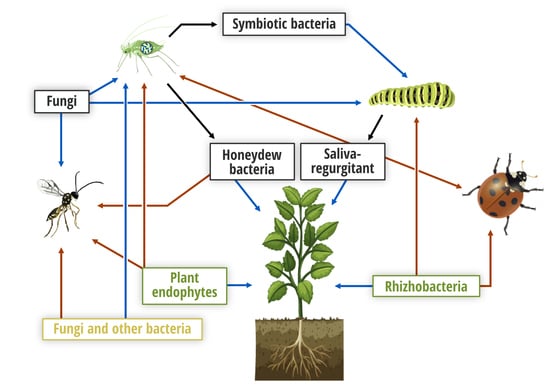
From Diverse Origins to Specific Targets: Role of Microorganisms in Indirect Pest Biological Control
Abstract
:1. Introduction
2. Microorganisms: Key Elements in Indirect Biological Pest Control
2.1. Rhizosphere Microorganisms
2.2. Phyllosphere Microorganisms
2.3. Endophytes
2.4. Insect Microbes
2.5. Nectar and Honeydew Microbes
3. Production and Formulation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nature Ecol. Evolut. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.M.; Hovmøller, M.S. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Stukenbrock, E.H.; McDonald, B.A. The origins of plant pathogens in agro-ecosystems. Annu. Rev. Phytopathol. 2008, 46, 75–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Lehmann, P.; Ammunet, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemela, P.; Terblanche, J.S.; et al. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D. Environmental and economic costs of the application pesticides primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Benbrook, C.M.; Groth, E.; Halloran, J.M.; Hansen, M.K.; Marquardt, S. Pest Management at the Crossroads; Consumers Union: New York, NY, USA, 1996. [Google Scholar]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef]
- Hatting, J.L.; Moore, S.D.; Malan, A.P. Microbial control of phytophagous invertebrate pests in South Africa: Current status and future prospects. J. Invertebr. Pathol. 2019, 165, 54–66. [Google Scholar] [CrossRef]
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef] [Green Version]
- van Lenteren, J.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2017, 63, 39–59. [Google Scholar] [CrossRef] [Green Version]
- Perović, D.J.; Gámez-Virués, S.; Landis, D.A.; Wäckers, F.; Gurr, G.M.; Wratten, S.D.; You, M.S.; Desneux, N. Managing biological control services through multi-trophic trait interactions: Review and guidelines for implementation at local and landscape scales. Biol. Rev. 2018, 93, 306–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karp, D.S.; Chaplin-Kramer, R.; Meehan, T.D.; Martin, E.A.; DeClerck, F.; Grab, H.; Gratton, C.; Hunt, L.; Larsen, A.E.; Martínez-Salinas, A.; et al. Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl. Acad. Sci. USA 2018, 115, E7863–E7870. [Google Scholar] [CrossRef] [Green Version]
- Egli, L.; Meyer, C.; Scherber, C.; Kreft, H.; Tscharntke, T. Winners and Losers of National and Global Efforts to Reconcile Agricultural Intensification and Biodiversity Conservation. Global Chang. Biol. 2018, 24, 2212–2228. [Google Scholar] [CrossRef]
- Myers, J.H.; Cory, J.S. Biological control agents: Invasive species or valuable solutions? In Impact of Biological Invasions on Ecosystem Services; Vilà, M., Hulme, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 12, pp. 191–202. [Google Scholar]
- De Clercq, P.; Mason, P.G.; Babendreier, D. Benefits and risks of exotic biological control agents. BioControl 2011, 56, 681–698. [Google Scholar] [CrossRef]
- Ruiu, L. Microbial biopesticides in agroecosystems. Agronomy 2018, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- Lacey, L.L.A.; Frutos, R.; Kaya, H.K. Insect Pathogens as Biological Control Agents: Do They Have a Future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef] [Green Version]
- de Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Rangel, D.E. Mutants and isolates of Metarhizium anisopliae are diverse in their relationships between conidial pigmentation and stress tolerance. J. Invertebr. Pathol. 2006, 93, 170–182. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Braga, G.U.L.; Anderson, A.J.; Roberts, D.W. Variability in conidial thermotolerance of Metarhizium anisopliae isolates from different geographic origins. J. Invertebr. Pathol. 2005, 88, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R.; Araj, S.E. Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biol. Control 2018, 116, 53–61. [Google Scholar] [CrossRef]
