Egg Hatching and First Instar Falling Models of Metcalfa pruinosa (Hemiptera: Flatidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection for Model Development
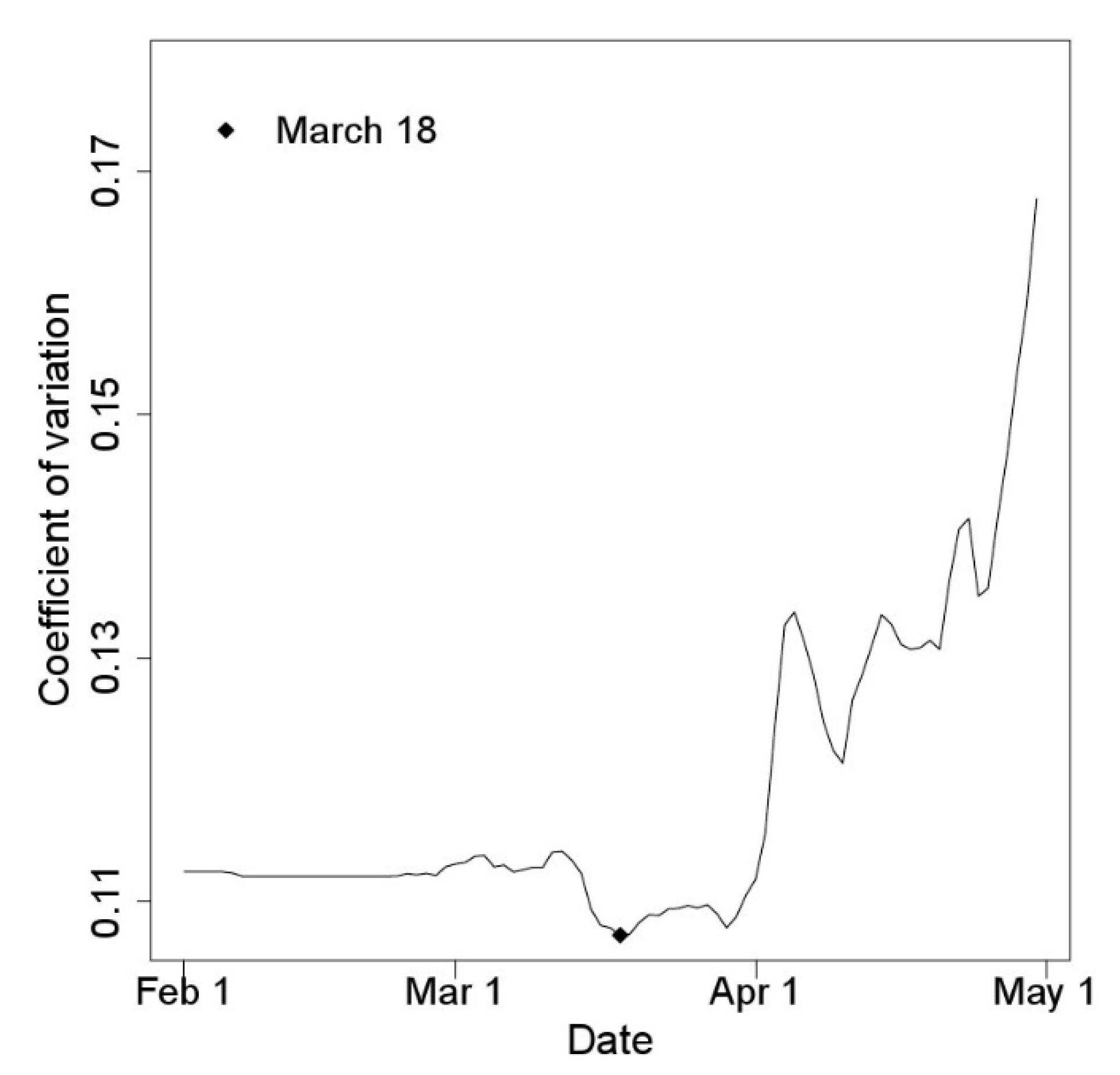
2.2. Starting Point of Degree-Days Models
2.3. Estimation of Model Parameters
2.4. Validation and Accuracy of Models
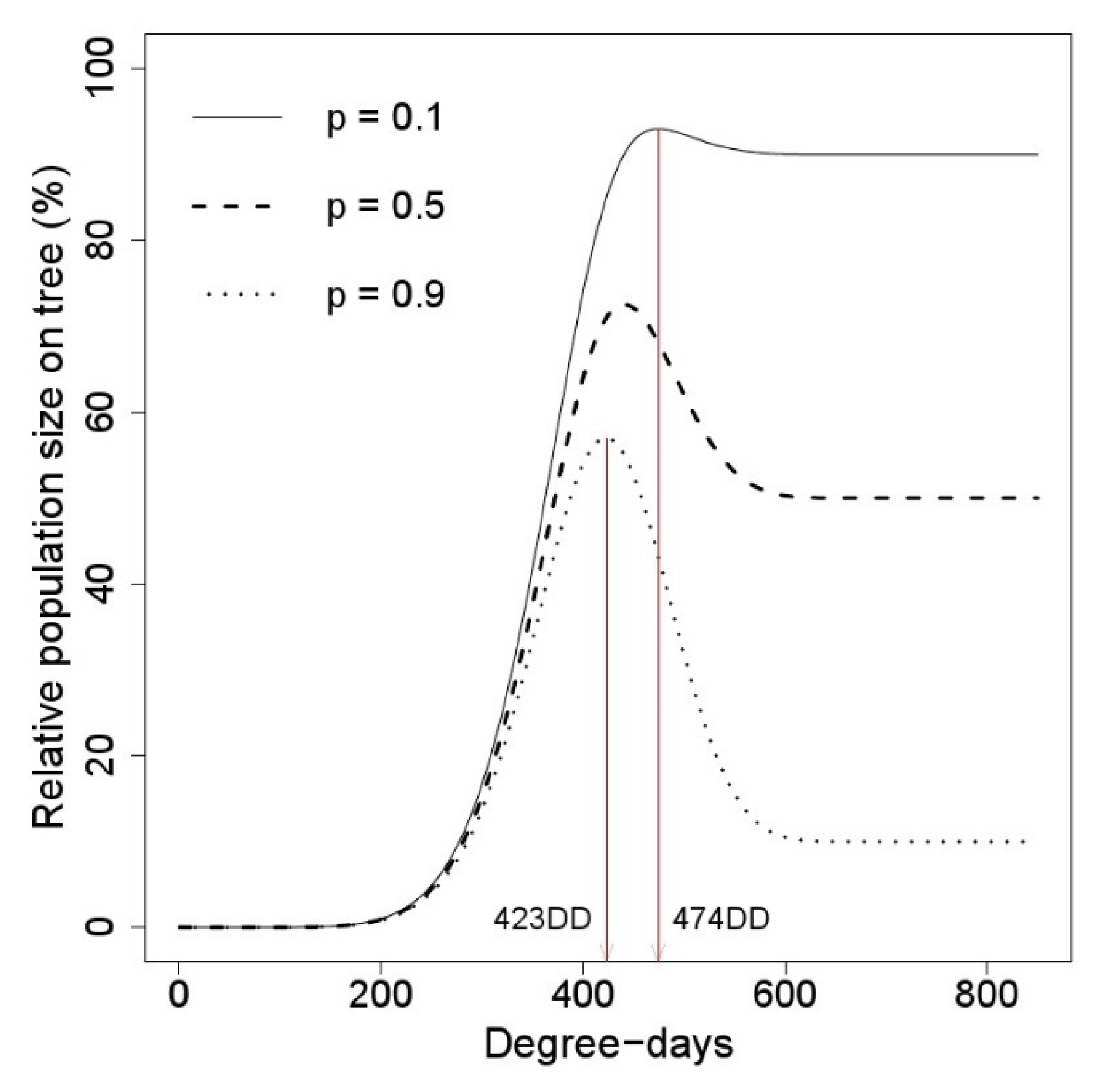
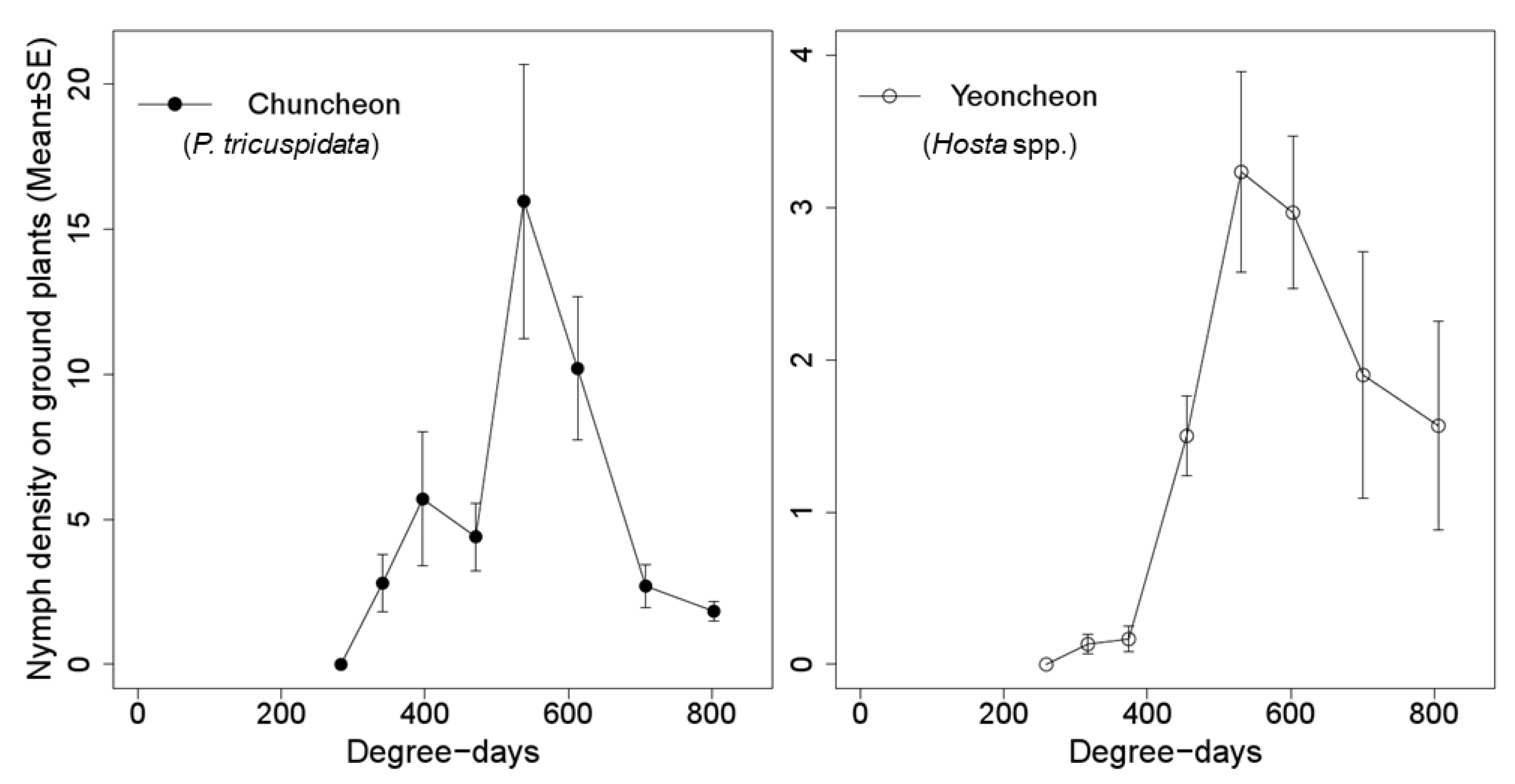
2.5. Change of Nymphal Density
3. Results
3.1. Starting Date of the Degree-Days Models
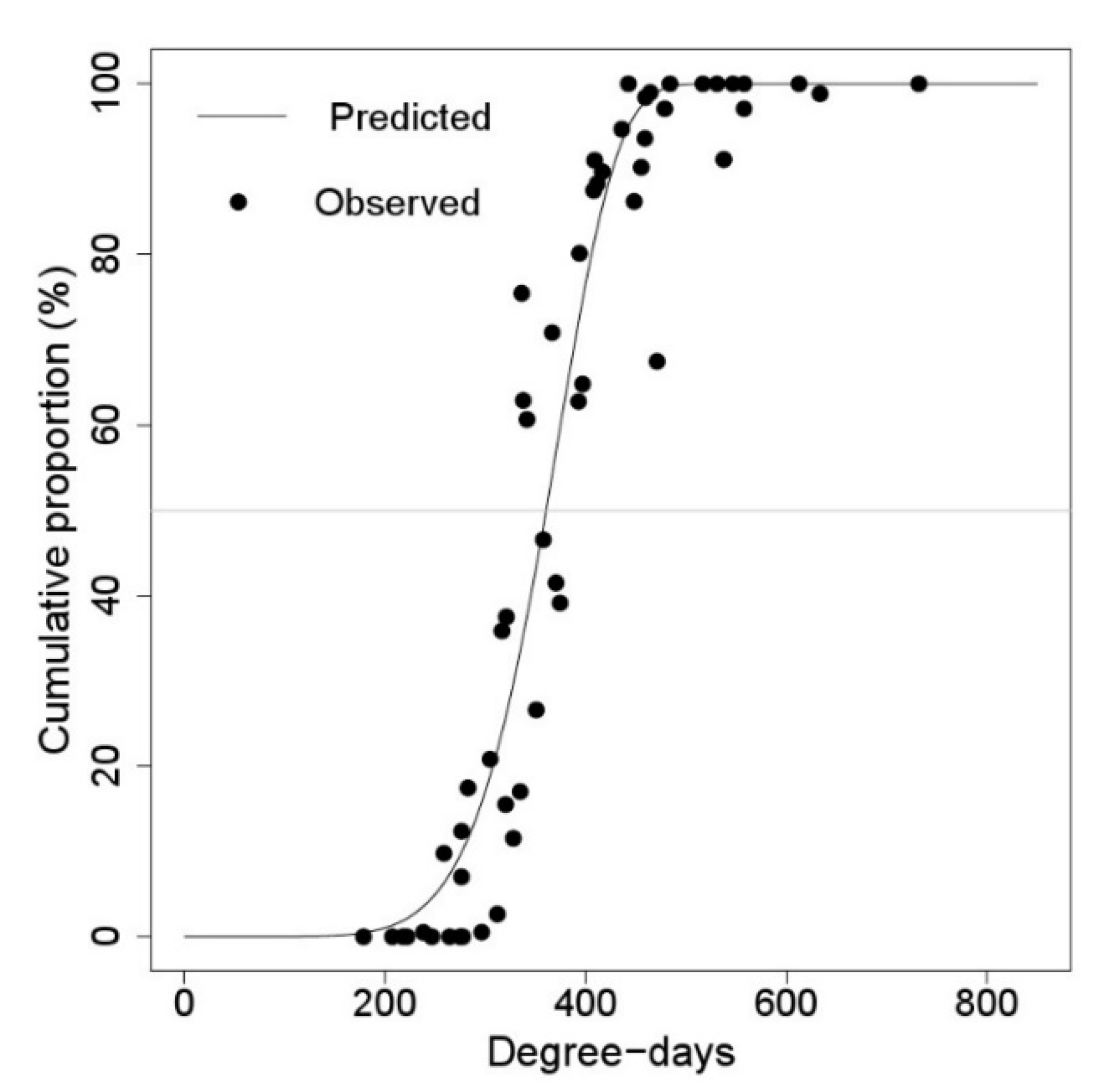
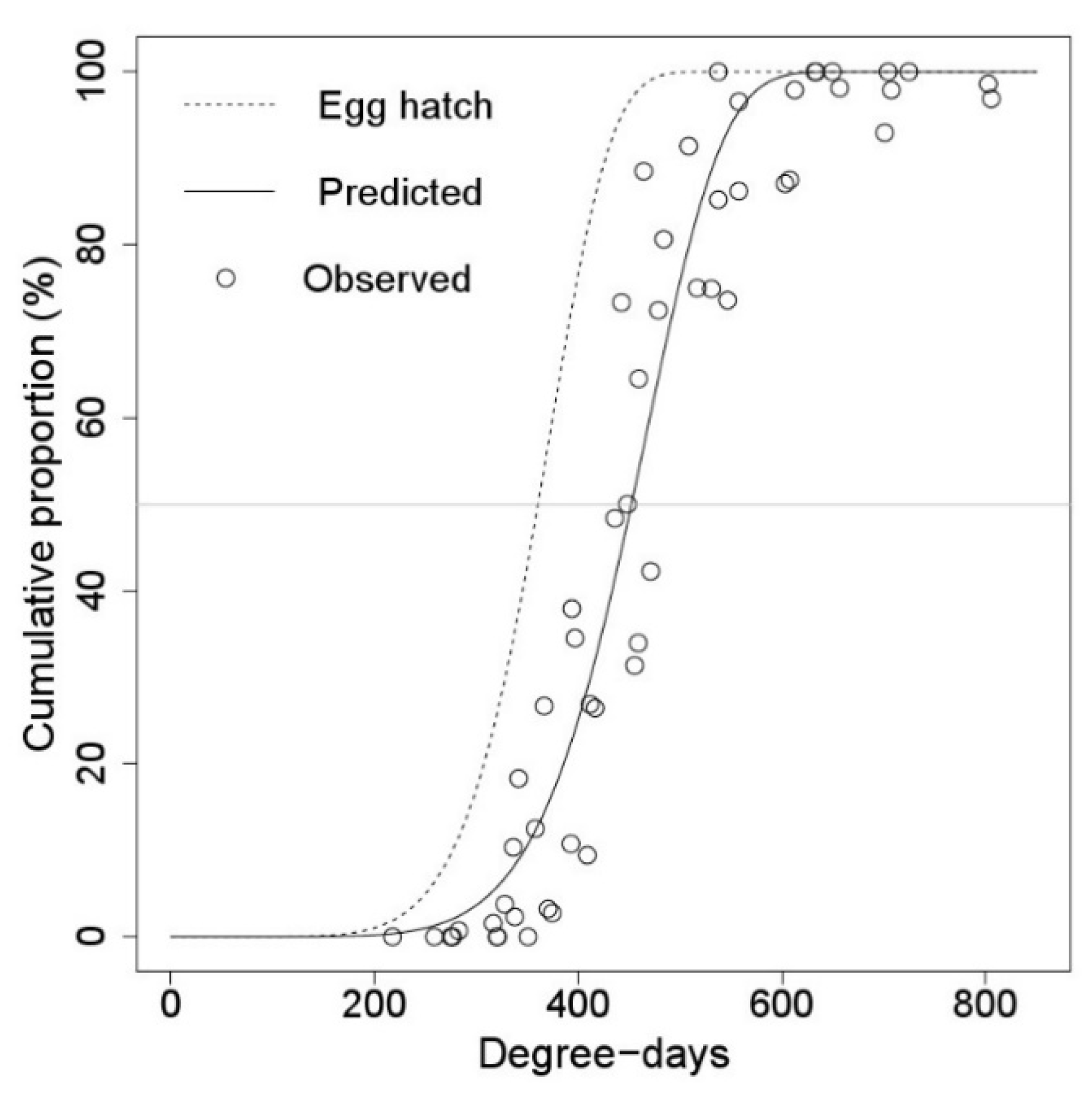
3.2. Egg Hatching and First Instar Falling Models
3.3. Change in Nymphal Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Metcalf, Z.P.; Bruner, S.C. Cuban flatidae with new species from adjacent regions. Ann. Entomol. Soc. Am. 1948, 41, 63–118. [Google Scholar] [CrossRef]
- Preda, C.; Skolka, M. Range expansion of Metcalfa pruinosa (Homoptera: Fulgoroidea) in southeastern Europe. Ecol. Balk. 2011, 3, 79–87. [Google Scholar]
