The Incidence of Wolbachia Bacterial Endosymbiont in Bisexual and Parthenogenetic Populations of the Psyllid Genus Cacopsylla (Hemiptera, Psylloidea)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Taxon Sampling, Wolbachia Screening, and Sequencing
2.2. Molecular Data Analysis
2.3. Sample Karyotyping
3. Results
3.1. PCR Screening and Geographical Pattern of Wolbachia Incidence
3.2. Wolbachia Alleles Identified in Cacopsylla Species
3.3. Phylogenetic Inferences
4. Discussion
4.1. Wolbachia Infection in Hemiptera
4.2. Patterns of Wolbachia Infection in Cacopsylla
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, M.Z.; Araujo-Jnr, E.V.; Welch, J.J.; Kawahara, A.Y. Wolbachia in butterflies and moths: Geographic structure in infection frequency. Front. Zool. 2015, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Hertig, M.; Wolbach, S.B. Studies on Rickettsia-like microorganisms in insects. J. Med. Res. 1924, 44, 329–374. [Google Scholar]
- Hertig, M. The Rickettsia, Wolbachia pipientis (gen. et sp.n.) and associated inclusions of the Mosquito, Culex pipiens. Parasitology 1936, 28, 453–486. [Google Scholar] [CrossRef]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia? —A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefoulon, E.; Clark, T.; Borveto, F.; Perriat-Sanguinet, M.; Moulia, C.; Slatko, B.E.; Gavotte, L. Pseudoscorpion Wolbachia symbionts: Diversity and evidence for a new supergroup S. BMC Microbiol. 2020, 20, 188. [Google Scholar] [CrossRef] [PubMed]
- Bandi, C.; Anderson, T.J.; Genchi, C.; Blaxter, M.L. Phylogeny of Wolbachia in filarial nematodes. Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 2407–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casiraghi, M.; Bain, O.; Guerrero, R.; Martin, C.; Pocacqua, V.; Gardner, S.L.; Franceschi, A.; Bandi, C. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: Evidence for symbiont loss during evolution. Int. J. Parasitol. 2004, 34, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Lefoulon, E.; Bain, O.; Makepeace, B.L.; d’Haese, C.; Uni, S.; Martin, C.; Gavotte, L. Breakdown of coevolution between symbiotic bacteria Wolbachia and their filarial hosts. PeerJ 2016, 4, e1840. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.M.; Wasala, S.K.; Howe, D.K.; Peetz, A.B.; Zasada, I.A.; Denver, D.R. Genomic evidence for plant-parasitic nematodes as the earliest Wolbachia hosts. Sci. Rep. 2016, 6, 34955. [Google Scholar] [CrossRef]
- Haegeman, A.; Vanholme, B.; Jacob, J.; Vandekerckhove, T.T.; Claeys, M.; Borgonie, G.; Gheysen, G. An endosymbiotic bacterium in a plant-parasitic nematode: Member of a new Wolbachia supergroup. Int. J. Parasitol. 2009, 39, 1045–1054. [Google Scholar] [CrossRef]
- Ferri, E.; Bain, O.; Barbuto, M.; Martin, C.; Lo, N.; Uni, S.; Landmann, F.; Baccei, S.G.; Guerrero, R.; de Souza, L.S.; et al. New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PLoS ONE 2011, 6, e20843. [Google Scholar] [CrossRef] [Green Version]
- Lefoulon, E.; Gavotte, L.; Junker, K.; Barbuto, M.; Uni, S.; Landmann, F.; Laaksonen, S.; Saari, S.; Nikander, S.; de Souza, L.S.; et al. A new type F Wolbachia from Splendidofilariinae (Onchocercidae) supports the recent emergence of this supergroup. Int. J. Parasitol. 2012, 42, 1025–1036. [Google Scholar] [CrossRef]
- Baldo, L.; Werren, J.H. Revisiting Wolbachia supergroup typing based on WSP: Spurious lineages and discordance with MLST. Curr. Microbiol. 2007, 55, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.; Rosengaus, R.B. Discovery of a novel Wolbachia supergroup in Isoptera. Curr. Microbiol. 2005, 51, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Glowska, E.; Dragun-Damian, A.; Dabert, M.; Gerth, M. New Wolbachia supergroups detected in quill mites (Acari: Syringophilidae). Infect. Genet. Evol. 2015, 30, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.; Casiraghi, M.; Salati, E.; Bazzocchi, C.; Bandi, C. How many Wolbachia supergroups exist? Mol. Biol. Evol. 2002, 19, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, N.; Paraskevopoulos, C.; Bourtzis, K.; O’Neill, S.L.; Werren, J.H.; Bordenstein, S.R.; Bandi, C. Taxonomic status of the intracellular bacterium Wolbachia pipientis. Int. J. Syst. Evol. Microbiol. 2007, 57, 654–657. [Google Scholar] [CrossRef] [Green Version]
