Developmental Temperature Affects Life-History Traits and Heat Tolerance in the Aphid Parasitoid Aphidius colemani
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material and Acclimation Treatment
2.2. Water and Lipid Contents
2.3. Life-History Traits
2.4. Critical Thermal Limits
2.5. Statistical Analyses
3. Results
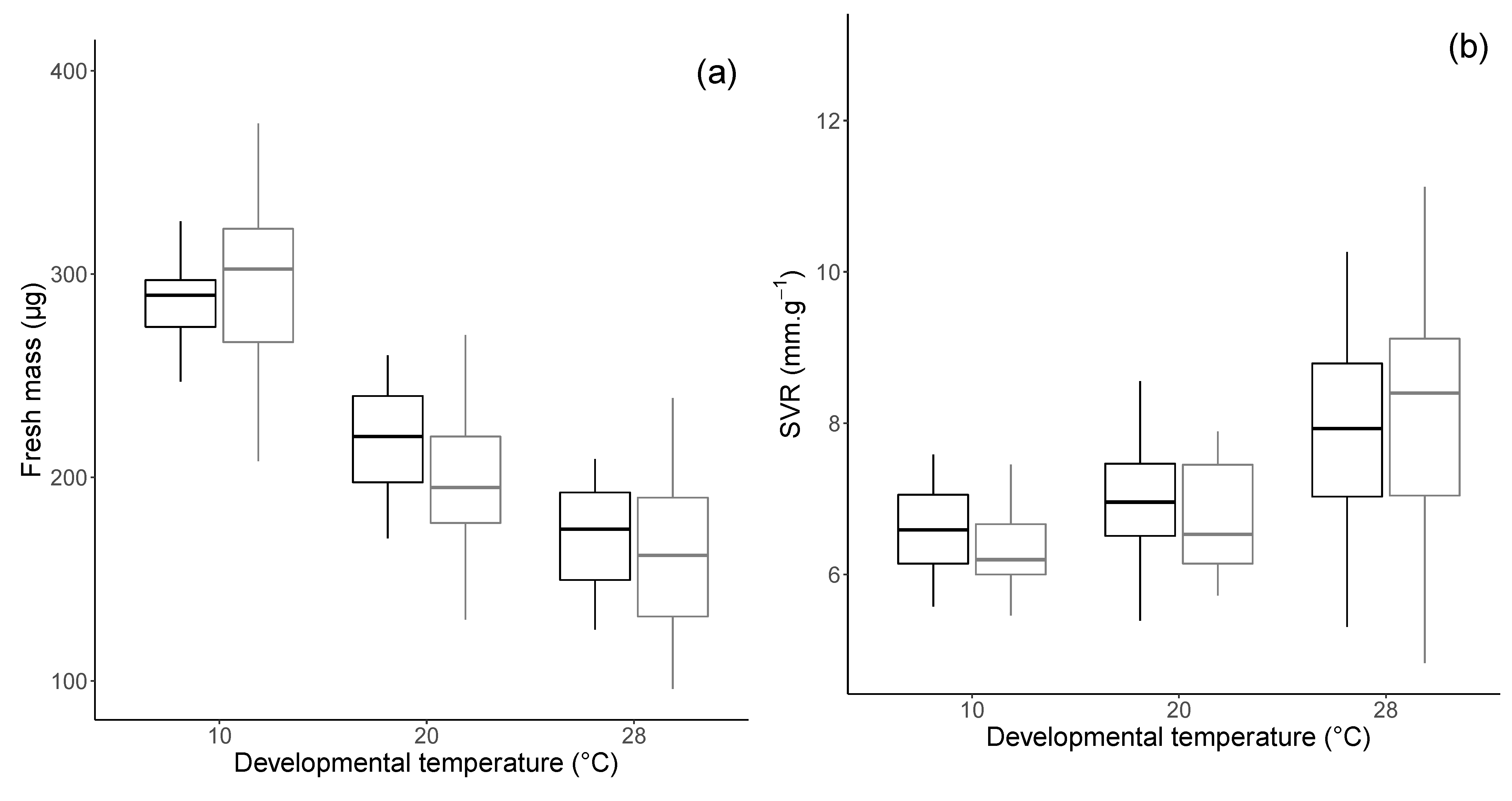
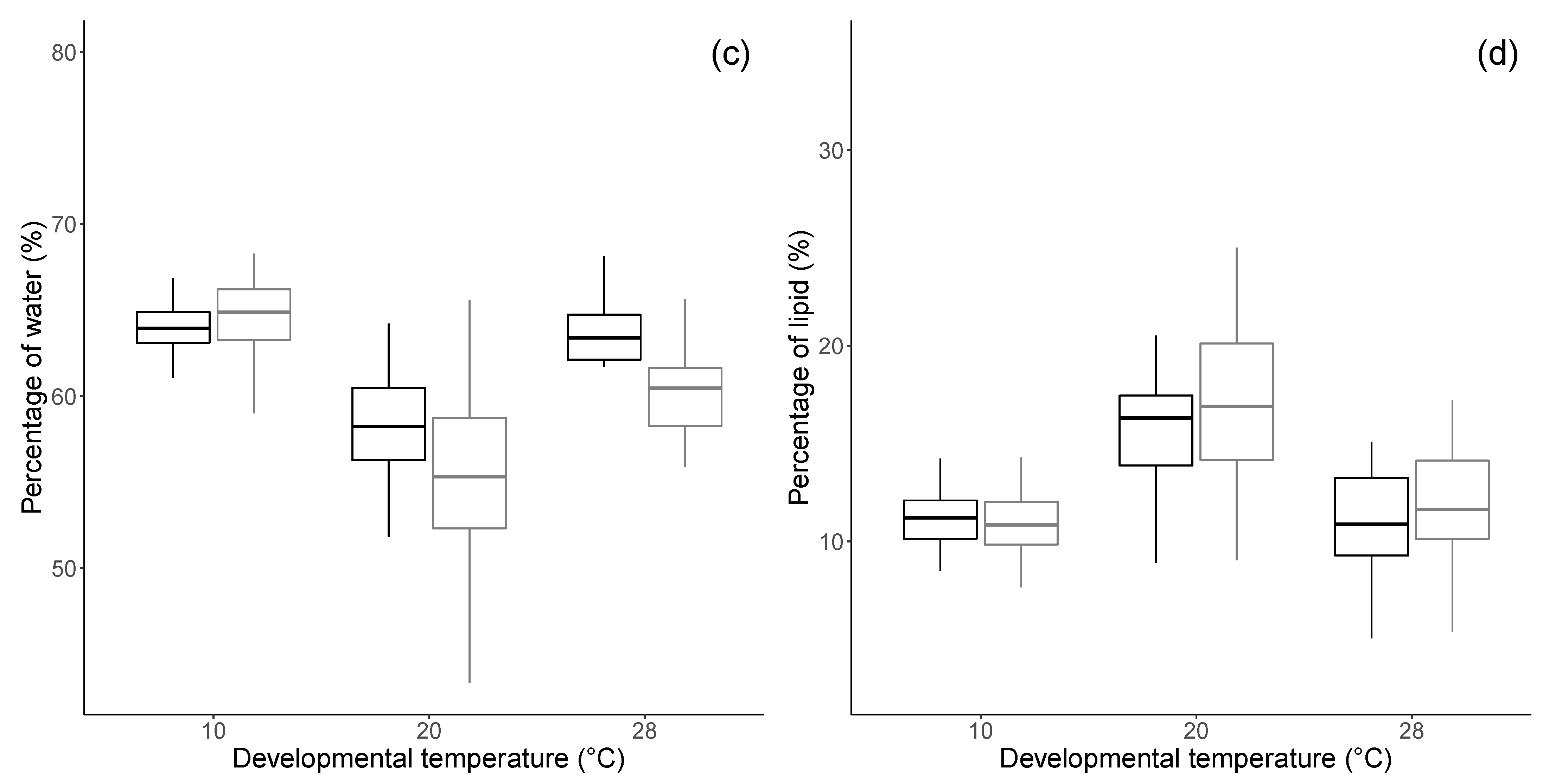
3.1. Morphology and Physiology
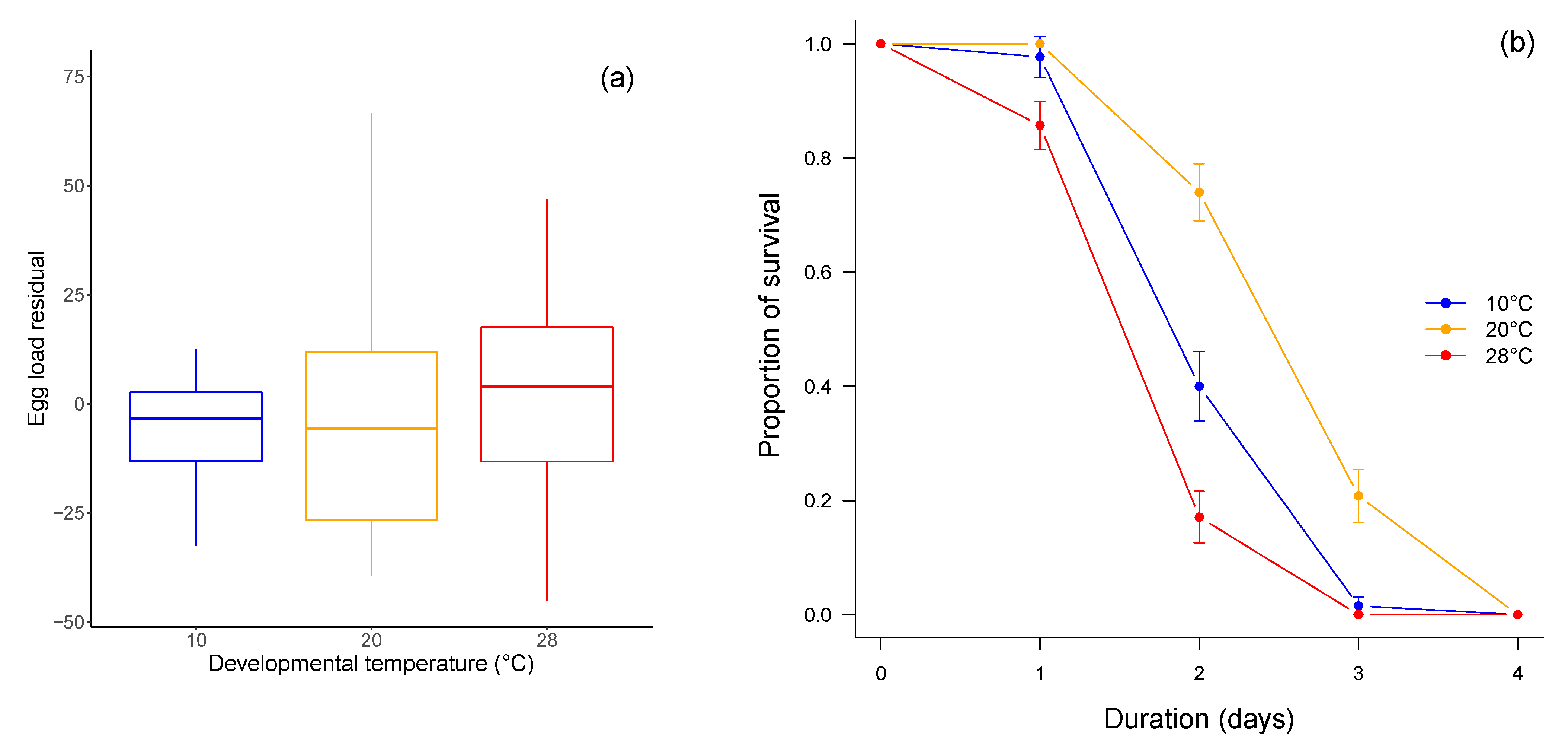
3.2. Fitness-Related Traits
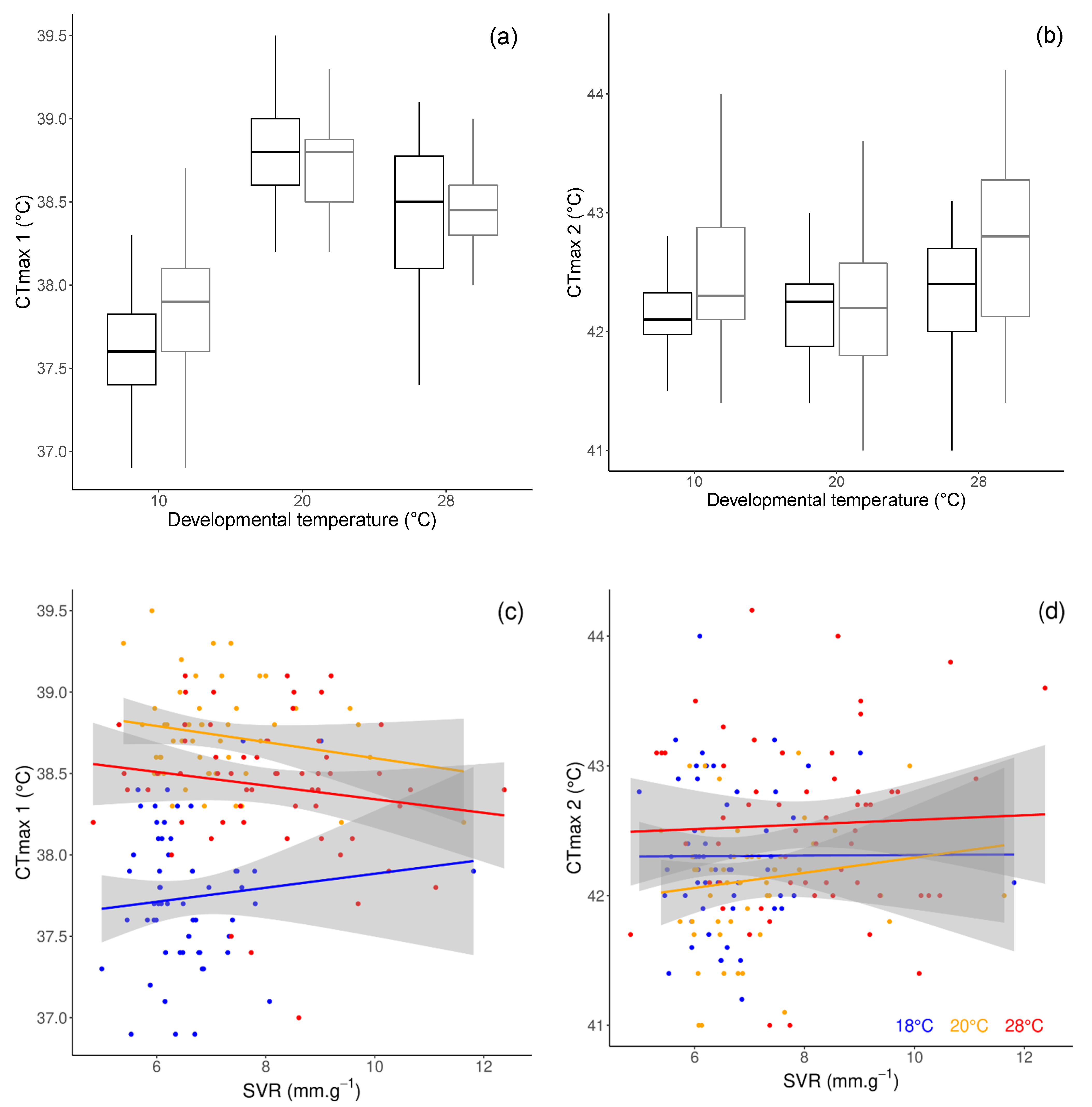
3.3. Critical Thermal Limits
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chown, S.L.; Terblanche, J.S. Physiological Diversity in Insects: Ecological and Evolutionary Contexts. Adv. Insect Physiol. 2006, 33, 50–152. [Google Scholar]
- Irlich, U.M.; Terblanche, J.S.; Blackburn, T.M.; Chown, S.L. Insect rate-temperature relationships: Environmental variation and the metabolic theory of ecology. Am. Nat. 2009, 174, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Abram, P.K.; Boivin, G.; Moiroux, J.; Brodeur, J. Behavioural effects of temperature on ectothermic animals: Unifying thermal physiology and behavioural plasticity. Biol. Rev. 2017, 92, 1859–1876. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Huey, R.B.; Kingsolver, J.G. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol. Evol. 1989, 4, 131–135. [Google Scholar] [CrossRef]
- Milosavljević, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effects of Constant and Fluctuating Temperatures on Development Rates and Longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.A.; Hoddle, M.S. The Influence of Temperature Variation on Life History Parameters and Thermal Performance Curves of Tamarixia radiata (Hymenoptera: Eulophidae), a Parasitoid of the Asian Citrus Psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef]
- Angilletta, M.J.; Dunham, A.E. The Temperature-Size Rule in Ectotherms: Simple Evolutionary Explanations May Not Be General. Am. Nat. 2003, 162, 332–342. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, D. Temperature and Organism Size-A Law for Ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar]