- Friesen, M.L. Microbially mediated plant functional traits. In Molecular Microbial Ecology of the Rhizosphere, Two Volume Set; De Bruijn, F.J., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2013; pp. 87–102. [Google Scholar]
- Pineda, A.; Zheng, S.J.; van Loon, J.J.A.; Pieterse, C.M.J.; Dicke, M. Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.; De Moraes, C.M.; Stephenson, A.G.; Mescher, M.C. Pathogen effects on vegetative and floral odours mediate vector attraction and host exposure in a complex pathosystem. Ecol. Lett. 2012, 15, 1430–1438. [Google Scholar] [CrossRef]
- Beck, J.J.; Vannette, R.L. Harnessing insect-microbe chemical communications to control insect pests of agricultural systems. J. Agric. Food Chem. 2017, 65, 23–28. [Google Scholar] [CrossRef]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2017, 107, 50–59. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Rachid, M.H.; Chung, Y.R. Induction of Systemic Resistance against Insect Herbivores in Plants by Beneficial Soil Microbes. Front. Plant Sci. 2017, 8, 1816. [Google Scholar]
- Van Oosten, V.R.; Bodenhausen, N.; Reymond, P.; Van Pelt, J.A.; Van Loon, L.L.; Dicke, M.; Pieterse, C.M.J. Differential Effectiveness of Microbially Induced Resistance Against Herbivorous Insects in Arabidopsis. Mol. Plant Microbe Interact. 2008, 7, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Curtis, T.P.; Sloan, W.T.; Scannell, J.W. Estimating prokaryotic diversity and its limits. Proc. Natl. Acad. Sci. USA 2002, 99, 10494–10499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, T.P.; Sloan, W.T. Exploring microbial diversity: A vast below. Science 2005, 309, 1331–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koide, R.T.; Mosse, B. A History of Research on Arbuscular Mycorrhiza. Mycorrhiza 2004, 14, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, D.; Bossi, S.; Cascone, P.; Digilio, M.C.; Prieto, J.D.; Fanti, P.; Guerrieri, E.; Iodice, L.; Lingua, G.; Lorito, M.; et al. Tomato below ground-above ground interactions: Trichoderma longibrachiatum affects the performance of Macrosiphum euphorbiae and its natural antagonists. Mol. Plant Microbe Interact. 2013, 26, 1249–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pangesti, N.; Weldegergis, B.T.; Langendorf, B.; van Loon, J.J.; Dicke, M.; Pineda, A. Rhizobacterial colonization of roots modulates plant volatile emission and enhances the attraction of a parasitoid wasp to host-infested plants. Oecologia 2015, 178, 1169–1180. [Google Scholar] [CrossRef] [Green Version]
- Aloo, B.N.; Makumba, B.A.; Mbega, E.R. The potential of Bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol. Res. 2019, 219, 26–39. [Google Scholar] [CrossRef]
- Rasmann, S.; Bennett, A.; Biere, A.; Karley, A.; Guerrieri, E. Root symbionts: Powerful drivers of plant above- and belowground indirect defenses. Insect. Sci. 2017, 24, 947–960. [Google Scholar] [CrossRef]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Current Opinion Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Bukovinszky, T.; van Veen, F.F.; Jongema, Y.; Dicke, M. Direct and indirect effects of resource quality on food web structure. Science 2008, 319, 804–807. [Google Scholar] [CrossRef]
- Kessler, A.; Heil, M. The multiple faces of indirect defences and their agents of natural selection. Funct. Ecol. 2011, 25, 348–357. [Google Scholar] [CrossRef]