- Kim, M.-J.; Baek, S.; Lee, S.-B.; Park, B.; Lee, S.-K.; Lee, Y.S.; Ahn, K.-S.; Choi, Y.-S.; Seo, H.-Y.; Lee, J.-H. Current and future distribution of Metcalfa pruinosa (Say) (Hemiptera: Flatidae) in Korea: Reasoning of fast spreading. J. Asia Pac. Entomol. 2019, 22, 933–940. [Google Scholar] [CrossRef]
- Zangheri, S.; Donadini, P. Comparsa nel Veneto di un omottero neartico: Metcalfa pruinosa Say (Homoptera; Flatidae). Redia 1980, 63, 301–305. [Google Scholar]
- Kim, Y.; Kim, M.; Hong, K.-J.; Lee, S. Outbreak of an exotic flatid; Metcalfa pruinosa (Say) (Hemiptera: Flatidae); in the capital region of Korea. J. Asia Pac. Entomol. 2011, 14, 473–478. [Google Scholar] [CrossRef]
- Strauss, G. Pest risk analysis of Metcalfa pruinosa in Austria. J. Pest. Sci. 2010, 83, 381–390. [Google Scholar] [CrossRef]
- Lee, D.-S.; Bae, Y.-S.; Byun, B.-K.; Lee, S.; Park, J.; Park, Y.-S. Occurrence prediction of the citrus flatid planthopper (Metcalfa pruinosa (Say; 1830)) in South Korea using a random forest model. Forests 2019, 10, 583. [Google Scholar] [CrossRef] [Green Version]
- Souliotis, C.; Papanikolaou, N.E.; Papachristos, D.; Fatouros, N. Host plants of the planthopper Metcalfa pruinosa (Say) (Hemiptera: Flatidae) and observations on its phenology in Greece. Hell. Plant Protect. J. 2008, 1, 39–41. [Google Scholar]
- Seo, H.-Y.; Park, D.-K.; Hwang, I.-S.; Choi, Y.-S. Host plants of Metcalfa pruinosa (Say) (Hemiptera: Flatidae) nymphs and adult. Korean J. Appl. Entomol. 2019, 58, 363–380. [Google Scholar]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Rebek, E.J.; Herms, D.A.; Smitley, D.R. Interspecific variation in resistance to emerald ash borer (Coleoptera: Buprestidae) among North American and Asian ash (Fraxinus spp.). Environ. Entomol. 2008, 37, 242–246. [Google Scholar] [CrossRef] [Green Version]
