | 10.93 | 0.0001 |
| T × S | 12 | 29.1 | 0.0001 |
| L × T × S | 60 | 3.41 | 0.0001 |
| Error | 63 | ||
| Corrected total | 188 |
| Larval Stages | EPNs | LT50 (h) | 95% CI (Lower-Upper) | Slope (±SE) | χ2 (df) |
|---|---|---|---|---|---|
| First instar | H. bacteriophora | 80 b | (50–141) | 4.52 (1.13) | 15.96 (1) |
| H. indica | 34 ab | (29–39) | 5.26 (0.80) | 43.64 (1) | |
| S. arenarium | 84 bc | (72–99) | 3.68 (0.66) | 31.35 (1) | |
| S. carpocapsae | 32 a | (28–36) | 6.91 (1.12) | 37.85 (1) | |
| S. longicaudum | 57 ab | (19–103) | 4.41 (1.19) | 13.74 (1) | |
| Second instar | H. bacteriophora | 51 e | (44–57) | 5.66 (0.79) | 51.35 (1) |
| H. indica | 24 b | (na) | na | 0.00 (1) | |
| S. arenarium | 42 d | (36–46) | 9.45 (1.83) | 26.60 (1) | |
| S. carpocapsae | 23 a | (na) | na | 0.00 (1) | |
| S. longicaudum | 26 c | (na) | na | 0.00 (1) | |
| Third instar | H. bacteriophora | 64 ab | (23–115) | 3.37 (0.90) | 14.05 (1) |
| H. indica | 27 ab | (21–31) | 5.07 (0.96) | 28.14 (1) | |
| S. arenarium | 27 ab | (24–31) | 7.61 (1.59) | 22.85 (1) | |
| S. carpocapsae | 24 a | (na) | na | 0.00 (1) | |
| S. longicaudum | 28 ab | (23–31) | 7.32 (1.58) | 21.57 (1) | |
| Fourth instar | H. bacteriophora | 51 d | (44–57) | 5.66 (0.79) | 51.35 (1) |
| H. indica | 35 c | (31–40) | 7.11 (1.09) | 42.42 (1) | |
| S. arenarium | 24 ab | (18–28) | 5.77 (1.49) | 14.95 (1) | |
| S. carpocapsae | 28 b | (24–31) | 6.34 (1.25) | 26.01 (1) | |
| S. longicaudum | 23 a | (na) | na | 0.00 (1) | |
| Fifth instar | H. bacteriophora | 75 d | (64–89) | 3.25 (0.58) | 31.83 (1) |
| H. indica | 27 b | (24–31) | 7.61 (1.59) | 22.85 (1) | |
| S. arenarium | 31 bc | (27–36) | 6.58 (1.09) | 36.54 (1) | |
| S. carpocapsae | 25 ab | (20–30) | 5.17 (1.04) | 24.75 (1) | |
| S. longicaudum | 23 a | (17–28) | 5.19 (1.17) | 19.70 (1) | |
| Sixth instar | H. bacteriophora | 75 d | (63–91) | 3.04 (0.56) | 29.77 (1) |
| H. indica | 46 c | (45–48) | 36.79 (0.00) | 0.00 (1) | |
| S. arenarium | 31 ab | (27–36) | 5.99 (0.96) | 39.25 (1) | |
| S. carpocapsae | 31 ab | (26–36) | 6.00 (0.99) | 36.97 (1) | |
| S. longicaudum | 27 a | (20–33) | 4.02 (0.70) | 32.60 (1) |
| Larval Stages | Nematode Reproduction (IJs/larva) (Mean ± SE) | ||||
|---|---|---|---|---|---|
| Hb | Hi | Sa | Sc | Sl | |
| L1 | 639 ± 123 Cb | 2079 ± 282 Ca | 858 ± 148C ab | 1836 ± 456 Dab | 981 ± 182 Dab |
| L2 | 2213 ± 225 Cb | 4356 ±527 Ca | 3308 ± 312 Cab | 4523 ± 290 DEa | 3431 ± 244 DEab |
| L3 | 4447 ± 401 Cb | 7411 ± 819 Ca | 5957 ± 480 Cab | 7980 ± 854 Da | 6597 ± 912 Dab |
| L4 | 14,083 ± 1433 BCab | 30,963 ± 3961 Ba | 24,987 ± 2031 Ba | 26,200 ± 1010 Ca | 25,706 ± 1244 Ca |
| L5 | 18,443 ±1499 Bab | 47,930 ± 2323 Aa | 24,988 ± 2031 ABa | 36,537 ± 3002 Bbc | 39,822 ± 1504 Bab |
| L6 | 30,308 ± 1789 Aab | 58,753 ± 4047 Aa | 35,867 ± 1975 Ab | 58,433 ± 3349 Aa | 57,990 ± 2837 Aa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, R.; Hwang, H.-S.; Mostafiz, M.M.; Yu, Y.-S.; Lee, K.-Y. Susceptibility of Various Developmental Stages of the Fall Armyworm, Spodoptera frugiperda, to Entomopathogenic Nematodes. Insects 2020, 11, 868. https://doi.org/10.3390/insects11120868
Acharya R, Hwang H-S, Mostafiz MM, Yu Y-S, Lee K-Y. Susceptibility of Various Developmental Stages of the Fall Armyworm, Spodoptera frugiperda, to Entomopathogenic Nematodes. Insects. 2020; 11(12):868. https://doi.org/10.3390/insects11120868
Chicago/Turabian StyleAcharya, Rajendra, Hwal-Su Hwang, Md Munir Mostafiz, Yeon-Su Yu, and Kyeong-Yeoll Lee. 2020. "Susceptibility of Various Developmental Stages of the Fall Armyworm, Spodoptera frugiperda, to Entomopathogenic Nematodes" Insects 11, no. 12: 868. https://doi.org/10.3390/insects11120868
APA StyleAcharya, R., Hwang, H.-S., Mostafiz, M. M., Yu, Y.-S., & Lee, K.-Y. (2020). Susceptibility of Various Developmental Stages of the Fall Armyworm, Spodoptera frugiperda, to Entomopathogenic Nematodes. Insects, 11(12), 868. https://doi.org/10.3390/insects11120868