- Schausberger, P.; Peneder, S.; Juerschik, S.; Hoffmann, D. Mycorrhiza changes plant volatiles to attract spider mite enemies. Funct. Ecol. 2012, 26, 441–449. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.H.; Harris, R.F. The ecology and biogeography of microorganisms on plant surfaces. Annu. Rev. Phytopathol. 2000, 38, 145–180. [Google Scholar] [CrossRef] [PubMed]
- Lambais, M.R. Bacterial diversity in tree canopies of the atlantic forest. Science 2006, 312, 1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knief, C.; Delmotte, N.; Chaffron, S.; Stark, M.; Innerebner, G.; Wassmann, R. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISMEJ 2012, 6, 1378–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatachalam, S.; Ranjan, K.; Prasanna, R.; Ramakrishnan, B.; Thapa, S.; Kanchan, A. Diversity and functional traits of culturable microbiome members, including cyanobacteria in the rice phyllosphere. Plant Biol. 2016, 18, 627–637. [Google Scholar] [CrossRef]
- Saleem, M.; Meckes, N.; Pervaiz, Z.H.; Traw, M.B. Microbial interactions in the phyllosphere increase plant performance under herbivore biotic stress. Front. Microbiol. 2017, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Montarry, J.; Cartolaro, P.; Delmotte, F.; Jolivet, J.; Willocquet, L. Genetic structure and aggressiveness of Erysiphe necator populations during grapevine powdery mildew epidemics. Appl. Environ. Microbiol. 2008, 74, 6327–6332. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, G.; Coaker, G.L.; Leveau, J.H.J. New insights into the structure and function of phyllosphere microbiota through high-throughput molecular approaches. FEMS Microbiol. Lett. 2013, 348, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Berg, M.; Koskella, B. Nutrient- and dose-dependent microbiome-mediated protection against a plant pathogen. Curr. Biol. 2018, 28, 2487–2492. [Google Scholar] [CrossRef] [Green Version]
- Innerebner, G.; Knief, C.; Vorholt, J.A. Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl. Environ. Microbiol. 2011, 77, 3202–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritpitakphong, U.; Falquet, L.; Vimoltust, A.; Berger, A.; Metraux, J.P.; L’Haridon, F. The microbiome of the leaf surface of Arabidopsis protects against a fungal pathogen. New Phytol. 2016, 210, 1033–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.S.; Jones, C.P.; Yao, K.F.; Lee, L.E. An epiphytic yeast (Sporobolomyces roseus) influencing in oviposition preference of the European corn borer (Ostrinia nubilalis) on maize. Acta Oecol. 1993, 14, 563–574. [Google Scholar]
- Bai, Y. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V. Potential impact of soil microbiomes on the leaf metabolome and on herbivore feeding behavior. New Phytol. 2013, 198, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Muvea, A.M. Colonization of onions by endophytic fungi and their impacts on the biology of Thrips tabaci. PLoS ONE 2014, 9, e108242. [Google Scholar] [CrossRef] [Green Version]
- Saikkonen, K.; Lehtonen, P.; Helander, M.; Koricheva, J.; Faeth, S.H. Model systems in ecology: Dissecting the endophyte-grass literature. Trends Plant Sci. 2006, 11, 428–433. [Google Scholar] [CrossRef]
- Vega, F.E. Insect pathology and fungal endophytes. J. Invertebr. Pathol. 2008, 98, 277–279. [Google Scholar] [CrossRef]
- Behie, S.W. Endophytic insect-parasitic fungi trans-locate nitrogen directly from insects to plants. Science 2012, 336, 1576–1577. [Google Scholar] [CrossRef] [Green Version]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Wang, L. Fungal Endophytes: Beyond Herbivore Management. Front. Microbiol. 2018, 9, 544. [Google Scholar] [CrossRef] [Green Version]