- Alma, A.; Ferracini, C.; Burgio, G. Development of a sequential plan to evaluate Neodryinus typhlocybae (Ashmead) (Hymenoptera: Dryinidae) population associated with Metcalfa pruinosa (Say) (Homoptera: Flatidae) infestation in northwestern Italy. Environ. Entomol. 2005, 34, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.W.; Lucchi, A. Distribution and ecology of Metcalfa pruinosa and associated planthoppers in North America (Hemiptera: Fulgoroidea). Atti. Accad. Naz. Ital. Entomol. Rend. Anno. 2001, 49, 121–130. [Google Scholar]
- Ciampolini, M.; Grossi, A.; Zottarelli, G. Damage to soyabean through attack by Metcalfa pruinosa. L’Inf. Agrar. 1987, 43, 101–103. [Google Scholar]
- Kim, D.-E.; Kil, J. Occurrence and host plant of Metcalfa pruinosa (Say) (Hemipter: Flatidae) in Korea. J. Environ. Sci. Int. 2014, 23, 1385–1394. [Google Scholar] [CrossRef] [Green Version]
- Dean, H.A.; Bailey, J.C. A flatid planthopper; Metcalfa pruinosa. J. Econ. Entomol. 1961, 54, 1104–1106. [Google Scholar] [CrossRef]