- Ros, V.I.D.; Fleming, V.M.; Feil, E.J.; Breeuwer, J.A.J. How diverse is the genus Wolbachia? Multiple-gene sequencing reveals a putatively new Wolbachia supergroup recovered from spider mites (Acari: Tetranychidae). Appl. Environ. Microbiol. 2009, 75, 1036–1043. [Google Scholar] [CrossRef] [Green Version]
- Stahlhut, J.K.; Desjardins, C.A.; Clark, M.E.; Baldo, L.; Russell, J.A.; Werren, J.H.; Jaenike, J. The mushroom habitat as an ecological arena for global exchange of Wolbachia. Mol. Ecol. 2010, 19, 1940–1952. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.M.; Pantoja, N.A.; O’Grady, P.M. Diversity and phylogenetic relationships of Wolbachia in Drosophila and other native Hawaiian insects. Fly 2012, 6, 273–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoh, N.; Tanaka, K.; Shibata, F.; Kondo, N.; Hizume, M.; Shimada, M.; Fukatsu, T. Wolbachia genome integrated in an insect chromosome: Evolution and fate of laterally transferred endosymbiont genes. Genome Res. 2008, 18, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, S.; Thézé, T.; Chebbi, M.A.; Giraud, I.; Moumen, B.; Ernenwein, L.; Grève, P.; Gilbert, C.; Cordaux, R. Birth of a W sex chromosome by horizontal transfer of Wolbachia bacterial symbiont genome. Proc. Natl. Acad. Sci. USA 2016, 113, 15036–15041. [Google Scholar] [CrossRef] [Green Version]
- Fenn, K.; Blaxter, M. Are filarial nematode Wolbachia obligate mutualist symbionts? Trends Ecol. Evol. 2004, 19, 163–166. [Google Scholar] [CrossRef]
- Balvín, O.; Roth, S.; Talbot, B.; Reinhardt, K. Co-speciation in bedbug Wolbachia parallel the pattern in nematode hosts. Sci. Rep. 2018, 8, 8797. [Google Scholar] [CrossRef]
- Serbus, L.R.; Casper-Lindley, C.; Landmann, F.; Sullivan, W. The genetics and cell biology of Wolbachia-host interactions. Annu. Rev. Genet. 2008, 42, 683–707. [Google Scholar] [CrossRef] [Green Version]
- Gebiola, M.; Giorgini, M.; Kelly, S.E.; Doremus, M.R.; Ferree, P.M.; Hunter, M.S. Cytological analysis of cytoplasmic incompatibility induced by Cardinium suggests convergent evolution with its distant cousin Wolbachia. Proc. Biol. Sci. 2017, 284, 20171433. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.D. Wolbachia infection associated with increased recombination in Drosophila. G3 (Bethesda) 2019, 9, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becking, T.; Chebbi, M.A.; Giraud, I.; Moumen, B.; Laverré, T.; Caubet, Y.; Peccoud, J.; Gilbert, C.; Cordaux, R. Sex chromosomes control vertical transmission of feminizing Wolbachia symbionts in an isopod. PLoS Biol. 2019, 17, e3000438. [Google Scholar] [CrossRef] [Green Version]
- Xi, Z.; Khoo, C.C.; Dobson, S.L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 2005, 310, 326–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, J.; Ganatra, M.; Kamal, I.; Ware, J.; Makarova, K.; Ivanova, N.; Bhattacharyya, A.; Kapatral, V.; Kumar, S.; Posfai, J.; et al. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005, 3, e12. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.K.; Lohman, D.J.; Wahlberg, N.; Müller, C.J.; Brattström, O.; Collins, S.C.; Peggie, D.; Aduse-Poku, K.; Kodandaramaiah, U. Evolution of Hypolimnas butterflies (Nymphalidae): Out-of-Africa origin and Wolbachia-mediated introgression. Mol. Phylogenet. Evol. 2018, 123, 50–58. [Google Scholar] [CrossRef]
- Ouvrard, D. Psyl’list—The World Psylloidea Database. 2021. Available online: http://www.hemiptera-databases.com/psyllist (accessed on 20 July 2021).
- Guidolin, A.S.; Cônsoli, F.L. Molecular characterization of Wolbachia strains associated with the invasive Asian citrus psyllid Diaphorina citri in Brazil. Microb. Ecol. 2013, 65, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Fromont, C.; Riegler, M.; Cook, J.M. Phylogeographic analyses of bacterial endosymbionts in fig homotomids (Hemiptera: Psylloidea) reveal codiversification of both primary and secondary endosymbionts. FEMS Microbiol. Ecol. 2016, 92, fiw205. [Google Scholar] [CrossRef] [Green Version]
- Hodkinson, I. Present-Day Distribution patterns of the Holarctic Psylloidea (Homoptera: Insecta) with particular reference to the origin of the Nearctic fauna. J. Biogeogr. 1980, 7, 127–146. [Google Scholar] [CrossRef]
- Hollis, D. Australian Psylloidea: Jumping Plantlice and Lerp Insects; Australian Biological Resources Study: Canberra, Australia, 2004; p. 216.
