Potential of Cucurbitacin B and Epigallocatechin Gallate as Biopesticides against Aphis gossypii
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Maintenance
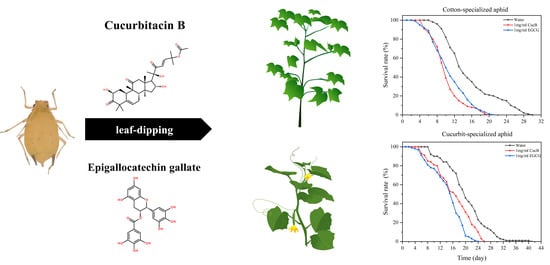
2.2. Preparation of CucB and EGCG
2.3. Toxicity Analysis of CucB and EGCG on A. gossypii
2.3.1. Effects of CucB and EGCG on the Life History Traits of A. gossypii
2.3.2. Effects of CucB and EGCG on the Nonhost Adaptation of F1 Generations
2.3.3. Effects of CucB and EGCG on Detoxifying Enzymes of A. Gossypii
2.4. Statistical Analyses
3. Results
3.1. Effects of CucB and EGCG on the Life History Traits of A. gossypii
3.2. Effects of CucB and EGCG on the Nonhost Adaptation of F1 Generations
3.3. Effects of CucB and EGCG on Detoxifying Enzymes of A. Gossypii
3.3.1. Acetylcholinesterase
3.3.2. Carboxylesterase
3.3.3. Acid Phosphatase
3.3.4. Cytochrome P450 Monooxygenases
3.3.5. Glutathione S-Transferases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen-Perkins, A.; Estrada, E. Mathematical modelling for sustainable aphid control in agriculture via intercropping. Proc. R. Soc. A 2019, 475, 20190136. [Google Scholar] [CrossRef] [Green Version]
- Züst, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 15206. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.; Eastop, V.F. Aphids on The World’s Trees—An Identification and Information Guide; John Wiley & Sons Ltd.: London, UK, 2000. [Google Scholar]
- Mossa, A.T.H.; Mohafrash, S.M.M.; Chandrasekaran, N. Safety of Natural Insecticides: Toxic Effects on Experimental Animals. Biomed. Res. Int. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, F. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Heckel, D.G. Insecticide Resistance After Silent Spring. Science 2012, 337, 1612–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubran, E.; Delorme, R.; Augé, D.; Moreau, J.-P. Insecticide resistance in Cotton Aphid Aphis gossypii (Glover) in the Sudan Gezira. Pestic. Sci. 1992, 35, 101–107. [Google Scholar] [CrossRef]
- Constantine, K.L.; Kansiime, M.K.; Mugambi, I.; Nunda, W.; Chacha, D.; Rware, H.; Makale, F.; Mulema, J.; Lamontagne-Godwin, J.; Williams, F.; et al. Why don’t smallholder farmers in Kenya use more biopesticides? Pest Manag. Sci. 2020, 76, 3615–3625. [Google Scholar] [CrossRef]
- Wilson, K.; Benton, T.G.; Graham, R.I.; Grzywacz, D. Pest Control: Biopesticides’ Potential. Science 2013, 342, 799. [Google Scholar] [CrossRef]
- Cheke, R.A. New pests for old as GMOs bring on substitute pests. Proc. Natl. Acad. Sci. USA 2018, 115, 8239–8240. [Google Scholar] [CrossRef] [Green Version]
