Augmenting Nesidiocoris tenuis (Nesidiocoris) with a Factitious Diet of Artemia Cysts to Control Bemisia tabaci (Gennadius) on Tomato Plants under Greenhouse Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Factitious Dietary, Banker Plants and Insect Rearing
2.2. Greenhouse Preparations and Experiment Settings
2.3. Experimental Treatment Settings
2.4. Sampling and Quantification of Test Subjects
2.5. Statistical Analyses
3. Results
3.1. Nesidiocoris tenuis Population Dynamics on Tomato Plants
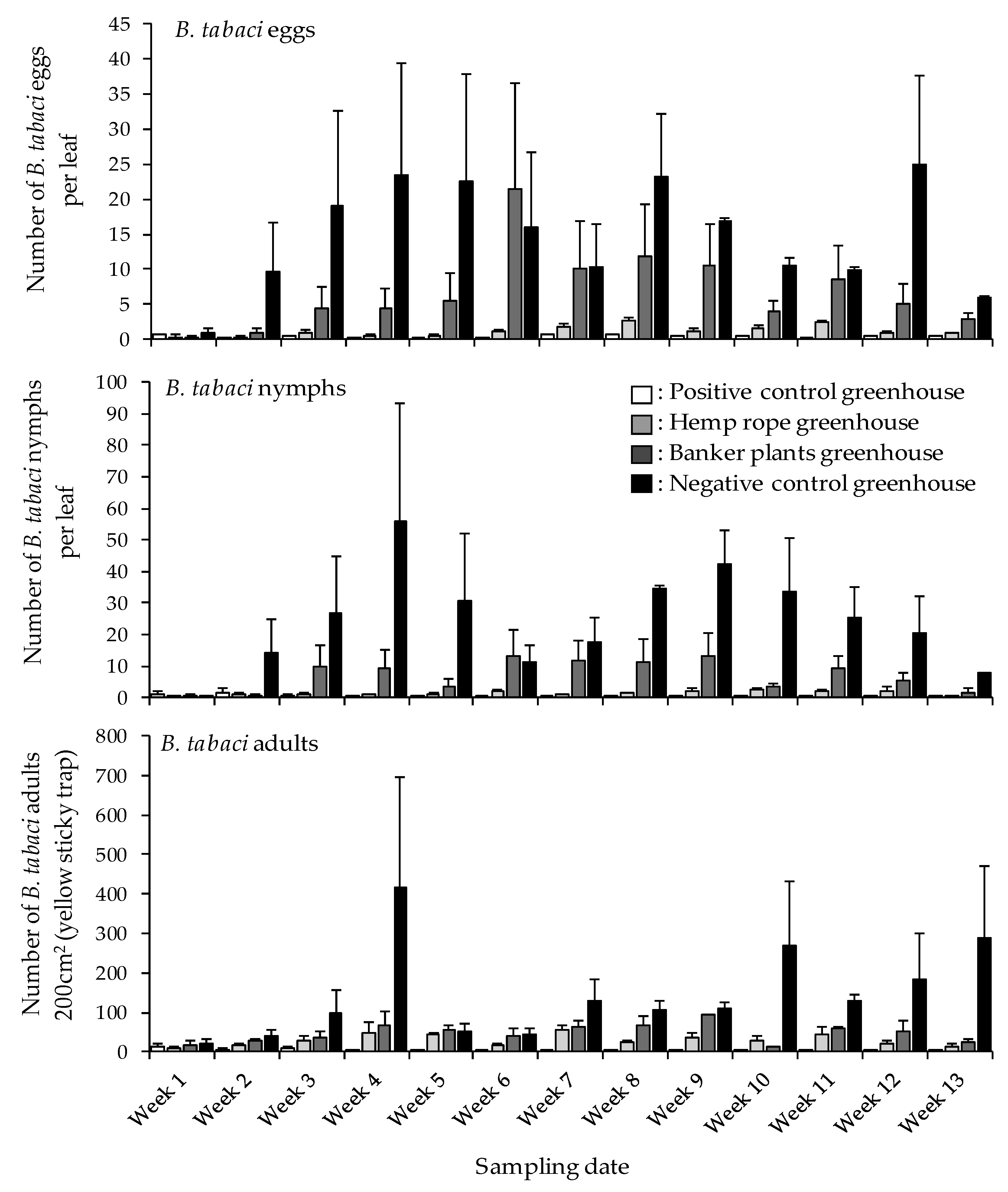
3.2. Impact of N. tenuis on B. tabaci Eggs, Nymphs, and Adults on Tomato Plants
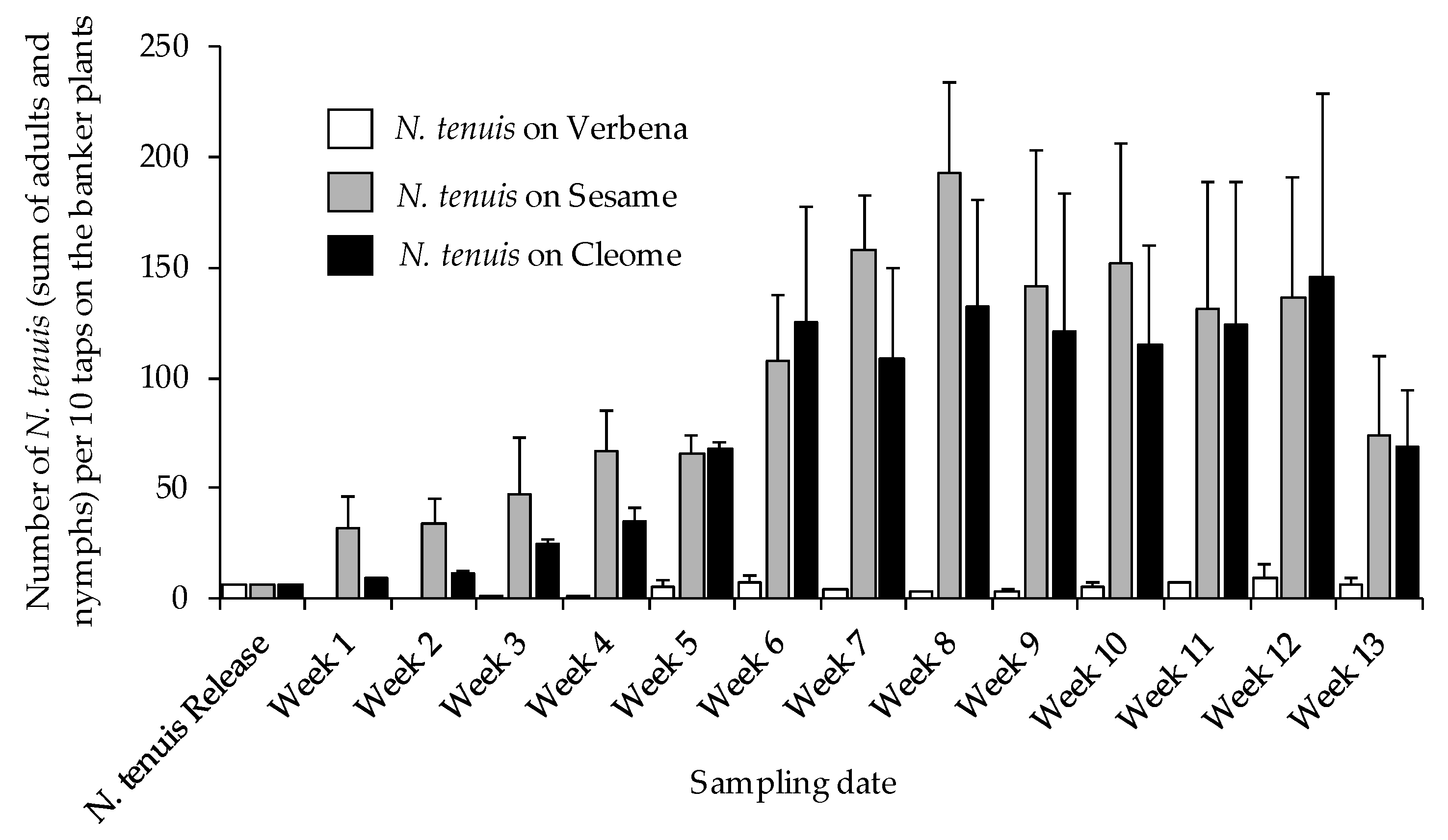
3.3. Nesidiocoris tenuis Population Trends on Banker Plants
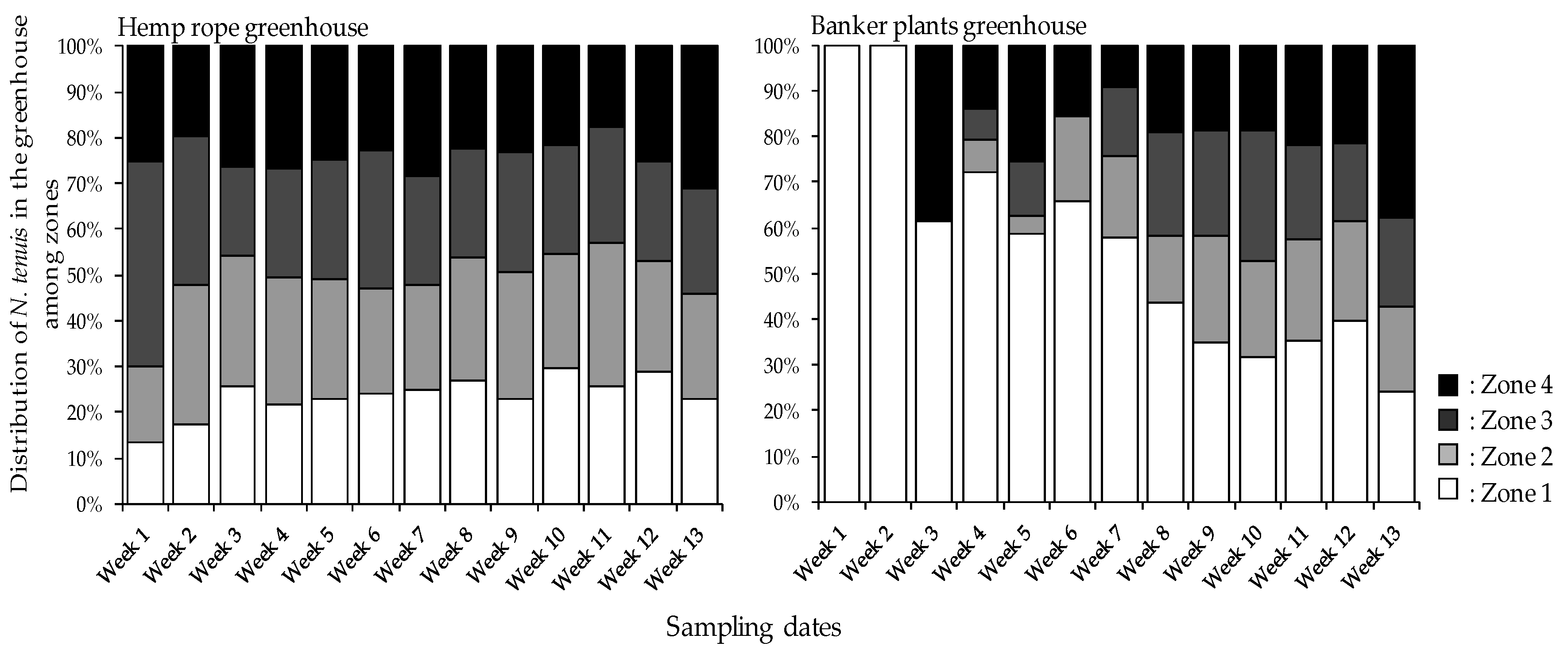