- Drohojowska, J.; Kalandyk-Kołodziejczyk, M.; Simon, E. Thorax morphology of selected species of the genus Cacopsylla (Hemiptera, Psylloidea). ZooKeys 2013, 319, 27–35. [Google Scholar] [CrossRef]
- Hodkinson, I.D. New psyllids from Canada (Homoptera: Psyllidae). Zool. J. Linn. Soc. 1976, 58, 321–330. [Google Scholar] [CrossRef]
- Hodkinson, I.D. The psyllids (Homoptera-Psyllidae) of Alaska. Syst. Entomol. 1978, 3, 333–360. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Facultative parthenogenesis in Psylla myrtilli Wagner (Hom., Psyllidae): The saga continues in Norway. Fauna Norvegica (B) 1983, 30, 1–2. [Google Scholar]
- Nokkala, S.; Maryańska-Nadachowska, A.; Kuznetsova, V.G. First evidence of polyploidy in Psylloidea (Homoptera, Sternorrhyncha): A parthenogenetic population of Cacopsylla myrtilli (W. Wagner, 1947) from northeast Finland is apomictic and triploid. Genetica 2008, 133, 201–205. [Google Scholar] [CrossRef]
- Nokkala, C.; Kuznetsova, V.G.; Nokkala, S. Meiosis in rare males in parthenogenetic Cacopsylla myrtilli (Wagner, 1947) (Hemiptera, Psyllidae) populations from northern Europe. Comp. Cytogenet. 2013, 7, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nokkala, C.; Kuznetsova, V.G.; Nokkala, S. Rare diploid females coexist with rare males: A novel finding in triploid parthenogenetic populations in the psyllid Cacopsylla myrtilli (W.Wagner, 1947) (Hemiptera, Psylloidea) in northern Europe. Genetica 2015, 143, 589–595. [Google Scholar] [CrossRef]
- Nokkala, S.; Kuznetsova, V.G.; Nokkala, C. Characteristics of parthenogenesis in Cacopsylla ledi (Flor, 1861) (Hemiptera, Sternorryncha, Psylloidea): Cytological and molecular approaches. Comp. Cytogenet. 2017, 11, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Nokkala, C.; Kuznetsova, V.G.; Rinne, V.; Nokkala, S. Description of two new species of the genus Cacopsylla Ossiannilsson, 1970 (Hemiptera, Psylloidea) from northern Fennoscandia recognized by morphology, cytogenetic characters and COI barcode sequence. Comp. Cytogenet. 2019, 13, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Labina, E.S.; Nokkala, S.; Maryańska-Nadachowska, A.; Kuznetsova, V.G. The distribution and population sex ratio of Cacopsylla myrtilli (W. Wagner, 1947) (Hemiptera: Psylloidea). Folia Biol. (Krakow) 2009, 57, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsova, V.G.; Labina, E.S.; Shapoval, N.A.; Maryańska-Nadachowska, A.; Lukhtanov, V.A. Cacopsylla fraudatrix sp.n. (Hemiptera: Psylloidea) recognised from testis structure and mitochondrial gene COI. Zootaxa 2012, 3547, 55–63. [Google Scholar] [CrossRef]