- Marrone, P.G. Pesticidal natural products—Status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Keesey, I.W.; Jiang, N.; Weißflog, J.; Winz, R.; Svatoš, A.; Wang, C.-Z.; Hansson, B.S.; Knaden, M. Plant-Based Natural Product Chemistry for Integrated Pest Management of Drosophila suzukii. J. Chem. Ecol. 2019, 45, 626–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerda, H.; Carpio, C.; Ledezma-Carrizalez, A.C.; Sánchez, J.; Ramos, L.; Muñoz-Shugulí, C.; Andino, M.; Chiurato, M. Effects of Aqueous Extracts from Amazon Plants on Plutella xylostella (Lepidoptera: Plutellidae) and Brevicoryne brassicae (Homoptera: Aphididae) in Laboratory, Semifield, and field trials. J. Insect Sci. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R. Effects of Catechin in Culture and in Cotton Seedlings on the Growth and Polygalacturonase Activity of Rhizoctonia solani. Phytopathology 1978, 68. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, R.L.; Metcalf, R.A.; Rhodes, A.M. Cucurbitacins as kairomones for diabroticite beetles. Proc. Natl. Acad. Sci. USA 1980, 77, 3769–3772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, S.; Kaul, S.C.; Wadhwa, R. Cucurbitacin B and cancer intervention: Chemistry, biology and mechanisms (Review). Int. J. Oncol. 2018, 52, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, X.; Ho, C.-T.; Huang, Q. Chemistry and Health Effect of Tea Polyphenol (−)-Epigallocatechin 3-O-(3-O-Methyl)gallate. J. Agric. Food Chem. 2019, 67, 5374–5378. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.R.; Hoffman, C.A. Chemical Feeding Deterrent Mobilized in Response to Insect Herbivory and Counteradaptation by Epilachna tredecimnotata. Science 1980, 209, 414–416. [Google Scholar] [CrossRef]
- Da Costa, C.P.; Jones, C.M. Cucumber Beetle Resistance and Mite Susceptibility Controlled by the Bitter Gene in Cucumis sativus L. Science 1971, 172, 1145–1146. [Google Scholar] [CrossRef]
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins—An insight into medicinal leads from nature. Pharmacogn. Rev. 2015, 9, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Yousaf, H.K.; Shan, T.; Chen, X.; Ma, K.; Shi, X.; Desneux, N.; Biondi, A.; Gao, X. Impact of the secondary plant metabolite Cucurbitacin B on the demographical traits of the melon aphid, Aphis gossypii. Sci. Rep. 2018, 8, 16473. [Google Scholar] [CrossRef]
- Howell, C.R.; Bell, A.A.; Stipanovic, R.D. Effect of aging on flavonoid content and resistance of cotton leaves to Verticillium wilt. Physiol. Plant Pathol. 1976, 8, 181–188. [Google Scholar] [CrossRef]
- Shikano, I.; Rosa, C.; Tan, C.-W.; Felton, G.W. Tritrophic Interactions: Microbe-Mediated Plant Effects on Insect Herbivores. Annu. Rev. Phytopathol. 2017, 55, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Naasani, I.; Seimiya, H.; Tsuruo, T. Telomerase inhibition, telomere shortening, and senescence of cancer cells by tea catechins. Biochem. Biophys. Res. Commun. 1998, 249, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Cuzzolin, L.; Zaffani, S.; Benoni, G. Safety implications regarding use of phytomedicines. Eur. J. Clin. Pharmacol. 2006, 62, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Green, P.W.; Stevenson, P.C.; Simmonds, M.S.; Sharma, H.C. Phenolic compounds on the pod-surface of pigeonpea, Cajanus cajan, mediate feeding behavior of Helicoverpa armigera larvae. J. Chem. Ecol. 2003, 4, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, J.S.; Rider, D.S.; Walsh, T.K.; De Vos, M.; Gordon, K.H.; Ponnala, L.; Macmil, S.L.; Roe, B.A.; Jander, G. Comparative analysis of detoxification enzymes in Acyrthosiphon pisum and Myzus persicae. Insect Mol. Biol. 2010, 19 (Suppl. 2), 155–164. [Google Scholar] [CrossRef]