- Van der Have, T.M.; De Jong, G. Adult size in ectotherms: Temperature effects on growth and differentiation. J. Theor. Biol. 1996, 183, 329–340. [Google Scholar] [CrossRef]
- Colinet, H.; Boivin, G.; Hance, T. Manipulation of parasitoid size using the temperature-size rule: Fitness consequences. Oecologia 2007, 152, 425–433. [Google Scholar] [CrossRef]
- Bale, J.S. Insects and low temperatures: From molecular biology to distributions and abundance. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2002, 357, 849–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terblanche, J.S.; Deere, J.A.; Clusella-Trullas, S.; Janion, C.; Chown, S.L. Critical thermal limits depend on methodological context. Proc. R. Soc. B Biol. Sci. 2007, 274, 2935–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, S.J.; Bale, J.S. Effect of long-term and rapid cold hardening on the cold torpor temperature of an aphid. Physiol. Entomol. 2006, 31, 348–352. [Google Scholar] [CrossRef]
- Nylin, S.; Gotthard, K. Plasticity in Life-History Traits. Annu. Rev. Entomol. 1998, 43, 63–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laughton, A.M.; O’Connor, C.O.; Knell, R.J. Responses to a warming world: Integrating life history, immune investment, and pathogen resistance in a model insect species. Ecol. Evol. 2017, 7, 9699–9710. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D. Ectotherm Life-History Responses to Developmental Temperature. In Animals and Temperature: Phenotypic and Evolutionary Adaptation; Cambridge University Press: Cambridge, UK, 1996; pp. 183–204. [Google Scholar]
- Huey, R.B.; Stevenson, R. Integrating Thermal Physiology and Ecology of Ectotherms: A Discussion of Approaches Department. Am. Zool. 1979, 19, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Sgrò, C.M.; Terblanche, J.S.; Hoffmann, A.A. What Can Plasticity Contribute to Insect Responses to Climate Change? Annu. Rev. Entomol. 2016, 61, 433–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohr, J.R.; Civitello, D.J.; Cohen, J.M.; Roznik, E.A.; Sinervo, B.; Dell, A.I. The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. 2018, 21, 1425–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, R.S.; Franklin, C.E. Testing the beneficial acclimation hypothesis. Trends Ecol. Evol. 2002, 17, 66–70. [Google Scholar] [CrossRef]