3.4. Nesidiocoris tenuis Distribution among Tomato Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Treatment x Zones | N. tenuis Adults | N. tenuis Nymphs | ||||
|---|---|---|---|---|---|---|
| χ2 | d.f. | p Value | χ2 | d.f. | p Value | |
| Week 1 | 111.50 | 1 | <0.001 | 162.50 | 1 | <0.001 |
| Week 2 | 117.28 | 1 | <0.001 | 148.89 | 1 | <0.001 |
| Week 3 | 50.51 | 1 | <0.001 | 60.85 | 1 | <0.001 |
| Week 4 | 38.05 | 1 | <0.001 | 73.48 | 1 | <0.001 |
| Week 5 | 31.51 | 1 | <0.001 | 60.34 | 1 | <0.001 |
| Week 6 | 43.41 | 1 | <0.001 | 81.52 | 1 | <0.001 |
| Week 7 | 11.12 | 1 | 0.011 | 64.73 | 1 | <0.001 |
| Week 8 | 2.61 | 1 | 0.456 | 18.65 | 1 | <0.001 |
| Week 9 | 6.23 | 1 | 0.101 | 5.26 | 1 | 0.154 |
| Week 10 | 1.91 | 1 | 0.592 | 2.19 | 1 | 0.534 |
| Week 11 | 4.01 | 1 | 0.261 | 8.74 | 1 | 0.033 |
| Week 12 | 1.11 | 1 | 0.775 | 5.97 | 1 | 0.113 |
| Week 13 | 3.45 | 1 | 0.370 | 1.61 | 1 | 0.657 |
| 2019 | 2020 | ||||||
|---|---|---|---|---|---|---|---|
| Application Week | Insecticide | IRAC Code | Application Rate * | Application Week | Insecticide | IRAC Code | Application Rate * |
| Week 1 | Spinetoram | 5 | 0.4 µL mL−1 | Week 1 | Spinetoram | 5 | 0.4 µL mL−1 |
| Week 2 | Abamectin | 6 | 1.0 µL mL−1 | Week 2 | Abamectin | 6 | 1.0 µL mL−1 |
| Week 3 | Pyrifluquinazon | 9B | 0.25 mg mL−1 | Week 3 | Pyrifluquinazon | 9B | 0.25 mg mL−1 |
| Week 4 | Dinotefuran | 4A | 0.5 mg mL−1 | Week 4 | Dinotefuran | 4A | 0.5 mg mL−1 |
| Week 5 | Cyantraniliprole | 28 | 0.5 µL mL−1 | Week 5 | Cyantraniliprole | 28 | 0.5 µL mL−1 |
| Week 6 | Spinetoram | 5 | 0.4 µL mL−1 | Week 6 | Spinetoram | 5 | 0.4 µL mL−1 |
| Week 7 | Milbemectin | 6 | 0.75 µL mL−1 | Week 7 | Pyrifluquinazon | 9B | 0.25 mg mL−1 |
| Week 8 | Pyrifluquinazon | 9B | 0.25 mg mL−1 | Week 8 | Milbemectin | 6 | 0.75 µL mL−1 |
| Week 9 | Spinetoram | 5 | 0.4 µL mL−1 | Week 9 | Cyantraniliprole | 28 | 0.5 µL mL−1 |
| Week 10 | Dinotefuran | 4A | 0.5 mg mL−1 | Week 10 | Dinotefuran | 4A | 0.5 mg mL−1 |
| Week 11 | Lepimectin | 6 | 0.5 µL mL−1 | Week 11 | Lepimectin | 6 | 0.5 µL mL−1 |
| Week 12 | Flonicamid | 29 | 0.5 mg mL−1 | Week 12 | Milbemectin | 6 | 0.75 µL mL−1 |
References
- Japan Government Statics (e-Stat). Available online: https://www.e-stat.go.jp/ (accessed on 24 August 2020).