- Gong, Y.-H.; Yu, X.-R.; Shang, Q.-L.; Shi, X.-Y.; Gao, X.-W. Oral delivery mediated RNA interference of a carboxylesterase gene results in reduced resistance to organophosphorus insecticides in the cotton Aphid, Aphis gossypii Glover. PLoS ONE 2014, 9, e102823. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, A.M.; Moreira, A.C.; Lopes, B.R.; Fracola, M.F.; de Almeida, F.G.; Bueno, O.C.; Cass, Q.B.; Souza, D.H.F. Acetylcholinesterases from Leaf-Cutting ant Atta sexdens: Purification, Characterization, and Capillary Reactors for On-Flow Assays. Enzym. Res. 2019, 2019, 6139863. [Google Scholar] [CrossRef]
- Mostafiz, M.; Alam, M.; Chi, H.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Effects of Sublethal Doses of Methyl Benzoate on the Life History Traits and Acetylcholinesterase (AChE) Activity of Aphis gossypii. Agronomy 2020, 10, 1313. [Google Scholar] [CrossRef]
- Furk, C.; Hines, C.M. Aspects of insecticide resistance in the melon and cotton aphis, Aphis gossypii (Hemiptera: Aphididae). Ann. Appl. Biol. 1993, 123, 9–17. [Google Scholar] [CrossRef]
- Carletto, J.; Martin, T.; Vanlerberghe-Masutti, F.; Brévault, T. Insecticide resistance traits differ among and within host races in Aphis gossypii. Pest Manag. Sci. 2010, 66, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Lombaert, E.; Boll, R.; Lapchin, L. Dispersal strategies of phytophagous insects at a local scale: Adaptive potential of aphids in an agricultural environment. BMC Evol. Biol. 2006, 6, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walling, L.L. The Myriad Plant Responses to Herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef]
- Liu, X.D.; Zhai, B.P.; Zhang, X.X. Specialized host-plant performance of the cotton aphid is altered by experience. Ecol. Res. 2008, 23, 919–925. [Google Scholar] [CrossRef]
- Agarwala, B.K.; Choudhury, P.R. Host races of the cotton aphid, Aphis gossypii, in asexual populations from wild plants of Taro and Brinjal. J. Insect Sci. 2013, 13, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Liang, X.L.; Zhao, H.Y.; Xu, T.T.; Liu, X.D. Special plant species determines diet breadth of phytophagous insects: A study on host plant expansion of the host-specialized Aphis gossypii Glover. PLoS ONE 2013, 8, e60832. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Tang, C.; Ma, K.; Xia, J.; Song, D.; Gao, X.-W. Overexpression of UDP-glycosyltransferase potentially involved in insecticide resistance in Aphis gossypii Glover collected from Bt cotton fields in China. Pest Manag. Sci. 2019. [Google Scholar] [CrossRef]