- Tougeron, K.; Devogel, M.; van Baaren, J.; Le Lann, C.; Hance, T. Trans-generational effects on diapause and life-history-traits of an aphid parasitoid. J. Insect Physiol. 2020, 121, 104001. [Google Scholar] [CrossRef]
- Alford, L.; Blackburn, T.M.; Bale, J.S. Effects of acclimation and latitude on the activity thresholds of the aphid Myzus persicae in Europe. J. Appl. Entomol. 2012, 136, 332–346. [Google Scholar] [CrossRef]
- Chown, S.L.; Jumbam, K.R.; Sørensen, J.G.; Terblanche, J.S. Phenotypic variance, plasticity and heritability estimates of critical thermal limits depend on methodological context. Funct. Ecol. 2009, 23, 133–140. [Google Scholar] [CrossRef]
- Heerwaarden, B.; Kellermann, V.; Sgrò, C.M. Limited scope for plasticity to increase upper thermal limits. Funct. Ecol. 2016, 30, 1947–1956. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezende, E.L.; Tejedo, M.; Santos, M. Estimating the adaptive potential of critical thermal limits: Methodological problems and evolutionary implications. Funct. Ecol. 2011, 25, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Kingsolver, J.G.; Arthur Woods, H.; Buckley, L.B.; Potter, K.A.; MacLean, H.J.; Higgins, J.K. Complex Life Cycles and the Responses of Insects to Climate Change. Integr. Comp. Biol. 2011, 51, 719–732. [Google Scholar] [CrossRef] [Green Version]
- Bowler, K.; Terblanche, J.S. Insect thermal tolerance: What is the role of ontogeny, ageing and senescence? Biol. Rev. 2008, 83, 339–355. [Google Scholar] [CrossRef]
- Pincebourde, S.; Casas, J. Warming tolerance across insect ontogeny: Influence of joint shifts in microclimates and thermal limits. Ecology 2015, 96, 986–997. [Google Scholar] [CrossRef]
- Santos, M.A.; Carromeu-Santos, A.; Quina, A.S.; Santos, M.; Matos, M.; Simões, P. High developmental temperature leads to low reproduction despite adult temperature. J. Therm. Biol. 2021, 95, 102794. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Buckley, L.B. Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160147. [Google Scholar] [CrossRef]
