Chemosensory and Behavioural Responses of Ixodes scapularis to Natural Products: Role of Chemosensory Organs in Volatile Detection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ticks
2.2. Chemicals
2.3. Electroscutumography (ESG) Recording
2.4. Y-Tube Behavioural Bioassay
2.5. Statistical Analysis
3. Results
3.1. Electroscutumography (ESG)
3.2. Behavioural Bioassays
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sonenshine, D.E.; Roe, R.M. Biology of Ticks, 2nd ed.; Oxford University Press: Oxford, UK, 2014; Volume 1. [Google Scholar]
- Sonenshine, D.E. Biology of Ticks; Oxford University Press: Oxford, UK, 1992; Volume 1, pp. 122–157. [Google Scholar]
- Iovinella, I.; Ban, L.; Song, L.; Pelosi, P.; Dani, F.R. Proteomic analysis of castor bean tick Ixodes ricinus: A focus on chemosensory organs. Insect Biochem. Mol. Biol. 2016, 78, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Josek, T.; Walden, K.K.; Allan, B.F.; Alleyne, M.; Robertson, H.M. A foreleg transcriptome for Ixodes scapularis ticks: Candidates for chemoreceptors and binding proteins that might be expressed in the sensory Haller’s organ. Ticks Tick Borne Dis. 2018, 9, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.M.F.; Li, A.Y.; Olafson, P.U.; Renthal, R.; Bauchan, G.R.; Lohmeyer, K.H.; León, A.A.P.D. Neuronal projections from the Haller’s organ and palp sensilla to the synganglion of Amblyomma americanum. Rev. Bras. Parasitol. Vet. 2016, 25, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.L.; Mitchell, R.D., III; Dhammi, A.; Bissinger, B.W.; Sonenshine, D.E.; Roe, R.M. Tick haller’s organ, a new paradigm for arthropod olfaction: How ticks differ from insects. Int. J. Mol. Sci. 2017, 18, 1563. [Google Scholar] [CrossRef]
- Carr, A.L.; Roe, R.M.; Arellano, C.; Sonenshine, D.E.; Schal, C.; Apperson, C.S. Responses of Amblyomma americanum and Dermacentor variabilis to odorants that attract haematophagous insects. Med. Vet. Entomol. 2013, 27, 86–95. [Google Scholar] [CrossRef]
- Carr, A.L.; Roe, M. Acarine attractants: Chemoreception, bioassay, chemistry and control. Pestic. Biochem. Phys. 2016, 131, 60–79. [Google Scholar] [CrossRef]
- Sonenshine, D.E. Pheromones and other semiochemicals of ticks and their use in tick control. Parasitology 2004, 129, S405–S425. [Google Scholar] [CrossRef] [PubMed]
- Van Duijvendijk, G.; Gort, G.; Sprong, H.; Takken, W. Behavioural responses of Ixodes ricinus nymphs to carbon dioxide and rodent odor. Med. Vet. Entomol. 2017, 31, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Garner, K.D.; Payton, M.E.; Talley, J.L.; Noden, B.H. Olfactory responses of Amblyomma maculatum to rumen fluid and other odorant that attract blood-seeking arthropods. Med. Vet. Entomol. 2019, 34, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.L.; de Oliveira Filho, J.G.; de Oliveira Silva, F.; Ferraz, A.L.L.; Mascarin, G.M. Attract or repel Amblyomma sculptum ticks: Screening of semiochemicals. Vet. Parasitol. 2020, 278, 109036. [Google Scholar] [CrossRef]
- Faraone, N.; MacPherson, S.; Hillier, N.K. Behavioral responses of Ixodes scapularis tick to natural products: Development of novel repellents. Exp. Appl. Acarol. 2019, 79, 195–207. [Google Scholar] [CrossRef]