- Zhou, W.; Rousset, F.; O’Neil, S. Phylogeny and PCR- based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Windsor, D.M. Wolbachia infection frequency in insects: Evidence of a global equilibrium? Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 1277–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic infer- ence and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werren, J.H.; Zhang, W.; Guo, L.R. Evolution and phylogeny of Wolbachia: Reproductive parasites of arthropods. Proc. Biol. Sci. 1995, 261, 55–63. [Google Scholar] [CrossRef]
- Ritter, S.; Michalski, S.G.; Settele, G.; Wiemers, M.; Fric, Z.F.; Sielezniew, M.; Šašic, M.; Rozier, Y. Wolbachia infections mimic cryptic speciation in two parasitic butterfly species, Phengaris teleius and P. nausithous (Lepidoptera: Lycaenidae). PLoS ONE 2013, 8, e78107. [Google Scholar] [CrossRef] [Green Version]
- Sucháčková Bartoňová, A.; Konvička, M.; Marešová, J.; Wiemers, M.; Ignatev, N.; Wahlberg, N.; Schmitt, T.; Fric, Z.F. Wolbachia affects mitochondrial population structure in two systems of closely related Palaearctic blue butterflies. Sci. Rep. 2021, 11, 3019. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F.; de Stordeur, E. Properties of Drosophila simulans strains experimentally infected by different clones of the bacterium Wolbachia. Heredity 1994, 72, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detcharoen, M.; Arthofer, W.; Schlick-Steiner, B.C.; Steiner, F.M. Wolbachia megadiversity: 99% of these microorganismic manipulators unknown. FEMS Microbiol. Ecol. 2019, 95, fiz151. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications [version 1; peer review: 2 approved]. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Camerota, C.; Raddadi, N.; Pizzinat, A.; Gonella, E.; Crotti, E.; Tedeschi, R.; Mozes-Daube, N.; Ember, I.; Acs, Z.; Kolber, M.; et al. Incidence of ‘Candidatus Liberibacter europaeus’ and phytoplasmas in Cacopsylla species (Hemiptera: Psyllidae) and their host/shelter plants. Phytoparasitica 2012, 40, 213–221. [Google Scholar] [CrossRef]
- Cen, Y.; Zhang, L.; Xia, Y.; Guo, J.; Deng, X.; Zhou, W.; Sequeira, R.; Gao, J.; Wang, Z.; Yue, J.; et al. Detection of ‘Candidatus Liberibacter Asiaticus’ in Cacopsylla (Psylla) citrisuga (Hemiptera: Psyllidae). Fla. Entomol. 2012, 95, 304–311. [Google Scholar] [CrossRef]