- Jacas, J.A.; Urbaneja, A. Control Biológico de Plagas Agrícolas; Phytoma: Valencia, Spain, 2009. [Google Scholar]
- Nakaishi, K.; Fukui, Y.; Arakawa, R. Reproduction of Nesidiocoris tenuis (Reuter) on Sesame. Jpn. J. Appl. Entomol. Zool. 2011, 55, 199–205, (In Japanese with English abstract). [Google Scholar] [CrossRef]
- Nakano, R.; Tsuchida, Y.; Doi, M.; Ishikawa, R.; Tatara, A.; Amano, Y.; Muramatsu, Y. Control of Bemisia tabaci (Gennadius) on tomato in greenhouse by a combination of Nesidiocoris tenuis (Reuter) and banker plants. Ann. Rep. Kansai Plant Prot. 2016, 58, 65–72, (In Japanese with English abstract). [Google Scholar] [CrossRef]
- Uehara, T.; Ogino, T.; Nakano, A.; Tezuka, T.; Yamaguchi, T.; Kaino, Y.; Shimoda, M. Violet light is the most effective wavelength for recruiting the predatory bug Nesidiocoris tenuis. BioControl 2019, 64, 139–147. [Google Scholar] [CrossRef]
- Yano, E.; Nakauchi, M.; Watanabe, T.; Watanabe, H.; Hosaka, S.; Nishimori, S.; Miura, S.; Kandori, I.; Hinomoto, N. Life history traits of Nesidiocoris tenuis on Bemisia tabaci and Thrips palmi. BioControl 2020, 65, 155–164. [Google Scholar] [CrossRef]
- Owashi, Y.; Hayashi, M.; Abe, J.; Miura, K. Effects of an alternative diet of Artemia cysts on the development and reproduction of Nesidiocoris tenuis (Hemiptera: Miridae). Appl. Entomol. Zool. 2020, 55, 121–127. [Google Scholar] [CrossRef]
- Calvo, J.; Bolckmans, K.; Stansly, P.A.; Urbaneja, A. Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 2009, 54, 237–246. [Google Scholar] [CrossRef]
- NARO. New Manual for Tomato Pest Control System that Reduces the Use of Synthetic Insecticides. 2019. Available online: https://www.naro.affrc.go.jp/publicity_report/publication/files/SIPtomatomanual190404-2205s.pdf (accessed on 24 August 2020). (In Japanese)
- Bonte, M.; Samih, M.A.; De Clercq, P. Development and reproduction of Adalia bipunctata on factitious and artificial foods. BioControl 2010, 55, 485–491. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Vangansbeke, D.; De Clercq, P. Artificial and factitious foods support the development and reproduction of the predatory mite Amblyseius swirskii. Exp. Appl. Acarol. 2014, 62, 181–194. [Google Scholar] [CrossRef]
- Castañé, C.; Iriarte, J.; Lucas, E. Comparison of prey consumption by Dicyphus tamaninii reared conventionally, and on a meat-based diet. Biocontrol 2020, 47, 657–666. [Google Scholar] [CrossRef]
- Vangansbeke, D.; Nguyen, D.T.; Audenaert, J.; Verhoeven, R.; Gobin, B.; Tirry, L.; De Clercq, P. Performance of the predatory mite Amblydromalus limonicus on factitious foods. BioControl 2014, 59, 67–77. [Google Scholar] [CrossRef]
- Hongo, T.; Obayashi, N. Use of diapause eggs of brine shrimp, Artemia salina (Linnaeus), for artificial diet of coccinellid beetle, Harmonia axyridis (Pallas). Jpn. J. Appl. Entomol. Zool. 1997, 41, 101–105. (In Japanese) [Google Scholar] [CrossRef]
- Seko, T.; Abe, J.; Miura, K. Effect of supplementary food containing Artemia salina on the development and survival of flightless Harmonia axyridis in greenhouses. BioControl 2019, 64, 333–341. [Google Scholar] [CrossRef]