- Ward, S.A.; Leather, S.; Pickup, J.; Harrington, R. Mortality during dispersal and the cost of host specificity in parasites: How many aphids find hosts? J. Anim. Ecol. 2001, 67, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, S.; Luo, J.Y.; Wang, C.Y.; Lv, L.M.; Zhu, X.Z.; Li, C.H.; Cui, J.J. Identification of Aphis gossypii Glover (Hemiptera: Aphididae) Biotypes from Different Host Plants in North China. PLoS ONE 2016, 11, e0146345. [Google Scholar] [CrossRef] [Green Version]
- Abe, M.; Matsuda, K. Feeding responses of four phytophagous lady beetle species (Coleoptera: Coccinellidae) to cucurbitacins and alkaloids. Appl. Entomol. Zool. 2000, 35, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Tallamy, D.; Stull, J.; Ehresman, N.; Gorski, P.; Mason, C. Cucurbitacins as Feeding and Oviposition Deterrents to Insects. Environ. Entomol. 1997, 26, 678–683. [Google Scholar] [CrossRef]
- Mostafiz, M.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Insecticidal efficacy of three benzoate derivatives against Aphis gossypii and its predator Chrysoperla carnea. Ecotoxicol. Environ. Saf. 2019, 184, 109653. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Qi, H.; Yang, D.; Yuan, H.; Rui, C. Cycloxaprid: A novel cis-nitromethylene neonicotinoid insecticide to control imidacloprid-resistant cotton aphid (Aphis gossypii). Pestic. Biochem. Physiol. 2016, 132, 96–101. [Google Scholar] [CrossRef] [PubMed]
- El-Nahhal, Y.; El-Dahdouh, N. Toxicity of Amoxicillin and Erythromycin to Fish and Mosquitoes. Ecotoxicol. Environ. Saf. 2015, 10, 13–21. [Google Scholar] [CrossRef]
- Chi, H. Life-Table Analysis Incorporating Both Sexes and Variable Development Rates Among Individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Mignani, S.; Rosa, R. The moving block bootstrap to assess the accuracy of statistical estimates in Ising model simulations. Comput. Phys. Commun. 1995, 92, 203–213. [Google Scholar] [CrossRef]
- Johnson, R.W. An Introduction to the Bootstrap. Teach. Stat. 2010, 23, 49–54. [Google Scholar] [CrossRef]
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): With additional comments on life table research criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef]
- Akkopru, E.P.; Atlihan, R.; Okut, H.; Chi, H. Demographic Assessment of Plant Cultivar Resistance to Insect Pests: A Case Study of the Dusky-Veined Walnut Aphid (Hemiptera: Callaphididae) on Five Walnut Cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef]
- Elfekih, S.; Chen, C.-Y.; Hsu, J.-C.; Belcaid, M.; Haymer, D. Identification and preliminary characterization of chemosensory perception-associated proteins in the melon fly Bactrocera cucurbitae using RNA-seq. Sci. Rep. 2016, 6, 19112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parimai, S.; Meinke, L.J.; Nowatzki, T.M.; Chandler, L.D.; French, B.W.; Siegfried, B.D. Toxicity of insecticide-bait mixtures to insecticide resistant and susceptible western corn rootworms (Coleoptera:: Chrysomelidae). Crop Prot. 2003, 22, 781–786. [Google Scholar] [CrossRef]