- Cooper, W.R.; Garczynski, S.F.; Horton, D.R.; Unruh, T.R.; Beers, E.H.; Peter, W.S.; Hilton, R.J. Bacterial Endosymbionts of the Psyllid Cacopsylla pyricola (Hemiptera: Psyllidae) in the Pacific Northwestern United States. Environ. Entomol. 2017, 46, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.L.; Om, N.; Beattie, G.A.C.; Chambers, G.A.; Donovan, N.J.; Liefting, L.W.; Riegler, M.; Holford, P. Characterization of the bacterial communities of psyllids associated with Rutaceae in Bhutan by high throughput sequencing. BMC Microbiol. 2020, 20, 215. [Google Scholar] [CrossRef]
- Sun, X.; Cui, L.; Li, Z. Diversity and phylogeny of Wolbachia infecting Bactrocera dorsalis (Diptera: Tephritidae) populations from China. Environ. Entomol. 2007, 36, 1283–1289. [Google Scholar] [CrossRef]
- Arthofer, W.; Riegler, M.; Schneider, D.; Krammer, M.; Miller, W.J.; Stauffer, C. Hidden Wolbachia diversity in field populations of the European cherry fruit fly, Rhagoletis cerasi (Diptera, Tephritidae). Mol. Ecol. 2009, 18, 3816–3830. [Google Scholar] [CrossRef]
- Hughes, G.L.; Allsopp, P.G.; Brumbley, S.M.; Woolfit, M.; McGraw, E.A.; O’Neill, S.L. Variable infection frequency and high diversity of multiple strains of Wolbachia pipientis in Perkinsiella planthoppers. Appl. Environ. Microbiol. 2011, 77, 2165–2168. [Google Scholar] [CrossRef] [Green Version]
- Arthofer, W.; Riegler, M.; Avtzis, D.N.; Stauffer, C. Evidence for low-titre infections in insect symbiosis: Wolbachia in the bark beetle Pityogenes chalcographus (Coleoptera, Scolytinae). Environ. Microbiol. 2009, 11, 1923–1933. [Google Scholar] [CrossRef]
- Mascarenhas, R.O.; Prezotto, L.F.; Perondini, A.L.P.; Marino, C.L.; Selivon, D. Wolbachia in guilds of Anastrepha fruit flies (Tephritidae) and parasitoid wasps (Braconidae). Genet. Mol. Biol. 2016, 39, 600–610. [Google Scholar] [CrossRef]
- Chu, C.; Hoffmann, M.; Braswell, W.E.; Pelz-Stelinski, K.S. Genetic variation and potential coinfection of Wolbachia among widespread Asian citrus psyllid (Diaphorina citri Kuwayama) populations. Insect Sci. 2019, 26, 671–682. [Google Scholar] [CrossRef] [Green Version]
- Sintupachee, S.; Milne, J.R.; Poonchaisri, S.; Baimai, V.; Kittayapong, P. Closely related Wolbachia strains within the pumpkin arthropod community and the potential for horizontal transmission via the plant. Microb. Ecol. 2006, 51, 294–301. [Google Scholar] [CrossRef]
- Gutzwiller, F.; Dedeine, F.; Kaiser, W.; Giron, D.; Lopez-Vaamonde, C. Correlation between the green-island phenotype and Wolbachia infections during the evolutionary diversification of Gracillariidae leaf-mining moths. Ecol. Evol. 2015, 5, 18, 4049–4062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