- Buckley, L.B.; Huey, R.B. Temperature extremes: Geographic patterns, recent changes, and implications for organismal vulnerabilities. Glob. Chang. Biol. 2016, 22, 3829–3842. [Google Scholar] [CrossRef] [PubMed]
- Woods, H.A.; Harrison, J.F. Interpreting rejections of the beneficial hypothesis: When is physiological plasticity adaptive? Evol. N. Y. 2002, 56, 1863–1866. [Google Scholar] [CrossRef]
- Terblanche, J.S.; Hoffmann, A.A. Validating measurements of acclimation for climate change adaptation. Curr. Opin. Insect Sci. 2020, 41, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hance, T.; van Baaren, J.; Vernon, P.; Boivin, G. Impact of extreme temperatures on parasitoids in a climate change perspective. Annu. Rev. Entomol. 2007, 52, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Boivin, G. Insect parasitoids cold storage: A comprehensive review of factors of variability and consequences. Biol. Control. 2011, 58, 83–95. [Google Scholar] [CrossRef]
- Buckley, L.B.; Urban, M.C.; Angilletta, M.; Crozier, L.G.; Rissler, L.J.; Sears, M.W. Can mechanism inform species’ distribution models? Ecol. Lett. 2010, 13, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, Y.K.; Beldade, P. Thermal Plasticity in Insects’ Response to Climate Change and to Multifactorial Environments. Front. Ecol. Evol. 2020, 8, 271. [Google Scholar] [CrossRef]
- Le Lann, C.; Visser, B.; Mériaux, M.; Moiroux, J.; van Baaren, J.; van Alphen, J.J.M.; Ellers, J. Rising temperature reduces divergence in resource use strategies in coexisting parasitoid species. Oecologia 2014, 174, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Foray, V.; Desouhant, E.; Voituron, Y.; Larvor, V.; Renault, D.; Colinet, H.; Gibert, P. Does cold tolerance plasticity correlate with the thermal environment and metabolic profiles of a parasitoid wasp? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Le Lann, C.; Roux, O.; Serain, N.; Van Alphen, J.J.M.; Vernon, P.; van Baaren, J. Thermal tolerance of sympatric hymenopteran parasitoid species: Does it match seasonal activity? Physiol. Entomol. 2011, 36, 21–28. [Google Scholar] [CrossRef]