- Dautel, H.; Dippel, C.; Werkhausen, A.; Diller, R. Efficacy testing of several Ixodes ricinus tick repellents: Different results with different assays. Ticks Tick Borne Dis. 2013, 4, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.F.; Klun, J.A.; Debboun, M. Repellency of deet and SS220 applied to skin involves olfactory sensing by two species of ticks. Med. Vet. Entomol. 2005, 19, 101–106. [Google Scholar] [CrossRef]
- Haggart, D.A.; Davis, E.E. Neurons sensitive to 2, 6-dichlorophenol on the tarsi of the tick Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 1981, 18, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Steullet, P.; Guerin, P.M. Identification of vertebrate volatiles stimulating olfactory receptors on tarsus I of the tick Amblyomma variegatum Fabricius (Ixodidae). J. Comp. Physiol. A 1994, 174, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Leonovich, S.A. Phenol and lactone receptors in the distal sensilla of the Haller’s organ in Ixodes ricinus ticks and their possible role in host perception. Exp. Appl. Acarol. 2004, 32, 89–102. [Google Scholar] [CrossRef]
- Soares, S.F.; Borges, L.M.F. Electrophysiological responses of the olfactory receptors of the tick Amblyomma cajennense (Acari: Ixodidae) to host-related and tick pheromone-related synthetic compounds. Acta Trop. 2012, 124, 192–198. [Google Scholar] [CrossRef]
- Romashchenko, A.V.; Ratushnyak, A.S.; Zapara, T.A.; Tkachev, S.E.; Moshkin, M.P. The correlation between tick (Ixodes persulcatus Sch.) questing behaviour and synganglion neuronal responses to odors. J. Insect Physiol. 2012, 58, 903–910. [Google Scholar] [CrossRef]
- Foelix, R.F.; Chu-Wang, I.W. Fine structural analysis of palpal receptors in the tick Amblyomma americanum (l.). Z. Zellforsch. Mikrosk. Anat. 1972, 129, 548–560. [Google Scholar] [CrossRef]
- Soares, S.F.; Louly, C.C.B.; Neves, C.A.; Marion-Poll, F.; Borges, L.M. Detection of phytoecdysteroids by gustatory sensilla on chelicerae of the brown dog tick Rhipicephalus sanguineus. Physiol. Entomol. 2012, 37, 241–249. [Google Scholar] [CrossRef]
- De Bruyne, M.D.; Guerin, P.M. Contact chemostimuli in the mating behaviour of the cattle tick, Boophilus microplus. Arch. Insect Biochem. Physiol. 1998, 39, 65–80. [Google Scholar] [CrossRef]
- Ferreira, L.L.; Soares, S.F.; de Oliveira Filho, J.G.; Oliveira, T.T.; de León, A.A.P.; Borges, L.M.F. Role of Rhipicephalus microplus cheliceral receptors in gustation and host differentiation. Ticks Tick Borne Dis. 2015, 6, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Rechav, Y.; Terry, S.; Knight, M.M.; Cross, R.H.M. Chemoreceptor organs used in detection of pheromone(s) of the tick Amblyomma hebraeum (Acarina: Ixodidae). J. Med. Entomol. 1977, 14, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, B.W.; Roe, R.M. Tick repellent research, methods, and development. Biology of ticks. In Biology of Ticks, 2nd ed.; Soneshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2013; Volume 2, pp. 380–390. [Google Scholar]
- Benelli, G.; Pavela, R.; Canale, A.; Mehlhorn, H. Tick repellents and acaricides of botanical origin: A green roadmap to control tick-borne diseases? Parasitol. Res. 2016, 115, 2545–2560. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R.; RStudio PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 24 July 2020).