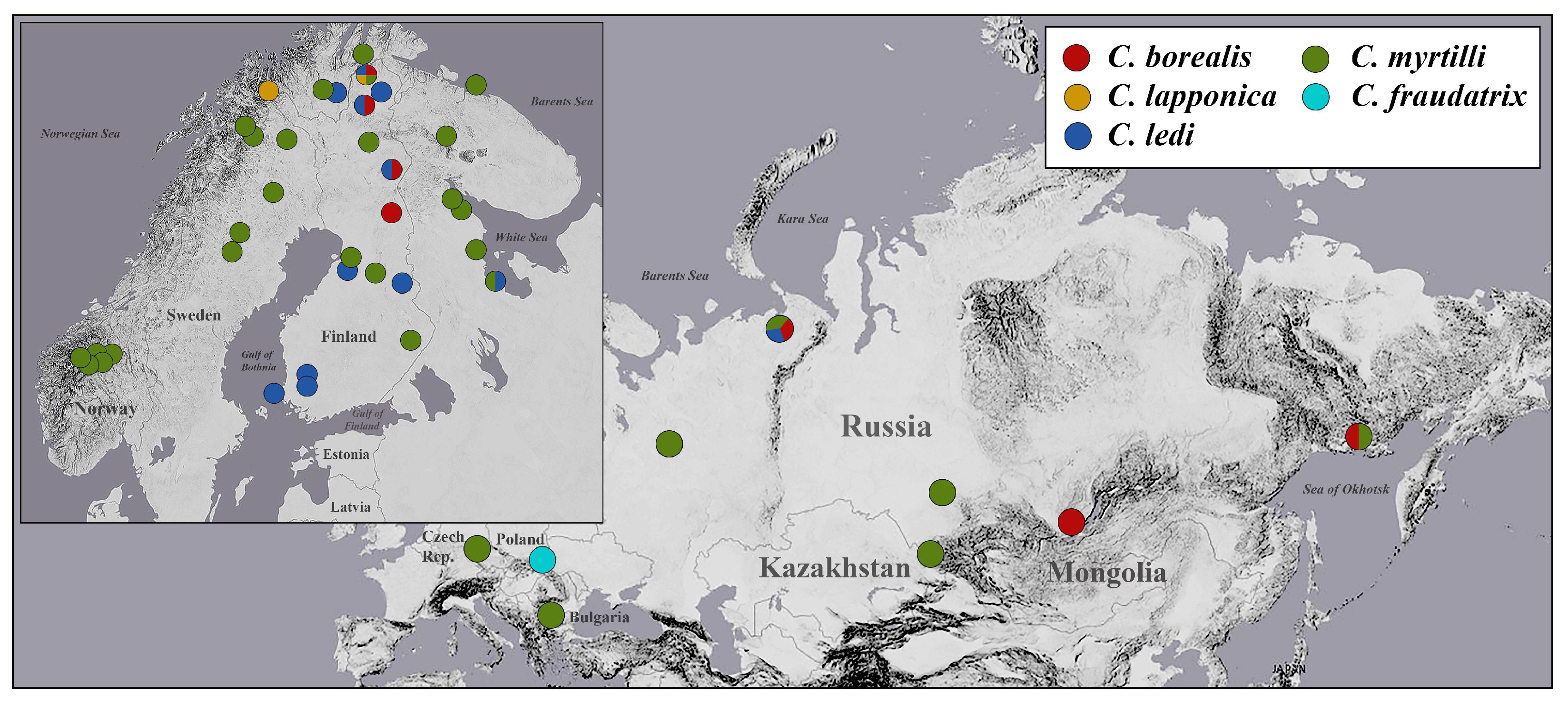


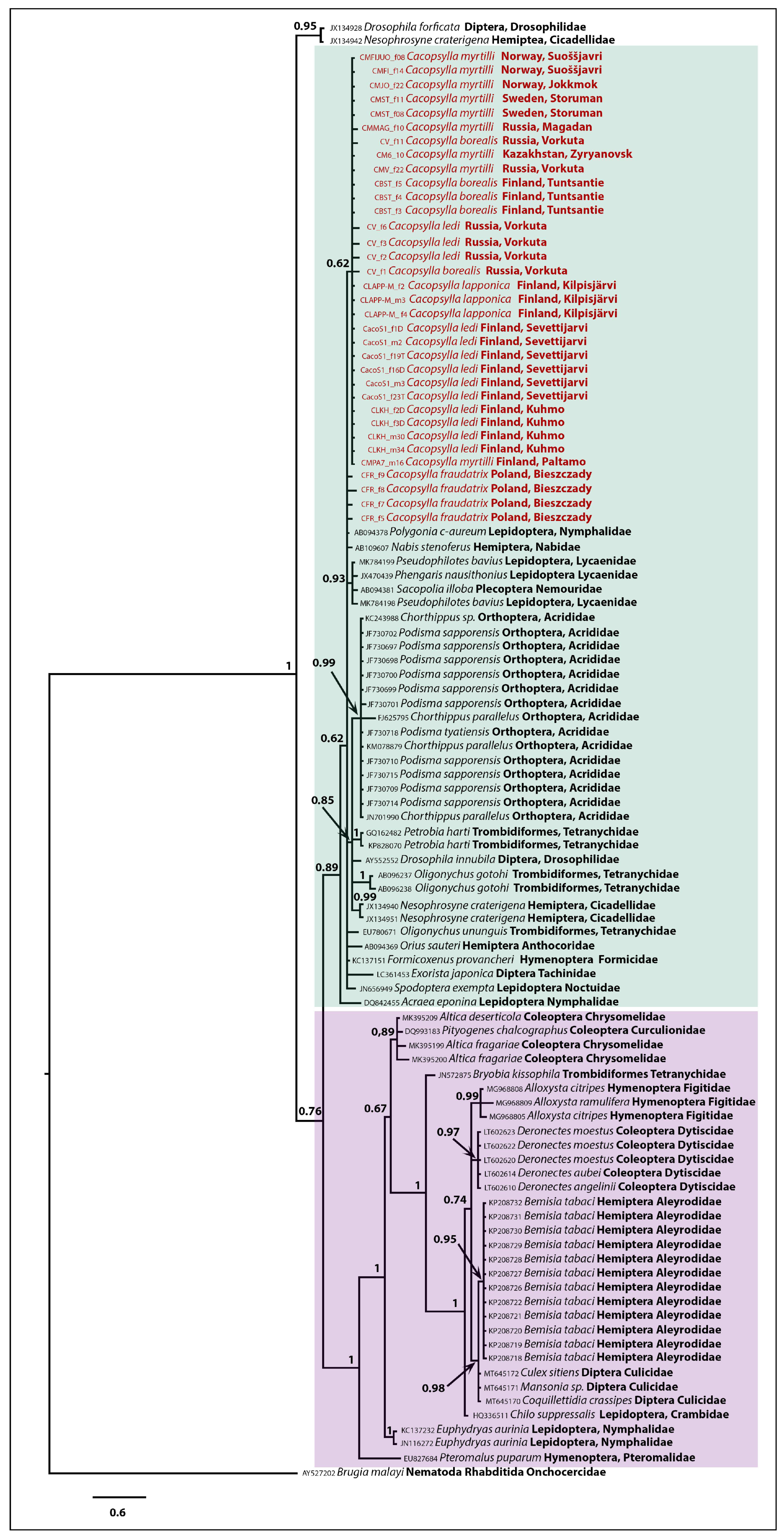
| Table | Samples | Locality |
|---|---|---|
| Cacopsylla lapponica | 2♂ + 4♀ | FINLAND, Lapland province, Kilpisjärvi, Pikku-Malla, 69°08′56″ N; 20°44′20″ E, h = 610 m, 27.07.2014, S. Nokkala & Ch. Nokkala leg. |
| 6♀ | FINLAND, Lapland province, Utsjoki, Ailigas, 69°53′51″ N; 27°03′32″ E, 28.07.2020, S. Nokkala & Ch. Nokkala leg. | |
| Cacopsylla fraudatrix | 1♂ + 5♀ | POLAND, Bieszczady Mts, Wielka Rawka, 49°06′ N; 22°35′ E, 07.08.2009, V. Kuznetsova & A. Maryańska-Nadachowska leg. |
| Cacopsylla borealis | 17♀ | FINLAND, Lapland province, Tuntsantie, 67°18′11″ N; 29°16′18″ E, 01.08.2019, S. Nokkala & Ch. Nokkala leg. |
| 5♀ | FINLAND, Lapland province, Inari, Pitkävuono, 68°58′56″ N; 26°57′18″ E, 16.08.2017, S. Nokkala & Ch. Nokkala leg. | |
| 3♀ | FINLAND, Lapland province, Utsjoki, Hietala 69°51′06″ N; 27°00′34″ E, 15.08.2017, S. Nokkala & Ch. Nokkala leg. | |
| 7♀ | FINLAND, Kuusamo, Kantojoki, 66°14′23″ N; 29°09′15″ E, 02.08.2019, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | RUSSIA, Magadan region, vic. Ola vill., 59°34′24″ N; 150°46′04″ E, 22.07.2020, Yu. Marusik & D. Berman leg. | |
| 2♀ | RUSSIA, Komi Republic, Vorkuta city, 67°27′34″ N; 63°59′01″ E, 06.08.2013, N. Khabazova & M. Mandelshtam leg. | |
| 12♀ | RUSSIA, Irkutsk region, Lake Baikal, vic. Bolshye Koty vill. 51°54′25″ N; 105°04′14″ E, 21.07.2007, E. Labina leg. | |
| Cacopsylla ledi | 2♂ + 10♀ | FINLAND, Lapland province, Sevettijärvi, 69°12′58″ N; 27°52′14″ E, 17.08.2017, S. Nokkala & Ch. Nokkala leg. |
| 3♀ | FINLAND, Oulu province, Siikajoki, 64°39′32″ N; 25°19′33″ E, 10.08.2018, S. Nokkala & Ch. Nokkala leg. | |
| 6♂ + 6♀ | FINLAND, Oulu province, Kuhmo, Syväjärvi, 64°11′40″ N; 29°57′49″ E, 04.08.2019, S. Nokkala & Ch. Nokkala leg. | |
| 6♂ | FINLAND, Western Finland province, Yläne, 60°53′10″ N; 22°26′41″ E, 15.08.2019, S. Nokkala & Ch. Nokkala leg. | |
| 6♂ | FINLAND, Western Finland province, Pöytyä, Lammenrahka, 60°44′37″ N; 22°25′32″ E, 16.08.2018, S. Nokkala & Ch. Nokkala leg. | |
| 6♂ | FINLAND, Western Finland province, Kustavi, 60°39′20″ N; 21°18′12″ E, 25.08.2019, S. Nokkala & Ch. Nokkala leg. | |
| 1♀ | FINLAND, Lapland province, Inari, Pitkävuono, 68°58′56″ N; 26°57′18″ E, 16.08.2017, S. Nokkala & Ch. Nokkala leg. | |
| 3♀ | FINLAND, Lapland province, Utsjoki, Hietala 69°51′06″ N; 27°00′34″ E, 15.08.2017, S. Nokkala & Ch. Nokkala leg. | |
| 1♀ | FINLAND, Lapland province, Tuntsantie, 67°18′11″ N; 29°16′18″ E, 01.08.2019, S. Nokkala & Ch. Nokkala leg. | |