- Le Lann, C.; Wardziak, T.; van Baaren, J.; van Alphen, J.J.M. Thermal plasticity of metabolic rates linked to life-history traits and foraging behaviour in a parasitic wasp. Funct. Ecol. 2011, 25, 641–651. [Google Scholar] [CrossRef]
- Moore, M.E.; Kester, K.M.; Kingsolver, J.G. Rearing temperature and parasitoid load determine host and parasitoid performance in Manduca sexta and Cotesia congregata. Ecol. Entomol. 2020, 45, 79–89. [Google Scholar] [CrossRef]
- Langer, A.; Boivin, G.; Hance, T. Oviposition, flight and walking capacity at low temperatures of four aphid parasitoid species (Hymenoptera: Aphidiinae). Eur. J. Entomol. 2004, 101, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Renoz, F.; Foray, V.; Ambroise, J.; Baa-Puyoulet, P.; Bearzatto, B.; Mendez, G.L.; Grigorescu, A.S.; Mahillon, J.; Mardulyn, P.; Gala, J.; et al. At the Gate of Mutualism: Identification of Genomic Traits Predisposing to Insect-Bacterial Symbiosis in Pathogenic Strains of the Aphid Symbiont Serratia symbiotica. Front. Cell. Infect. Microbiol. 2021, 11, 558. [Google Scholar] [CrossRef]
- Benelli, G.; Messing, R.H.; Wright, M.G.; Giunti, G.; Kavallieratos, N.G.; Canale, A. Cues triggering mating and host-seeking behavior in the aphid parasitoid Aphidius colemani (Hymenoptera: Braconidae: Aphidiinae): Implications for biological control. J. Econ. Entomol. 2014, 107, 2005–2022. [Google Scholar] [CrossRef]
- Colinet, H.; Hance, T.; Vernon, P. Water Relations, Fat Reserves, Survival, and Longevity of a Cold-exposed Parasitic Wasp Aphidius colemani (Hymenoptera: Aphidiinae). Environ. Entomol. 2006, 35, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Langhof, M.; Meyhöfer, R.; Poehling, H.M.; Gathmann, A. Measuring the field dispersal of Aphidius colemani (Hymenoptera: Braconidae). Agric. Ecosyst. Environ. 2005, 107, 137–143. [Google Scholar] [CrossRef]
- Zamani, A.A.; Talebi, A.; Fathipour, Y.; Baniameri, V. Effect of temperature on life history of Aphidius colemani and Aphidius matricariae (Hymenoptera: Braconidae), two parasitoids of Aphis gossypii and Myzus persicae (Homoptera: Aphididae). Environ. Entomol. 2007, 36, 263–271. [Google Scholar] [CrossRef]
- Colinet, H.; Vernon, P.; Hance, T. Does thermal-related plasticity in size and fat reserves influence supercooling abilities and cold-tolerance in Aphidius colemani (Hymenoptera: Aphidiinae) mummies? J. Therm. Biol. 2007, 32, 374–382. [Google Scholar] [CrossRef]