- Park, B.; Kim, M.-J.; Lee, S.-K.; Kim, G.-H. Analysis for dispersal and spatial pattern of Metcalfa pruinosa (Hemiptera: Flatidae) in southern sweet persimmon orchard. Korean J. Appl. Entomol. 2019, 58, 291–297. [Google Scholar]
- Lauterer, P.; Malenovsky, I. Metcalfa pruinosa (Say; 1830) introduced into the Czech Republic (Hemiptera: Flatidae). Beitr. Zikadenkunde 2002, 5, 10–13. [Google Scholar]
- Duso, C. Infestations by Metcalfa pruinosa in the Venice district. Inf. Fitopatol. 1984, 34, 11–14. [Google Scholar]
- Wilson, S.W.; McPherson, J.E. Life histories of Anormenis septentrionalis; Metcalfa pruinosa; and Ormenoides venusta with descriptions of immature stages. Ann. Entomol. Soc. Am. 1981, 74, 299–311. [Google Scholar] [CrossRef]
- Balakhnina, I.V.; Pastarnak, I.N.; Gnezdilov, V.M. Monitoring and control of Metcalfa pruinosa (Say) (Hemiptera; Auchenorrhyncha: Flatidae) in Krasnodar territory. Entomol. Rev. 2014, 94, 1067–1072. [Google Scholar] [CrossRef]
- Lee, W.; Park, C.-G.; Seo, B.Y.; Lee, S.-K. Development of an emergence model for overwintering eggs of Metcalfa pruinosa (Hemiptera: Flatidae). Korean J. Appl. Entomol. 2016, 55, 35–43. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Whang, I.-S.; Na, M.-S.; Park, D.-G.; Seo, H.-Y. Monitoring methods for Metcalfa pruinosa (Say) (Hemiptera: Flatidae) eggs on acacia branches. Korean J. Appl. Entomol. 2018, 57, 297–302. [Google Scholar]
- Jones, V.P.; Doerr, M.D.; Brunner, J.F. Is biofix necessary for predicting codling moth (Lepidoptera: Tortricidae) emergence in Washington State apple orchards? J. Econ. Entomol. 2008, 101, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, I.E.; Jermini, M.; Fuog, D.; Baumgärtner, J. Toward an improved understanding of the dynamics of vineyard-infesting Scaphoideus titanus leafhopper populations for better timing of management activities. Pest. Manag. Sci. 2011, 67, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 9 March 2020).