- Jaber, L.R.; Alananbeh, K.M. Fungal entomopathogens as endophytes reduce several species of Fusarium causing crown and root rot in sweet pepper (Capsicum annuum L.). Biol. Control 2018, 126, 117–126. [Google Scholar] [CrossRef]
- Barker, G.M.; Addison, P.J. Influence of clavicipitaceous endophyte infection in ryegrass on development of the parasitoid Microctonus hyperodae Loan (Hymenoptera: Braconidae) in Listronotus bonariensis (Kuschel) (Coleoptera: Curculionidae). Biol. Control 1996, 7, 281–287. [Google Scholar] [CrossRef]
- Urrutia, C.M.A.; Wade, M.R.; Phillips, C.B.; Wratten, S.D. Influence of host diet on parasitoid fitness: Unravelling the complexity of a temperate pastoral agroecosystem. Entomol. Exp. Appl. 2007, 123, 63–71. [Google Scholar] [CrossRef]
- Fuchs, B.; Krauss, J. Can Epichloë endophytes enhance direct and indirect plant defence? Fungal Ecol. 2019, 38, 98–103. [Google Scholar] [CrossRef]
- Harri, S.A.; Krauss, J.; Muller, C.B. Fungal endosymbionts of plants reduce lifespan of an aphid secondary parasitoid and influence host selection. Proc. Biol. Sci. 2018, 275, 2627–2632. [Google Scholar] [CrossRef] [Green Version]
- Hogenhout, S.A.; Bos, J.I.B. Effector proteins that modulate plant–insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Turlings, T.C.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Felton, G.W.; Tumlinson, J.H. Plant-insect dialogs: Complex interactions at the plant-insect interface. Curr. Opin. Plant Biol. 2008, 11, 457–463. [Google Scholar] [CrossRef]
- Tian, D.; Peiffer, M.; Shoemaker, E.; Tooker, J.; Haubruge, E.; Francis, F.; Luthe, D.S.; Felton, G.W. Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. PLoS ONE 2012, 7, e36168. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Peiffer, M.; Hoover, K.; Rosa, C.; Zeng, R.; Felton, G.W. Helicoverpa zea gut-associated bacteria indirectly induce defenses in tomato by triggering a salivary elicitor(s). New Phytol. 2017, 214, 1294–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, W.L.; Guo, G.X.; Ji, X.L. Volatile emission in wheat and parasitism by Aphidius avenae after exogenous application of salivary enzymes of Sitobion avenae. Entomol. Exp. Appl. 2009, 130, 215–221. [Google Scholar] [CrossRef]
- Ma, R.; Chen, J.L.; Cheng, D.F.; Sun, J.R. Activation of defense mechanism in wheat by polyphenol oxidase from aphid saliva. J. Agric. Food Chem. 2010, 58, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Harmel, N.; Letocart, E.; Cherqui, A.; Giordanengo, P.; Mazzucchelli, G.; Guillonneau, F.; De Pauw, E.; Haubruge, E.; Francis, F. Identification of aphid salivary proteins: A proteomic investigation of Myzus persicae. Insect. Mol. Biol. 2008, 17, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Ma, L.; Francis, F.; Yang, Y.; Chen, H.; Wu, H.; Chen, X. Proteins identified from saliva and salivary glands of the Chinese gall aphid Schlechtendalia chinensis. Proteomics 2018, 18, e1700378. [Google Scholar] [CrossRef]
- Bos, J.I.; Prince, D.; Pitino, M.; Maffei, M.E.; Win, J.; Hogenhout, S.A. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 2010, 6, e1001216. [Google Scholar] [CrossRef]
- Atamian, H.S.; Chaudhary, R.; Cin, V.D.; Bao, E.; Girke, T.; Kaloshian, I. In planta expression or delivery of potato aphid Macrosiphum euphorbiae effectors Me10 and Me23 enhances aphid fecundity. Mol. Plant Microbe. Interact. 2013, 26, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, P.A.; Stam, R.; Warbroek, T.; Bos, J.I. Mp10 and Mp42 from the aphid species Myzus persicae trigger plant defenses in Nicotiana benthamiana through different activities. Mol. Plant Microbe. Interact. 2014, 27, 30–39. [Google Scholar] [CrossRef] [Green Version]