- Jerbi-Elayed, M.; Lebdi-Grissa, K.; Le Goff, G.; Hance, T. Influence of Temperature on Flight, Walking and Oviposition Capacities of two Aphid Parasitoid Species (Hymenoptera: Aphidiinae). J. Insect Behav. 2015, 28, 157–166. [Google Scholar] [CrossRef]
- Starý, P. Taxonomy, origin, distribution and host range of Aphidius species (Hym., Aphidiidae) in relation to biological control of the pea aphid in Europe and North America. J. Appl. Entomol. 1974, 77, 141–171. [Google Scholar] [CrossRef]
- Boivin, G.; Hance, T.; Brodeur, J. Aphid parasitoids in biological control. Can. J. Plant. Sci. 2012, 92, 1–12. [Google Scholar] [CrossRef]
- Jerbi-Elayed, M.; Lebdi-Grissa, K.; Foray, V.; Muratori, F.; Hance, T. Using multiple traits to estimate the effects of heat shock on the fitness of Aphidius colemani. Entomol. Exp. Appl. 2015, 155, 18–27. [Google Scholar] [CrossRef]
- Le Ralec, A. Egg contents in relation to host-feeding in some parasitic Hymenoptera. BioControl 1995, 40, 87–93. [Google Scholar] [CrossRef]
- Lutterschmidt, W.I.; Hutchison, V.H. The critical thermal maximum: Data to support the onset of spasms as the definitive end point. Can. J. Zool. 1997, 75, 1553–1560. [Google Scholar] [CrossRef]
- Calosi, P.; Bilton, D.T.; Spicer, J.I.; Atfield, A. Thermal tolerance and geographical range size in the Agabus brunneus group of European diving beetles (Coleoptera: Dytiscidae). J. Biogeogr. 2008, 35, 295–305. [Google Scholar] [CrossRef]
- Godfray, H. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; ISBN 9780691000473. [Google Scholar]
- Foray, V.; Gibert, P.; Desouhant, E. Differential thermal performance curves in response to different habitats in the parasitoid Venturia canescens. Naturwissenschaften 2011, 98, 683–691. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Stastical Computing; R Core Team: Vienna, Austria, 2010; ISBN 3-900051-07-0. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- Rivero, A.; West, S. The physiological costs of being small in a parasitic wasp. Evol. Ecol. Res. 2002, 4, 407–420. [Google Scholar]