- Masler, E.; Rogers, S.; Chitwood, D. Developmental responses of heterodera glycines and meloidogyne incognita to fundamental environmental cues. J. Nematol. 2013, 45, 303. [Google Scholar]
- Lopez, T.E.; Pham, H.M.; Barbour, J.; Tran, P.; Van Nguyen, B.; Hogan, S.P.; Homo, R.L.; Coskun, V.; Schriner, S.E.; Jafari, M. The impact of green tea polyphenols on development and reproduction in Drosophila melanogaster. J. Funct. Foods 2016, 20, 556–566. [Google Scholar] [CrossRef] [Green Version]
- Chitwood, D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef] [Green Version]
- Ponsankar, A.; Sahayaraj, K.; Senthil-Nathan, S.; Vasantha-Srinivasan, P.; Karthi, S.; Thanigaivel, A.; Petchidurai, G.; Madasamy, M.; Hunter, W.B. Toxicity and developmental effect of cucurbitacin E from Citrullus colocynthis L. (Cucurbitales: Cucurbitaceae) against Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Environ. Sci. Pollut. Res. Int. 2019, 27, 23390–23401. [Google Scholar] [CrossRef]
- Mithofer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-D.; Xu, T.-T.; Lei, H.-X. Refuges and host shift pathways of host-specialized aphids Aphis gossypii. Sci. Rep. 2017, 7, 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanetti, R.; Zanuncio, J.; Santos, J.C.; Da Silva, W.L.P.; Ribeiro, G.; Tamara, G.; Lemes, P.G. An Overview of Integrated Management of Leaf-Cutting Ants (Hymenoptera: Formicidae) in Brazilian Forest Plantations. Forests 2014, 5, 439–455. [Google Scholar] [CrossRef] [Green Version]
- Hemingway, J.; Field, L.; Vontas, J. An Overview of Insecticide Resistance. Science 2002, 298, 96–97. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. Physiological and biochemical effect of neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front. Physiol. 2013, 4, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Rossiter, L.; Ortelli, F.; Jensen, B.; Wang, X.; Roth, C.W.; Collins, F.H.; Hemingway, J. Identification of a novel class of insect glutathione S-transferases involved in resistance to DDT in the malaria vector Anopheles gambiae. Biochem. J. 2001, 359, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ma, J.; Qin, X.; Tu, X.; Cao, G.; Wang, G.; Nong, X.; Zhang, Z. Biology, physiology and gene expression of grasshopper Oedaleus asiaticus exposed to diet stress from plant secondary compounds. Sci. Rep. 2017, 7, 8655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizutani, M.; Sato, F. Unusual P450 reactions in plant secondary metabolism. Arch. Biochem. Biophys. 2010, 507, 194–203. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Y.; Zeng, J.; Duan, L.; Xue, X.; Wang, H.; Lin, T.; Liu, Z.; Zeng, K.; Zhong, Y.; et al. Convergence and divergence of bitterness biosynthesis and regulation in Cucurbitaceae. Nat. Plants 2016, 2, 16183. [Google Scholar] [CrossRef] [Green Version]