- Mesplé, F.; Trousselier, M.; Casellas, C.; Legendre, P. Evaluation of simple statistical criteria to qualify a simulation. Ecol. Model. 1996, 88, 9–18. [Google Scholar] [CrossRef]
- Flint, M.L. IPM in Practice: Principles and Methods of Integrated Pest Management, 2nd ed.; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2012; pp. 182–187. [Google Scholar]

| Site | Year | Latitude and Longitude (°, min, sec.) | Egg Hatching | First Instar Falling | ||
|---|---|---|---|---|---|---|
| No. Hatchings | First Appearance | No. Trap Catches | First Catch | |||
| Yeoncheon | 2018 | 38°04′59.0″ N, 127°04′36.7″ E | 194 | 5/16 | 85 | 5/29 |
| Seoul | 2018 | 37°27′27.4″ N, 126°56′54.4″ E | 188 | 5/18 | 93 | 5/27 |
| Suwon | 2018 | 37°15′57.8″ N, 126°59′11.1″ E | 24 | 5/21 | 15 | 5/28 |
| Yesan | 2018 | 36°44′11.9″ N, 126°49′17.9″ E | 58 | 5/24 | 16 | 5/28 |
| Yeoncheon | 2019 | 38°04′59.0″ N, 127°04′36.7″ E | 92 | 5/21 | 255 | 5/27 |
| Chuncheon | 2019 | 37°45′16.8″ N, 127°44′10.9″ E | 338 | 5/20 | 142 | 5/20 |
| Seocheon | 2019 | 36°10′23.9″ N, 126°46′28.0″ E | 156 | 5/22 | 53 | 5/22 |
| Paju | 2019 | 37°57′00.8″ N, 126°55′20.9″ E | 171 | 5/21 | 29 | 5/27 |
| Model | Starting Date 1 | Parameter | |
|---|---|---|---|
| α (Mean ± SEM) | β (Mean ± SEM) | ||
| Egg hatching | 1 January | 379.554 ± 4.4365 *** | 7.115 ± 0.7276 *** |
| 18 March | 362.466 ± 4.2109 *** | 6.907 ± 0.6798 *** | |
| First instar falling | 1 January | 476.119 ± 5.2429 *** | 7.121 ± 0.6990 *** |
| 18 March | 460.282 ± 5.3532 *** | 6.819 ± 0.6776 *** | |
| Starting Date | N 1 | Linear Regression | RMSE 3 (Days) | |||
|---|---|---|---|---|---|---|
| Slope (95% CI 2) | Intercept (95% CI) | p-Value | r 2 | |||
| 1 January | 34 | 0.99 (0.98, 1.01) | 1.14 (−1.72, 4.00) | <0.001 | 0.997 | 6.0 |
| 18 March | 6.3 | |||||
| Model | Cumulative Proportion (%) | ||||
|---|---|---|---|---|---|
| 10 | 30 | 50 | 70 | 90 | |
| Hatching | 276.64 | 328.36 | 360.50 | 389.59 | 426.76 |
| Falling | 347.12 | 411.95 | 452.23 | 488.69 | 535.28 |
| Difference | 70.47 | 83.59 | 91.74 | 99.11 | 108.52 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Baek, S.; Lee, J.-H. Egg Hatching and First Instar Falling Models of Metcalfa pruinosa (Hemiptera: Flatidae). Insects 2020, 11, 345. https://doi.org/10.3390/insects11060345
Kim M-J, Baek S, Lee J-H. Egg Hatching and First Instar Falling Models of Metcalfa pruinosa (Hemiptera: Flatidae). Insects. 2020; 11(6):345. https://doi.org/10.3390/insects11060345
Chicago/Turabian StyleKim, Min-Jung, Sunghoon Baek, and Joon-Ho Lee. 2020. "Egg Hatching and First Instar Falling Models of Metcalfa pruinosa (Hemiptera: Flatidae)" Insects 11, no. 6: 345. https://doi.org/10.3390/insects11060345