- Ellers, J.; Van Alphen, J.J.M.; Sevenster, J.G. A field study of size-fitness relationships in the parasitoid Asobara tabida. J. Anim. Ecol. 1998, 67, 318–324. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Huey, R.B. Size, temperature, and fitness: Three rules. Evol. Ecol. Res. 2008, 10, 251–268. [Google Scholar]
- Foray, V.; Desouhant, E.; Gibert, P. The impact of thermal fluctuations on reaction norms in specialist and generalist parasitic wasps. Funct. Ecol. 2014, 28, 411–423. [Google Scholar] [CrossRef]
- Casas, J.; Driessen, G.; Mandon, N.; Wielaard, S.; Desouhant, E.; van Alphen, J.J.M.; Lapchin, L.; Rivero, A.; Christides, J.P.; Bernstein, C. Energy dynamics in a parasitoid foraging in the wild. J. Anim. Ecol. 2003, 72, 691–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, B.; Le Lann, C.; den Blanken, F.J.; Harvey, J.A.; van Alphen, J.J.M.; Ellers, J. Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. Proc. Natl. Acad. Sci. USA 2010, 107, 8677–8682. [Google Scholar] [CrossRef] [Green Version]
- Ruther, J.; Prager, L.; Pokorny, T. Parasitic wasps do not lack lipogenesis. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210548. [Google Scholar] [CrossRef]
- Jervis, M.; Ellers, J.; Harvey, J.A. Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu. Rev. Entomol. 2008, 53, 361–385. [Google Scholar] [CrossRef] [Green Version]
- Clarke, A. Seasonal Acclimatization and Latitudinal Compensation in Metabolism: Do They Exist? Funct. Ecol. 1993, 7, 139–149. [Google Scholar] [CrossRef]
- Klok, C.J.; Chown, S.L. Resistance to temperature extremes in sub-Antarctic weevils: Interspecific variation, population differentiation and acclimation. Biol. J. Linn. Soc. 2003, 78, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.A.; Shirriffs, J.; Scott, M. Relative importance of plastic vs genetic factors in adaptive differentiation: Geographical variation for stress resistance in Drosophila melanogaster from eastern Australia. Funct. Ecol. 2005, 19, 222–227. [Google Scholar] [CrossRef]
- Schou, M.F.; Mouridsen, M.B.; Sørensen, J.G.; Loeschcke, V. Linear reaction norms of thermal limits in Drosophila: Predictable plasticity in cold but not in heat tolerance. Funct. Ecol. 2017, 31, 934–945. [Google Scholar] [CrossRef]