- De Vos, M.; Jander, G. Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ. 2009, 32, 1548–1560. [Google Scholar] [CrossRef]
- Vandermoten, S.; Harmel, N.; Mazzucchelli, G.; De Pauw, E.; Haubruge, E.; Francis, F. Comparative analyses of salivary proteins from three aphid species. Insect. Mol. Biol. 2014, 23, 67–77. [Google Scholar] [CrossRef]
- Pozo, M.; Lievens, B.; Jacquemyn, H. Impact of microorganisms on nectar chemistry, pollinator attraction and plant fitness. In Nectar: Production, Chemical Composition and Benefits to Animals and Plants; Nova Science Publishers: Hauppauge, NY, USA, 2015; Volume 41. [Google Scholar]
- Lievens, B.; Hallsworth, J.E.; Pozo, M.I.; Belgacem, Z.; Stevenson, A.; Willems, K.; Jacquemyn, H. Microbiology of sugar-rich environments: Diversity, ecology, and system constraints. Environ. Microbiol. 2015, 17, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Vannette, R.L.; Fukami, T. Dispersal enhances beta diversity in nectar microbes. Ecol. Lett. 2017, 20, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Brysch-Herzberg, M. Ecology of yeasts in plant-bumblebee mutualism in Central Europe. FEMS Microbiol. Ecol. 2004, 50, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M.; Pozo, M.I. Nectar yeasts warm the flowers of a winter-blooming plant. Proc. R. Soc. B 2010, 277, 1827–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rering, C.C.; Beck, J.J.; Hall, G.W.; McCartney, M.M.; Vannette, R.L. Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol. 2018, 220, 750–759. [Google Scholar] [CrossRef] [Green Version]
- Sobhy, I.S.; Baets, D.; Goelen, T.; Herrera-Malaver, B.; Bosmans, L.; Van den Ende, W.; Verstrepen, K.J.; Wäckers, F.; Jacquemyn, H.; Lievens, B. Sweet scents: Nectar specialist yeasts enhance nectar attraction of a generalist aphid parasitoid without affecting survival. Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Herrera, C.M.; Pozo, M.I.; Medrano, M. Yeasts in nectar of an early-blooming herb: Sought by bumble bees, detrimental to plant fecundity. Ecology 2013, 94, 273–279. [Google Scholar] [CrossRef]
- Schaeffer, R.N.; Irwin, R.E. Yeasts in nectar enhance male fitness in a montane perennial herb. Ecology 2014, 95, 1792–1798. [Google Scholar] [CrossRef]
- Schaeffer, R.N.; Phillips, C.R.; Duryea, M.C.; Andicoechea, J.; Irwin, R.E. Nectar yeasts in the tall Larkspur Delphinium barbeyi (Ranunculaceae) and effects on components of pollinator foraging behavior. PLoS ONE 2014, 9, e108214. [Google Scholar] [CrossRef]
- Good, A.P.; Gauthier, M.-P.L.; Vannette, R.L.; Fukami, T. Honey bees avoid nectar colonized by three bacterial species, but not by a yeast species, isolated from the bee gut. PLoS ONE 2014, 9, e86494. [Google Scholar] [CrossRef]
- Junker, R.R.; Romeike, T.; Keller, A.; Langen, D. Density-dependent negative responses by bumblebees to bacteria isolated from flowers. Apidologie 2014, 45, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Sobhy, I.S.; Goelen, T.; Herrera-Malaver, B.; Verstrepen, K.J.; Wäckers, F.; Jacquemyn, H.; Lievens, B. Associative learning and memory retention of nectar yeast volatiles in a generalist parasitoid. Anim. Behav. 2019, 153, 137–146. [Google Scholar] [CrossRef]
- Lenaerts, M.; Goelen, T.; Paulussen, C.; Herrera-Malaver, B.; Steensels, J.; Van den Ende, W.; Verstrepen, K.J.; Wäckers, F.; Jacquemyn, H.; Lievens, B. Nectar bacteria affect life history of a generalist aphid parasitoid by altering nectar chemistry. Funct. Ecol. 2017, 31, 2061–2069. [Google Scholar] [CrossRef] [Green Version]