| Aphid | Parameters | Cucurbitacin B (CucB) | Epigallocatechin Gallate (EGCG) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Solvent Control | 1 mg/L CucB | 5 mg/L CucB | Control | 1 mg/L EGCG | 5 mg/L EGCG | 10 mg/L EGCG | ||
| Cucurbit-specialized aphids | Pre-adult duration | 4.02 ± 0.02 a | 4.03 ± 0.03 a | 4.16 ± 0.05 b | 4.17 ± 0.0 b | 4.02 ± 0.02 a | 4.05 ± 0.10 ab | 4.10 ± 0.03 b | 4.20 ± 0.04 b |
| APRP | 0.67 ± 0.05 a | 0.76 ± 0.05 a | 0.94 ± 0.04 b | 0.89 ± 0.04 b | 0.67 ± 0.05 a | 0.71 ± 0.04 b | 0.72 ± 0.04 b | 0.74 ± 0.03 b | |
| Fecundity | 47.49 ± 1.33 a | 50.05 ± 1.62 a | 26.78 ± 1.80 b | 22.56 ± 1.40 c | 47.49 ± 1.33 a | 32.64 ± 1.92 b | 32.86 ± 1.52 b | 30.04 ± 1.45 b | |
| Longevity | 15.7 ± 0.59 a | 16.4 ± 0.58 a | 10.27 ± 0.35 c | 9.9 ± 0.37 c | 15.7 ± 0.59 a | 11.0 ± 0.41 b | 10.2 ± 0.30 b | 10.0 ± 0.32 b | |
| TPRP | 4.7 ± 0.05 a | 4.8 ± 0.05 b | 4.9 ± 0.04 c | 4.9 ± 0.04 c | 4.2 ± 0.05 a | 4.2 ± 0.05 a | 4.6 ± 0.04 b | 4.7 ± 0.05 b | |
| Oviposition day | 11.0 ± 0.45 a | 9.9 ± 0.39 a | 5.35 ± 0.25 b | 4.8 ± 0.31 b | 9.9 ± 0.39 a | 6.6 ± 0.36 b | 6.0 ± 0.28 b | 5.9 ± 0.30 b | |
| Cotton-specialized aphids | Pre-adult duration | 4.80 ± 0.04 a | 4.98 ± 0.01 b | 5.00 ± 0.01 b | 5.00 ± 0.02 b | 4.8 ± 0.04 a | 5.89 ± 0.03 c | 5.69 ± 0.05 b | 6.00 ± 0.01 d |
| APRP | 0.24 ± 0.05 a | 0.37 ± 0.05 ab | 0.34 ± 0.05 ab | 0.80 ± 0.04 b | 0.24 ± 0.05 a | 0.71 ± 0.05 c | 0.60 ± 0.07 b | 1.63 ± 0.12 d | |
| Fecundity | 37.64 ± 0.48 a | 39.43 ± 0.76 a | 36.80 ± 1.43 a | 29.87 ± 1.12 b | 37.64 ± 0.69 a | 18.10 ± 0.78 b | 14.42 ± 0.68 c | 15.26 ± 0.88 c | |
| Longevity | 20.4 ± 0.63 a | 20.1 ± 0.66 a | 16.5 ± 0.59 b | 14.7 ± 0.56 c | 20.4 ± 0.64 a | 14.5 ± 0.50 b | 14.9 ± 0.56 b | 15.7 ± 0.64 b | |
| TPRP | 5.0 ± 0.04 a | 5.8 ± 0.04 b | 5.3 ± 0.05 b | 5.4 ± 0.05 b | 5.0 ± 0.04 a | 6.6 ± 0.05 c | 6.3 ± 0.07 b | 7.6 ± 0.12 d | |
| Oviposition day | 13.6 ± 0.36 a | 12.8 ± 0.45 b | 10.4 ± 0.48 c | 8.5 ± 0.36 d | 13.6 ± 0.36 a | 8.4 ± 0.36 b | 7.6 ± 0.39 b | 9.3 ± 0.41 b | |
| Aphid | Parameters | CucB | EGCG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Solvent Control | 1 mg/L CucB | 5 mg/L CucB | Control | 1 mg/L EGCG | 5 mg/L EGCG | 10 mg/L EGCG | ||
| Cucurbit-specialized aphids | λ | 1.5659 ± 0.003 a | 1.5371 ± 0.003 b | 1.5110 ± 0.009 c | 1.4802 ± 0.009 d | 1.5659 ± 0.003 a | 1.5049 ± 0.009 b | 1.5885 ± 0.007 a | 1.5702 ± 0.006 a |
| R0 | 47.49 ± 1.33 a | 50.05 ± 1.62 a | 25.44 ± 1.26 c | 22.33 ± 1.40 c | 47.49 ± 1.33 a | 31.01 ± 1.96 b | 32.53 ± 1.54 b | 29.44 ± 1.49 b | |
| r | 0.4484 ± 0.002 a | 0.4299 ± 0.002 b | 0.4128 ± 0.005 c | 0.3921 ± 0.006 d | 0.4484 ± 0.002 a | 0.4087 ± 0.006 b | 0.4628 ± 0.004 a | 0.4512 ± 0.004 a | |
| T | 8.6 ± 0.07 b | 9.1 ± 0.08 a | 7.8 ± 0.08 c | 7.9 ± 0.08 c | 8.6 ± 0.07 a | 8.4 ± 0.10 a | 7.5 ± 0.07 b | 7.5 ± 0.10 b | |