- Arijs, Y.; De Clercq, P. Rearing Orius laevigatus on cysts of the brine shrimp Artemia franciscana. Biol. Control 2001, 21, 79–83. [Google Scholar] [CrossRef]
- Nishimori, T.; Miura, K.; Seko, T. Rearing Orius strigicollis (Hemiptera: Anthocoridae) on an alternative diet of brine shrimp, Artemia salina (Anostraca: Artemiidae). Appl. Entomol. Zool. 2016, 51, 321–325. [Google Scholar] [CrossRef]
- Wari, D.; Okada, R.; Takagi, M.; Yaguchi, M.; Kashima, T.; Ogawara, T. Augmentation and compatibility of Beauveria bassiana with pesticides against different growth stages of Bemisia tabaci (Gennadius); an in vitro and field approach. Pest. Manag. Sci. 2020, 76, 3236–3252. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Urbaneja, A. The zoophytophagous predator Nesidiocoris tenuis: A successful but controversial biocontrol agent in tomato crops. In Advances in Insect Control and Resistance Management; Horowitz, A.R., Ishaaya, I., Eds.; Springer: Cham, Switzerland, 2016; pp. 9–26. [Google Scholar] [CrossRef]
- Urbaneja-Bernat, P.; Alonso, M.; Tena, A.; Bolckmans, K.; Urbaneja, A. Sugar as nutritional supplement for the zoophytophagous predator Nesidiocoris tenuis. BioControl 2013, 58, 57–64. [Google Scholar] [CrossRef]
- Gillespie, D.R.; McGregor, R.R. The functions of plant feeding in the omnivorous predator Dicyphus hesperus: Water places limits on predation. Ecol. Entomol. 2000, 25, 380–386. [Google Scholar] [CrossRef]
- Arno, J.; Castane, C.; Riudavets, J.; Roig, J.; Gabarra, R. Characterization of damage to tomato plants produced by the zoophytophagous predator Nesidiocoris tenuis. IOBC WPRS Bull. 2006, 29, 249–254. [Google Scholar]
- Messelink, G.J.; Bloemhard, C.M.J.; Hoogerbrugge, H.; van Schelt, J.; Ingegno, B.L.; Tavella, L. Evaluation of mirid predatory bugs and release strategy for aphid control in sweet pepper. J. Appl. Entomol. 2015, 139, 333–341. [Google Scholar] [CrossRef]
- Oveja, M.F.; Riudavets, J.; Arnó, J.; Gabarra, R. Does a supplemental food improve the effectiveness of predatory bugs on cucumber? Biocontrol 2016, 61, 47–56. [Google Scholar] [CrossRef]
- Leman, A.; Messelink, G.J. Supplemental food that supports both predator and pest: A risk for biological control? Exp. Appl Acarol 2015, 65, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Vangansbeke, D.; Nguyen, D.T.; Audenaert, J.; Verhoeven, R.; Gobin, B.; Tirrya, L.; De Clercq, P. Supplemental food for Amblyseius swirskii in the control of thrips: Feeding friend or foe? Pest. Manag. Sci. 2016, 72, 466–473. [Google Scholar] [CrossRef] [PubMed]

| N. tenuis Trends on Tomato Plants | F | d.f. | p |
|---|---|---|---|
| Treatments | 7.78 | 2, 49 | 0.0012 |
| Sampling interval | 4.65 | 12, 39 | 0.0001 |
| Treatment x sampling interval | 8.65 | 25, 26 | 0.0002 |
| Week 1 | 1.11 | 24, 27 | 0.0516 |
| Week 2 | 1.34 | 24, 27 | 0.0505 |
| Week 3 | 9.16 | 24, 27 | <0.0001 |
| Week 4 | 9.13 | 24, 27 | <0.0001 |
| Week 5 | 8.39 | 24, 27 | <0.0001 |
| Week 6 | 6.47 | 24, 27 | <0.0001 |
| Week 7 | 6.34 | 24, 27 | <0.0001 |
| Week 8 | 1.50 | 24, 27 | 0.0513 |