| 6♂ | NORWAY, Troms og Finnmark county, Mohkkejogas, 69°26′35″ N; 25°11′36″ E, 26.07.2020, S. Nokkala & Ch. Nokkala leg. | |
| 18♀ | RUSSIA, Karelia Republic, White Sea, vic. Kolezma vill., 64°14′46″ N 35°48′49″ E, 30.09.2020, V. Kuznetsova & P. Strelkov leg. | |
| 10♀ | RUSSIA, Komi Republic, Vorkuta city, 67°27′34″ N; 63°59′01″ E, 06.08.2013, N. Khabazova & M. Mandelshtam leg. | |
| Cacopsylla myrtilli | 1♂ + 12♀ | FINLAND, Kainuu province, Paltamo district, S of Törmänmäki, 64°33′28″ N; 27°43′41″ E, 16.07.2009, S. Nokkala & Ch. Nokkala leg. |
| 1♂ + 14♀ | FINLAND, Lapland province, Utsjoki, Ailigas, 69°53′51″ N; 27°03′32″ E, 16.08.2017, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | FINLAND, Lapland province, Utsjoki, Hietala 69°51′06″ N; 27°00′34″ E, 11.08.2012, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | FINLAND, Lapland province, Sodankylä, Puisuvanto, 67°46′52″ N; 26°46′09″ E, 03.08.2018, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | FINLAND, Oulu province, Liminka, 64°43′53″ N; 25°23′04″ E, 04.08.2009, S. Nokkala & Ch. Nokkala leg. | |
| 9♀ | FINLAND, Eastern Finland province, Tohmajärvi, 62°23′12″ N; 30°19′46″ E, 12.08.2012, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | SWEDEN, Swedish Lapland province, Abisko, Lapporten, 68°19′14″ N; 18°51′05″ E, h = 570 m, 26.07.2014, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | SWEDEN, Swedish Lapland province, Björkliden, Fjället, 68°24′32″ N; 18°39′55″ E, 03.08.2009, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | SWEDEN, Norbothnia province, Soppero, 68°00′39″ N; 21°39′25″ E, 10.08.2012, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | SWEDEN, Norbothnia province, Jokkmok, 66°35′36″ N; 19°49′20″ E, 08.08.2012, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | SWEDEN, Westrobothnia province, Sorsele, 65°29′07″ N; 17°33′36″ E, 17.08.2010, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | SWEDEN, Westrobothnia province, Storuman, 65°05′16″ N; 17°06′51″ E, 08.08.2012, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | NORWAY, Troms og Finnmark county, Suoššjavri, 69°22′11″ N; 24°18′20″ E, 18.08.2011, S. Nokkala & Ch. Nokkala leg. | |
| 6♀ | NORWAY, Innlandet county, Sjoa, Kringlothaugen mt., 61°43′06″ N; 09°22′40″ E, h = 700 m, 01.08.2009, S. Nokkala & Ch. Nokkala leg. | |
| 6♀ | NORWAY, Innlandet county, Sjoa, Kvernbrusaetrin, 61°42′27″ N; 09°19′25″ E, h = 1100 m, 01.08.2009, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | NORWAY, Innlandet county, Sjoa, Stålane, 61°41′15″ N; 09°14′27″ E, h = 1100 m, 01.08.2009, S. Nokkala & Ch. Nokkala leg. | |