- Renault, D.; Vernon, P.; Vannier, G. Critical thermal maximum and body water loss in first instar larvae of three Cetoniidae species (Coleoptera). J. Therm. Biol. 2005, 30, 611–617. [Google Scholar] [CrossRef]
- Mills, N.; Wajnberg, E. Optimal foraging behaviour and efficient biological control methods. In Behavioural Ecology of Insect Parasitoids–From Theoretical Approaches to Field Applications; Wajnberg, E., Bernstein, C., van Alphen, J., Eds.; Blackwell Oxford: Oxford, UK, 2008; pp. 3–30. [Google Scholar]
- Jerbi-Elayed, M.; Tougeron, K.; Grissa-Lebdi, K.; Hance, T. Effect of developmental temperatures on Aphidius colemani host-foraging behavior at high temperature. J. Therm. Biol. 2021. (in revision). [Google Scholar]
- Ismail, M.; Van Baaren, J.; Hance, T.; Pierre, J.-S.; Vernon, P. Stress intensity and fitness in the parasitoid Aphidius ervi (Hymenoptera: Braconidae): Temperature below the development threshold combined with a fluctuating thermal regime is a must. Ecol. Entomol. 2013, 38, 355–363. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerbi-Elayed, M.; Foray, V.; Tougeron, K.; Grissa-Lebdi, K.; Hance, T. Developmental Temperature Affects Life-History Traits and Heat Tolerance in the Aphid Parasitoid Aphidius colemani. Insects 2021, 12, 852. https://doi.org/10.3390/insects12100852
Jerbi-Elayed M, Foray V, Tougeron K, Grissa-Lebdi K, Hance T. Developmental Temperature Affects Life-History Traits and Heat Tolerance in the Aphid Parasitoid Aphidius colemani. Insects. 2021; 12(10):852. https://doi.org/10.3390/insects12100852
Chicago/Turabian StyleJerbi-Elayed, Mey, Vincent Foray, Kévin Tougeron, Kaouthar Grissa-Lebdi, and Thierry Hance. 2021. "Developmental Temperature Affects Life-History Traits and Heat Tolerance in the Aphid Parasitoid Aphidius colemani" Insects 12, no. 10: 852. https://doi.org/10.3390/insects12100852
APA StyleJerbi-Elayed, M., Foray, V., Tougeron, K., Grissa-Lebdi, K., & Hance, T. (2021). Developmental Temperature Affects Life-History Traits and Heat Tolerance in the Aphid Parasitoid Aphidius colemani. Insects, 12(10), 852. https://doi.org/10.3390/insects12100852