| Cotton-specialized aphids | λ | 1.5032 ± 0.004 a | 1.4772 ± 0.003 b | 1.5063 ± 0.006 a | 1.4697 ± 0.007 b | 1.5032 ± 0.004 a | 1.2988 ± 0.006 b | 1.2925 ± 0.005 b | 1.23272 ± 0.006 c |
| R0 | 37.64 ± 0.69 a | 39.42 ± 0.87 a | 36.80 ± 1.42 a | 28.38 ± 1.24 b | 37.64 ± 0.69 a | 16.47 ± 0.87 b | 13.84 ± 0.71 c | 13.89 ± 0.91 c | |
| r | 0.4076 ± 0.003 a | 0.3901 ± 0.002 b | 0.4097 ± 0.005 a | 0.3851 ± 0.05 b | 0.4076 ± 0.003 a | 0.2614 ± 0.004 b | 0.2566 ± 0.004 b | 0.2092 ± 0.006 c | |
| T | 8.9 ± 0.07 b | 9.4 ± 0.06 a | 8.8 ± 0.07 bc | 8.7 ± 0.04 c | 8.9 ± 0.07 a | 10.7 ± 0.10 c | 10.2 ± 0.11 b | 12.6 ± 0.19 d | |
| Treated aphid | Parameters | CucB | EGCG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Solvent Control | 1 mg/L CucB | 5 mg/L CucB | Control | 1 mg/L EGCG | 5 mg/L EGCG | 10 mg/L EGCG | ||
| Cucurbit-specialized aphids transferred to cotton | Pre-adult duration | 5.00 ± 0.01 a | 5.94 ± 0.06 b | 6.02 ± 0.05 b | 6.05 ± 0.06 b | 5.00 ± 0.01 a | 6.01 ± 0.01 b | 6.03 ± 0.03 b | 6.00 ± 0.01 b |
| APRP | 2.58 ± 0.43 a | 2.31 ± 0.53 a | 3.23 ± 0.37 a | 4.00 ± 0.75 a | 2.58 ± 0.43 a | 1.42 ± 0.09 b | 0.38 ± 0.12 c | 1.25 ± 0.07 b | |
| TPOP | 7.6 ± 0.54 a | 9.2 ± 0.38 b | 8.2 ± 0.56 a | 10.0 ± 0.76 b | 7.9 ± 0.54 a | 6.4 ± 0.13 b | 6.3 ± 0.08 b | 6.4 ± 0.10 b | |
| Fecundity | 0.7 ± 0.20 a | 0.7 ± 0.20 a | 4.6 ± 0.84 b | 5.5 ± 1.13 b | 0.7 ± 0.20 a | 9.4 ± 0.84 c | 5.8 ± 0.71 b | 6.4 ± 0.64 b | |
| Longevity | 5.5 ± 0.44 a | 5.8 ± 0.41 a | 4.9 ± 0.45 a | 2.9 ± 0.20 b | 5.5 ± 0.44 a | 7.3 ± 0.76 b | 6.0 ± 0.47 ab | 7.1 ± 0.66 b | |
| Oviposition day | 1.8 ± 0.22 a | 1.5 ± 0.24 a | 2.4 ± 0.38 b | 4.0 ± 0.76 b | 1.8 ± 0.22 a | 6.9 ± 0.66 c | 4.1 ± 0.38 b | 5.1 ± 0.43 b | |
| Cotton- specialized aphids transferred to cucumber | Pre-adult duration | 4.76 ± 0.08 a | 4.92 ± 0.79 a | 4.13 ± 0.05 b | 4.26 ± 0.06 b | 4.92 ± 0.03 a | 6.12 ± 0.04 c | 6.00 ± 0.01 b | 6.37 ± 0.07 d |
| APRP | 0.75 ± 0.11 a | 0.62 ± 0.08 a | 0.48 ± 0.09 a | 0.50 ± 0.08 a | 0.75 ± 0.11 a | 1.47 ± 0.10 b | 0.92 ± 0.12 a | 1.33 ± 0.13 b | |
| TPOP | 5.7 ± 0.12 a | 5.4 ± 0.10 a | 4.7 ± 0.09 b | 4.2 ± 0.06 c | 5.7 ± 0.12 a | 7.5 ± 0.10 c | 7.0 ± 0.12 b | 7.7 ± 0.16 c | |
| Fecundity | 12.6 ± 1.13 a | 11.3 ± 0.91 ab | 9.3 ± 0.77 bc | 8.5 ± 0.77 c | 12.6 ± 1.13 a | 21.8 ± 1.24 b | 13.3 ± 1.15 a | 15.7 ± 1.29 a | |
| Longevity | 10.5 ± 0.69 a | 10.1 ± 0.69 ab | 8.6 ± 0.57 b | 6.0 ± 0.45 c | 10.5 ± 0.69 a | 15.3 ± 1.02 b | 12.2 ± 0.83 ab | 13.5 ± 0.88 b | |
| Oviposition day | 8.5 ± 0.64 a | 7.3 ± 0.55 ab | 6.4 ± 0.48 bc | 5.3 ± 0.5 c | 8.5 ± 0.64 a | 8.4 ± 0.62 a | 12.6 ± 0.69 b | 9.8 ± 0.67 a | |
| Treated aphid | Parameters | CucB | EGCG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Solvent Control | 1 mg/L CucB | 5 mg/L CucB | Control | 1 mg/L EGCG | 5 mg/L EGCG | 10 mg/L EGCG | ||
| Cucurbit-specialized aphids transferred to cotton | λ | 0.8664 ± 0.03 a | 0.8808 ± 0.003 a | 0.97218 ± 0.03 b | 0.8521 ± 0.03 a | 0.8664 ± 0.03 a | 1.1153 ± 0.02 b | 1.0845 ± 0.02 b | 1.0962 ± 0.02 b |