| Week 9 | 1.15 | 24, 27 | 0.0523 |
| Week 10 | 4.79 | 24, 27 | <0.0001 |
| Week 11 | 5.71 | 24, 27 | <0.0001 |
| Week 12 | 6.65 | 24, 27 | <0.0001 |
| Week 13 | 9.22 | 24, 27 | <0.0001 |
| Experiment | Source | Response Variable | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. tabaci Eggs | B. tabaci Nymphs | B. tabaci Adults | ||||||||
| d.f. | F | p | d.f. | F | p | d.f. | F | p | ||
| Hemp rope vs. Banker plants: Tomato + N. tenuis + B. tabaci | Treatment | 1 | 0.95 | 0.508 | 1 | 1.12 | 0.482 | 1 | 2.04 | 0.389 |
| Sampling intervals | 12 | 1.07 | 0.453 | 12 | 1.18 | 0.390 | 12 | 0.92 | 0.557 | |
| Treatment x Sampling interval | 12 | 0.94 | 0.541 | 12 | 1.28 | 0.339 | 12 | 0.93 | 0.546 | |
| Hemp rope vs. Positive Control: Tomato + B. tabaci | Treatment | 1 | 13.93 | 0.167 | 1 | 3.26 | 0.322 | 1 | 36.59 | 0.104 |
| Sampling intervals | 12 | 1.20 | 0.376 | 12 | 0.22 | 0.992 | 12 | 0.45 | 0.911 | |
| Treatment x Sampling interval | 12 | 1.84 | 0.153 | 12 | 0.97 | 0.524 | 12 | 0.88 | 0.585 | |
| Hemp rope vs. Negative Control: Tomato + B. tabaci | Treatment | 1 | 3.99 | 0.298 | 1 | 31.96 | <0.001 | 1 | 57,465.69 | 0.003 |
| Sampling intervals | 12 | 0.42 | 0.928 | 12 | 0.41 | 0.537 | 12 | 0.47 | 0.899 | |
| Treatment x Sampling interval | 12 | 0.46 | 0.905 | 12 | 0.49 | 0.499 | 12 | 0.56 | 0.840 | |
| Treatment x Zones | χ2 | d.f. | p Value |
|---|---|---|---|
| Week 1 | 142.61 | 1 | <0.001 |
| Week 2 | 131.58 | 1 | <0.001 |
| Week 3 | 58.67 | 1 | <0.001 |
| Week 4 | 52.64 | 1 | <0.001 |
| Week 5 | 37.09 | 1 | <0.001 |
| Week 6 | 48.79 | 1 | <0.001 |
| Week 7 | 25.56 | 1 | <0.001 |
| Week 8 | 7.39 | 1 | 0.061 |
| Week 9 | 3.35 | 1 | 0.341 |
| Week 10 | 1.11 | 1 | 0.776 |
| Week 11 | 3.60 | 1 | 0.306 |
| Week 12 | 2.54 | 1 | 0.468 |
| Week 13 | 1.49 | 1 | 0.684 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, T.; Takagi, M.; Tezuka, T.; Ogawara, T.; Wari, D. Augmenting Nesidiocoris tenuis (Nesidiocoris) with a Factitious Diet of Artemia Cysts to Control Bemisia tabaci (Gennadius) on Tomato Plants under Greenhouse Conditions. Insects 2021, 12, 265. https://doi.org/10.3390/insects12030265
Saito T, Takagi M, Tezuka T, Ogawara T, Wari D. Augmenting Nesidiocoris tenuis (Nesidiocoris) with a Factitious Diet of Artemia Cysts to Control Bemisia tabaci (Gennadius) on Tomato Plants under Greenhouse Conditions. Insects. 2021; 12(3):265. https://doi.org/10.3390/insects12030265
Chicago/Turabian StyleSaito, Takeshi, Motonori Takagi, Toshiyuki Tezuka, Takashi Ogawara, and David Wari. 2021. "Augmenting Nesidiocoris tenuis (Nesidiocoris) with a Factitious Diet of Artemia Cysts to Control Bemisia tabaci (Gennadius) on Tomato Plants under Greenhouse Conditions" Insects 12, no. 3: 265. https://doi.org/10.3390/insects12030265
APA StyleSaito, T., Takagi, M., Tezuka, T., Ogawara, T., & Wari, D. (2021). Augmenting Nesidiocoris tenuis (Nesidiocoris) with a Factitious Diet of Artemia Cysts to Control Bemisia tabaci (Gennadius) on Tomato Plants under Greenhouse Conditions. Insects, 12(3), 265. https://doi.org/10.3390/insects12030265