- Hogervorst, P.A.; Wäckers, F.L.; Romeis, J. Effects of honeydew sugar composition on the longevity of Aphidius ervi. Entomol. Exp. Appl. 2007, 122, 223–232. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Van Rijn, P.C.; Heimpel, G.E. Honeydew as a food source for natural enemies: Making the best of a bad meal? Biol. Control 2008, 45, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, Y.; Cloutier, C. Honeydew as a source of host-searching kairomones for the aphid parasitoid Aphidius nigripes (Hymenoptera: Aphidiidae). Can. J. Zool. 1984, 62, 1513–1520. [Google Scholar] [CrossRef]
- Bargen, H.; Saudhof, K.; Poehling, H.M. Prey finding by larvae and adult females of Episyrphus balteatus. Entomol. Exp. Appl. 1998, 87, 245–254. [Google Scholar] [CrossRef]
- Leroy, P.; Sabri, A.; Heuskin, S.; Thonart, P.; Lognay, G.; Verheggen, F.; Francis, F.; Brostaux, Y.; Felton, G.; Haubruge, E. Microorganisms from Aphid Honeydew Attract and Enhance the Efficacy of Natural Enemies. Nat. Commun. 2011, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Goelen, T.; Sobhy, I.S.; Vanderaa, C.; de Boer, J.G.; Delvigne, F.; Francis, F.; Wäckers, F.; Rediers, H.; Verstrepen, K.J.; Wenseleers, T.; et al. Volatiles of bacteria associated with parasitoid habitats elicit distinct olfactory responses in an aphid parasitoid and its hyperparasitoid. Funct. Ecol. 2020, 34, 507–520. [Google Scholar] [CrossRef]
- Sabri, A.; Vandermoten, S.; Leroy, P.; Haubruge, E.; Hance, T.; Thonart, P.; De Pauw, E.; Francis, F. Proteomic Investigation of Aphid Honeydew Reveals an Unexpected Diversity of Proteins. PLoS ONE 2013, 8, e74656. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.S. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, A.; Forsgren, E.; Fries, I.; Paxton, R.J.; Flaberg, E.; Szekely, L.; Olofsson, T.C. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS ONE 2012, 7, e33188. [Google Scholar] [CrossRef]
- Christiaens, J.F.; Franco, L.M.; Cools, T.L.; De Meester, L.; Michiels, J.; Wenseleers, T.; Hassan, B.A.; Yaksi, E.; Verstrepen, K.J. The fungal aroma gene ATF1 promotes dispersal of yeast cells through insect vectors. Cell Rep. 2014, 9, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Martınez, E.S.; Bosque-Perez, N.A.; Berger, P.H.; Zemetra, R.S.; Ding, H.; Eigenbrode, S.D. Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to Barley yellow dwarf virus-infected transgenic and untransformed wheat. Environ. Entomol. 2004, 33, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Mann, R.S.; Ali, J.G.; Hermann, S.L.; Tiwari, S.; Pelz-Stelinski, K.S.; Alborn, H.T.; Stelinski, L.L. Induced release of a plant-defense volatile ‘deceptively’ attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathog. 2012, 8, e1002610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, F.; Druart, F.; Diana Di Mavungu, J.; De Boevre, M.; De Saeger, S.; Delvigne, F. Biofilm mode of cultivation leads to an improvement of the entomotoxic patterns of two Aspergillus species. Microorganisms 2020, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Bowers, M.D. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia 2015, 179, 1–14. [Google Scholar] [CrossRef]
- Pineda, A.; Soler, R.; Weldegergis, B.T.; Shimwela, M.M.; Van Loon, J.J.A.; Dicke, M. Non-pathogenic rhizobacteria interfere with the attraction of parasitoids to aphid-induced plant volatiles via jasmonic acid signalling. Plant Cell Environ. 2013, 36, 393–404. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Erb, M.; Ton, J.; Brandenburg, A.; Karlen, D.; Zopfi, J. Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ. 2014, 37, 813–826. [Google Scholar] [CrossRef]