- Marzouk, A.S.; Khalil, G.M.; Mohamed, F.S.A.; Farid, N. Hyalomma dromedarii (Acari: Ixodoidea: Ixodidae): Central and peripheral nervous system anatomy. Exp. Appl. Acarol. 1987, 3, 145–161. [Google Scholar] [CrossRef]
- Roma, G.C.; Nunes, P.H.; Remédio, R.N.; Camargo-Mathias, M.I. Synganglion histology in different stages of Rhipicephalus sanguineus ticks (Acari: Ixodidae). Parasitol. Res. 2012, 110, 2455–2463. [Google Scholar] [CrossRef]
- Sato, K.; Touhara, K. Insect olfaction: Receptors, signal transduction, and behavior. In Chemosensory Systems in Mammals, Fishes, and Insects, 1st ed.; Korsching, S., Meyerhof, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 203–220. [Google Scholar]
- Barth, F.G. A Spider’s World: Senses and Behavior; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2002. [Google Scholar]
- Uhl, G. Spider olfaction: Attracting, detecting, luring and avoiding. In Spider Ecophysiology, 1st ed.; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 141–157. [Google Scholar]
- Ignell, R.; Dekker, T.; Ghaninia, M.; Hansson, B.S. Neuronal architecture of the mosquito deutocerebrum. J. Comp. Neurol. 2005, 493, 207–240. [Google Scholar] [CrossRef]
- Del Fabbro, S.D.; Nazzi, F. From Chemistry to Behavior. Molecular structure and bioactivity of repellents against Ixodes ricinus ticks. PLoS ONE 2013, 8, e67832. [Google Scholar] [CrossRef]
- McMahon, C.; Kröber, T.; Guerin, P.M. In vitro assays for repellents and deterrents for ticks: Differing effects of products when tested with attractant or arrestment stimuli. Med. Vet. Entomol. 2003, 17, 370–378. [Google Scholar] [CrossRef]
- Allan, S.A. Chemical ecology of tick-host interactions. In Olfaction in Vector-Host Interactions, 1st ed.; Takken, W., Knols, B.G.J., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2010; Volume 2, pp. 327–347. [Google Scholar]