| R0 | 0.23 ± 0.07 a | 0.74 ± 0.21 b | 0.24 ± 0.08 a | 0.11 ± 0.07 b | 0.23 ± 0.07 a | 3.39 ± 0.54 b | 2.16 ± 0.38 b | 2.64 ± 0.41 b | |
| r | −0.1434 ± 0.04 a | −0.1269 ± 0.03 a | −0.0270 ± 0.02 b | −0.1601 ± 0.04 a | −0.1433 ± 0.04 a | 0.1091 ± 0.01 b | 0.0812 ± 0.02 b | 0.0918 ± 0.01 b | |
| T | 10.2 ± 0.63 a | 11.2 ± 0.43 a | 10.7 ± 1.07 b | 13.8 ± 0.17 b | 10.2 ± 0.63 ab | 11.2 ± 0.47 a | 9.5 ± 0.22 b | 10.6 ± 0.29 a | |
| Cotton- specialized aphids transferred to cucumber | λ | 1.2144 ± 0.01 a | 1.2207 ± 0.01 a | 1.2200 ± 0.01 a | 1.1708 ± 0.02 a | 1.2144 ± 0.013 a | 1.2103 ± 0.008 a | 1.1946 ± 0.010 a | 1.1966 ± 0.009 a |
| R0 | 8.18 ± 0.95 a | 7.81 ± 0.82 ab | 5.74 ± 0.65 b | 3.38 ± 0.51 b | 8.18 ± 0.95 a | 14.58 ± 1.32 c | 9.07 ± 1.00 ab | 11.48 ± 1.17 bc | |
| r | 0.1942 ± 0.011 a | 0.1994 ± 0.009 a | 0.1989 ± 0.012 a | 0.1577 ± 0.020 a | 0.1942 ± 0.011 a | 0.1908 ± 0.006 a | 0.1778 ± 0.008 a | 0.1795 ± 0.008 a | |
| T | 10.8 ± 0.19 a | 10.3 ± 0.28 a | 8.8 ± 0.21 b | 7.7 ± 0.19 c | 10.8 ± 0.19 a | 14.0 ± 0.20d | 12.4 ± 0.25 b | 13.6 ± 0.27 c | |
| Detoxifying enzyme | Aphid | CucB (p) | EGCG (p) | ||||
|---|---|---|---|---|---|---|---|
| Concentration | Duration | Interaction | Concentration | Duration | Interaction | ||
| AChE | CU | <0.0001 | 0.0884 | 0.0861 | <0.0001 | 0.0298 | 0.1113 |
| CO | 0.5504 | 0.0388 | 0.0044 | <0.0001 | 0.0035 | <0.0001 | |
| CarE | CU | <0.0001 | 0.2753 | 0.0133 | <0.0001 | <0.0001 | 0.5676 |
| CO | <0.0001 | 0.9971 | 0.0157 | <0.0001 | 0.0442 | 0.5438 | |
| ACP | CU | 0.2109 | 0.0227 | 0.9992 | 0.0913 | 0.0881 | 0.9895 |
| CO | <0.0001 | 0.0036 | 0.8920 | <0.0001 | <0.0001 | 0.3220 | |
| P450 | CU | <0.0001 | 0.5598 | 0.8144 | <0.0001 | 0.5028 | 0.8965 |
| CO | 0.7502 | 00.0282 | 0.0909 | 0.0124 | 0.0412 | 0.0890 | |
| GST | CU | 0.1188 | <0.0001 | 0.0871 | 0.1413 | 0.0055 | 0.6950 |
| CO | <0.0001 | 0.0019 | 0.2817 | <0.0001 | 0.0104 | 0.0002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Ma, C.; Luo, J.; Niu, L.; Hua, H.; Zhang, S.; Cui, J. Potential of Cucurbitacin B and Epigallocatechin Gallate as Biopesticides against Aphis gossypii. Insects 2021, 12, 32. https://doi.org/10.3390/insects12010032
Zhao C, Ma C, Luo J, Niu L, Hua H, Zhang S, Cui J. Potential of Cucurbitacin B and Epigallocatechin Gallate as Biopesticides against Aphis gossypii. Insects. 2021; 12(1):32. https://doi.org/10.3390/insects12010032
Chicago/Turabian StyleZhao, Chenchen, Chao Ma, Junyu Luo, Lin Niu, Hongxia Hua, Shuai Zhang, and Jinjie Cui. 2021. "Potential of Cucurbitacin B and Epigallocatechin Gallate as Biopesticides against Aphis gossypii" Insects 12, no. 1: 32. https://doi.org/10.3390/insects12010032
APA StyleZhao, C., Ma, C., Luo, J., Niu, L., Hua, H., Zhang, S., & Cui, J. (2021). Potential of Cucurbitacin B and Epigallocatechin Gallate as Biopesticides against Aphis gossypii. Insects, 12(1), 32. https://doi.org/10.3390/insects12010032