- Song, G.C.; Ryu, C.M. Two volatile organic compounds trigger plant self- defense against a bacterial pathogen and a sucking insect in cucumber under open field conditions. Int. J. Mol. Sci. 2013, 14, 9803–9819. [Google Scholar] [CrossRef] [Green Version]
- Kaur, T.; Singh, B.; Kaur, A.; Kaur, S. Endophyte-mediated interactions between cauliflower, the herbivore Spodoptera litura, and the ectoparasitoid Bracon hebetor. Oecologia 2015, 179, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Bixby-Brosi, A.; Potter, D. Endophyte-mediated tritrophic interactions between a grass-feeding caterpillar and two parasitoid species with different life histories. Arthropod Plant Interact. 2012, 6, 27–34. [Google Scholar] [CrossRef]
- González-Mas, N.; Cuenca-Medina, M.; Gutiérrez-Sánchez, F.; Quesada-Moraga, E. Bottom-up effects of endophytic Beauveria bassiana on multitrophic interactions between the cotton aphid, Aphis gossypii, and its natural enemies in melon. J. Pest Sci. 2019, 92, 1271–1281. [Google Scholar] [CrossRef] [Green Version]

| Soilborne Microbes | Pests | Beneficials | Effects | |
|---|---|---|---|---|
| Pseudomonas fluorescens WCS417r | Myzus persicae Sultzer | Diaeretiella rapae M’Intosh | Increasing parasitoid attraction to plant | [112] |
| Pseudomonas fluorescens WCS417r | Mamestra brassicae L. | Microplitis mediator Haliday | Plant growth and parasitoid attraction | [38] |
| Enterobacter aerogenes | Spodoptera littoralis Boisduval | Cotesia marginiventris Cresson | Increasing parasitoid attraction to plant | [113] |
| Pseudononas syringae | Myzus persicae | Coccinella septempunctata L. | Plant pest resistance and parasitoid attraction | [114] |
| Endophytes and plant symbionts | ||||
| Beauveria bassiana, Metarhizium brunneum | Myzus persicae | Aphidius colemani Vierick | Reduction of pest development and parasitoid biology | [24] |
| Aspergillus flavus, A. niger | S. littoralis | Bracon hebetor Say | Pest resistance and beneficial attraction | [115] |
| Neotyphodium lolii | Agrotis ipsilon (Hufnagel) | Copidosoma bakeri (Howard) | Negative development of parasitoid | [116] |
| Beauveria bassiana | Aphis gossypii Glover | Chrysoperla carnea, A. colemani | Reductiion of pest and beneficial performances | [117] |
| Nectar and honeydew microbes | ||||
| Metschnikowia gruessii, M. reukaufii | Not studied | Aphidius ervi | Attraction and dispersal of parasitoids | [90] |
| Bacillus strains | Not studied | Aphidius colemani | Attraction of parasitoids | [103] |
| Staphylococcus sciuri | Acyrthosiphon pisum | Episyrphus balteatus Degeer | Attraction and longevity of parasitoids | [102] |
| Insect microbes | ||||
| Still no evidence on both trophic levels | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, F.; Jacquemyn, H.; Delvigne, F.; Lievens, B. From Diverse Origins to Specific Targets: Role of Microorganisms in Indirect Pest Biological Control. Insects 2020, 11, 533. https://doi.org/10.3390/insects11080533
Francis F, Jacquemyn H, Delvigne F, Lievens B. From Diverse Origins to Specific Targets: Role of Microorganisms in Indirect Pest Biological Control. Insects. 2020; 11(8):533. https://doi.org/10.3390/insects11080533
Chicago/Turabian StyleFrancis, Frédéric, Hans Jacquemyn, Frank Delvigne, and Bart Lievens. 2020. "From Diverse Origins to Specific Targets: Role of Microorganisms in Indirect Pest Biological Control" Insects 11, no. 8: 533. https://doi.org/10.3390/insects11080533
APA StyleFrancis, F., Jacquemyn, H., Delvigne, F., & Lievens, B. (2020). From Diverse Origins to Specific Targets: Role of Microorganisms in Indirect Pest Biological Control. Insects, 11(8), 533. https://doi.org/10.3390/insects11080533