- Dogan, E.B.; Ayres, J.W.; Rossignol, P.A. Behavioural mode of action of DEET: Inhibition of lactic acid attraction. Med. Vet. Entomol. 1999, 13, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, M.; Steinbach, N.; Stensmyr, M.C.; Hansson, B.S.; Vosshall, L.B. A natural polymorphism alters odor and DEET sensitivity in an insect odorant receptor. Nature 2011, 478, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zeng, F.; Bedoukian, R.H.; Leal, W.S. DEET and other repellents are inhibitors of mosquito odorant receptors for oviposition attractants. Insect Biochem. Mol. Biol. 2019, 113, 103224. [Google Scholar] [CrossRef] [PubMed]
- Hillier, N.K.; Vickers, N.J. Mixture interactions in moth olfactory physiology: Examining the effects of odorant mixture, concentration, distal stimulation, and antennal nerve transection on sensillar responses. Chem. Senses 2011, 36, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.F.; Solberg, V.B.; Klun, J.A.; Kramer, M.; Debboun, M. Comparative activity of DEET and AI3-37220 repellents against the ticks Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in laboratory bioassays. J. Med. Entomol. 2004, 41, 249–254. [Google Scholar] [CrossRef]
- Dautel, H. Test systems for tick repellents. Int. J. Med. Microbiol. Suppl. 2004, 293, 182–188. [Google Scholar] [CrossRef]

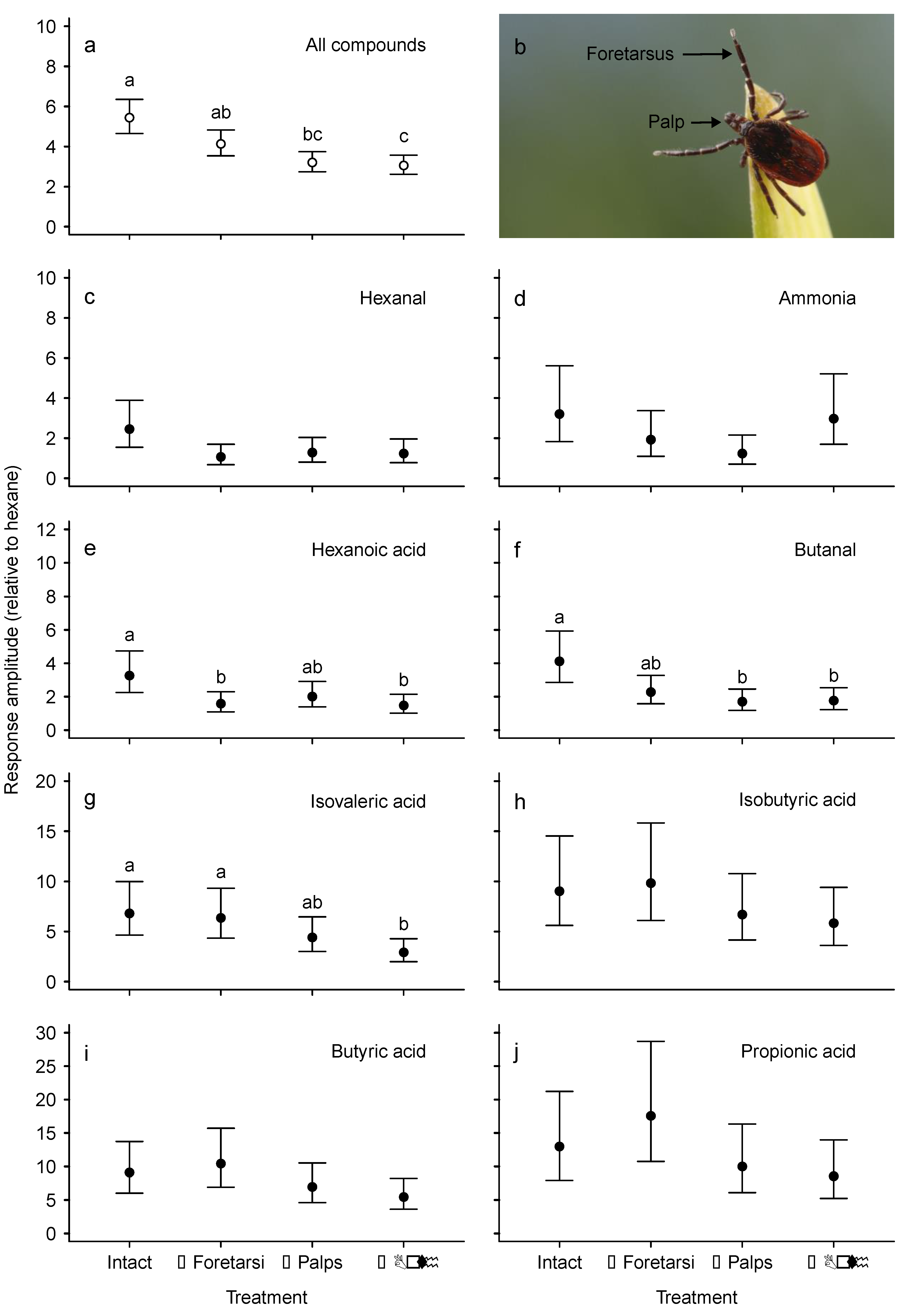
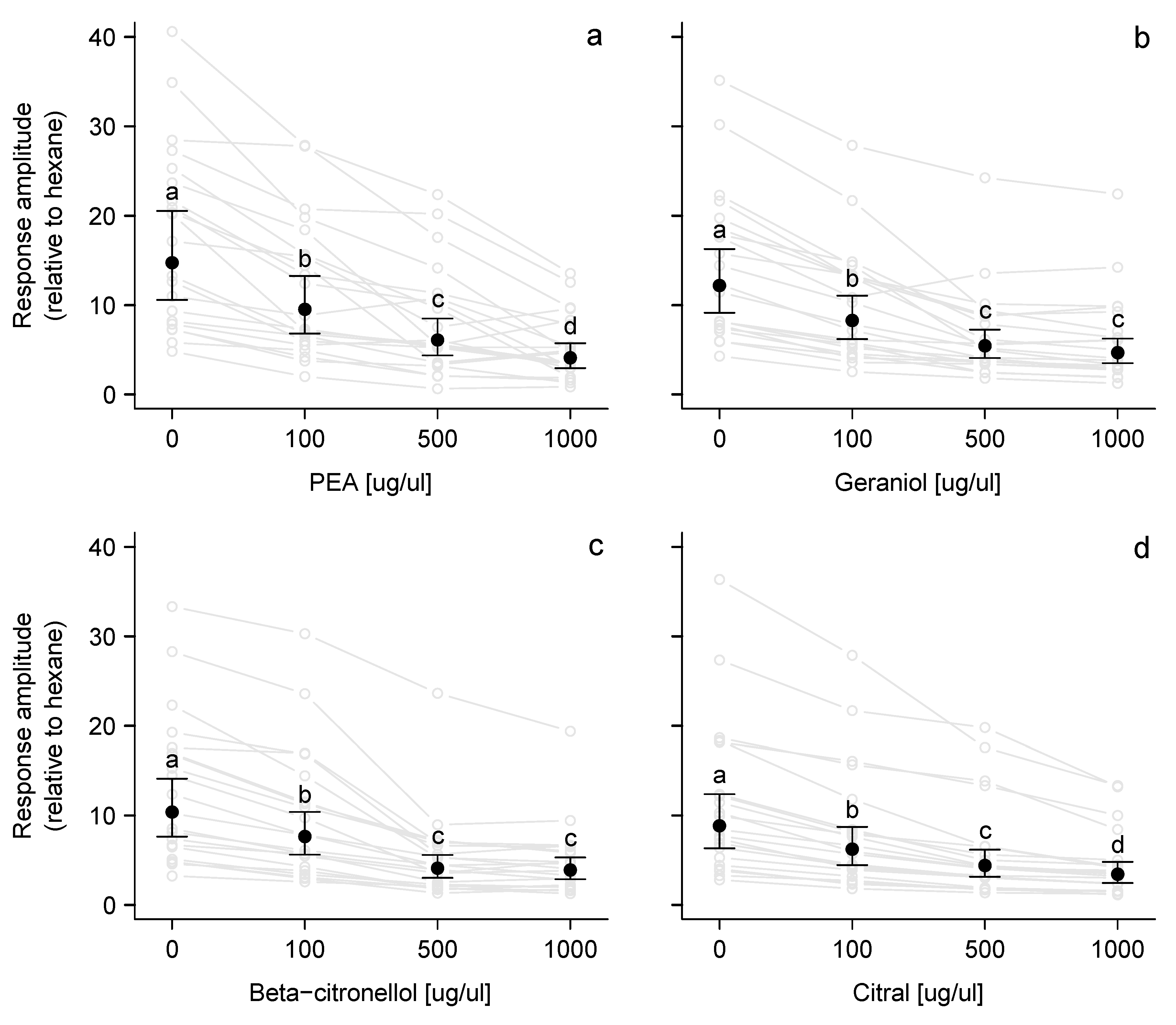

| Treatment | Concentration BTA | Concentration VOC † |
|---|---|---|
| control | 0 * | 0 * |
| 0 | 100 μg/μL | 0 * |
| 100 | 100 μg/μL | 100 μg/μL |
| 500 | 100 μg/μL | 500 μg/μL |
| 1000 | 100 μg/μL | 1000 μg/μL |
| VOC | Number of Carbons | 1 Functional Groups | mV | 95% CI | Tukey’s |
|---|---|---|---|---|---|
| 2n-Hexane | 6 | SH | 2.0 | (1.2, 3.3) | a |
| Pentanal | 5 | SH; A | 1.8 | (1.1, 2.9) | a |
| Octanal | 8 | SH; A | 1.8 | (1.1, 3.0) | a |
| Lactic acid | 3 | SH; CA; H | 2.1 | (1.3, 3.4) | a |
| Heptanal | 7 | SH; A | 2.6 | (1.6, 4.3) | a |
| Hexanal | 6 | SH; A | 5.0 | (3.1, 8.1) | b |
| NH3 | - | - | 6.5 | (4.0, 10.6) | bc |
| Hexanoic acid | 6 | SH; CA | 6.6 | (4.1, 10.8) | bc |
| Butanal | 4 | SH; A | 8.4 | (5.1, 13.6) | c |
| Isovaleric acid | 5 | SH; CA | 13.8 | (8.5, 22.6) | d |
| Isobutyric acid | 4 | SH; CA | 18.3 | (11.2, 29.9) | de |
| Butyric acid | 4 | SH; CA | 18.5 | (11.3, 30.2) | de |
| Propionic acid | 3 | SH; CA | 26.4 | (16.1, 43.0) | e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraone, N.; Light, M.; Scott, C.; MacPherson, S.; Hillier, N.K. Chemosensory and Behavioural Responses of Ixodes scapularis to Natural Products: Role of Chemosensory Organs in Volatile Detection. Insects 2020, 11, 502. https://doi.org/10.3390/insects11080502
Faraone N, Light M, Scott C, MacPherson S, Hillier NK. Chemosensory and Behavioural Responses of Ixodes scapularis to Natural Products: Role of Chemosensory Organs in Volatile Detection. Insects. 2020; 11(8):502. https://doi.org/10.3390/insects11080502
Chicago/Turabian StyleFaraone, Nicoletta, Michael Light, Catherine Scott, Samantha MacPherson, and N. Kirk Hillier. 2020. "Chemosensory and Behavioural Responses of Ixodes scapularis to Natural Products: Role of Chemosensory Organs in Volatile Detection" Insects 11, no. 8: 502. https://doi.org/10.3390/insects11080502
APA StyleFaraone, N., Light, M., Scott, C., MacPherson, S., & Hillier, N. K. (2020). Chemosensory and Behavioural Responses of Ixodes scapularis to Natural Products: Role of Chemosensory Organs in Volatile Detection. Insects, 11(8), 502. https://doi.org/10.3390/insects11080502