| 12♀ | NORWAY, Innlandet county, Sjoa, Rudihoe, 61°46′27″ N; 09°17′16″ E, h = 1100 m, 01.08.2009, S. Nokkala & Ch. Nokkala leg. | |
| 11♀ | NORWAY, Innlandet county, Sjoa, Rindhovda, 61°43′05″ N; 09°05′12″ E, h = 1080 m, 16.08.2010, S. Nokkala & Ch. Nokkala leg. | |
| 18♀ | RUSSIA, Murmansk region, Barents Sea, Kildin Island, 69°19′58″ N; 34°23′43″ E, 05.08.2016, P. Strelkov leg. | |
| 3♂ + 10♀ | RUSSIA, Murmansk region, Laplandsky Natural Reserve, 68°07′ N; 32°27′ E, 01.08.2019, A. Polevoi leg. | |
| 1♀ | RUSSIA, Karelia Republic, White Sea, vic. Kolezma vill., 64°14′46″ N 35°48′49″ E, 30.09.2020, V. Kuznetsova & P. Strelkov leg. | |
| 10♀ | RUSSIA, Karelia Republic, Louchskiy district, “Belomorskaya″ Research Station, 66°17′58″ N; 33°37′18″ E, 29.08.2019, G. Paskerova leg. | |
| 17♀ | RUSSIA, Karelia Republic, White Sea, Sredniy Island, 66°17′28″ N; 33°39′06″ E, 21.08.2017, G. Paskerova leg. | |
| 12♀ | RUSSIA, Karelia Republic, vic. Kem′ city, 2017. | |
| 15♀ | RUSSIA, Magadan region, vic. Ola vill., 59°34′24″ N; 150°46′04″ E, 22.07.2020, Yu. Marusik & D. Berman leg. | |
| 12♀ | RUSSIA, Komi Republic, Vorkuta city, 67°27′34″ N; 63°59′01″ E, 06.08.2013, N. Khabazova & M. Mandelshtam leg. | |
| 13♀ | RUSSIA, Komi Republic, 2 km W of Syktyvkar city, 61°38′57″ N; 50°44′09″ E, 02.09.2020, A. Zinovieva leg. | |
| 12♀ | RUSSIA, Kemerovo region, Kemerovskiy district, vic. Voskresenka vill., 55°19′ N; 86°48′ E, July 2019. A. Polevoi leg. | |
| 1♂ + 10♀ | KAZAKHSTAN, Zyryanovsky district, ca. 30 km N of Zyryanovsk city, 50°00′05″ N; 84°13′32″ E, 11.07.2012, V. Lukhtanov leg. | |
| 12♀ | CZECH REPUBLIC, Karlovy Vary region, Kleiner Kranichsee Natural Reserve, 50°24′56″ N; 12°40′30″ E, h = 925 m, 28.07.2020, I. Malinovsky leg. | |
| 12♀ | CZECH REPUBLIC, Karlovy Vary region, Kleiner Kranichsee Natural Reserve, 50°23′33″ N; 12°37′48″ E, h = 915 m, 29.07.2020, I. Malinovsky leg. | |
| 4♀ | BULGARIA, Kyustendil province, Rila, Stara planina Mts., 42°06′ N; 23°33′ E, 17.08.2020, I. Gjonov leg. |
| Host Taxon | Sample ID | GB Accession No. | Sex | Allele | Locality |
|---|---|---|---|---|---|
| Cacopsylla lapponica | CLAPP-M_f2 | MZ684116 | ♀ | wMyr01 | FINLAND, Kilpisjärvi, 69°08′56″ N; 20°44′20″ E |
| Cacopsylla lapponica | CLAPP-M_m3 | MZ684117 | ♀ | wMyr01 | FINLAND, Kilpisjärvi, 69°08′56″ N; 20°44′20″ E |
| Cacopsylla lapponica | CLAPP-M_f4 | MZ684118 | ♂ | wMyr01 | FINLAND, Kilpisjärvi, 69°08′56″ N; 20°44′20″ E |
| Cacopsylla fraudatrix | CFR_f5 | MZ684132 | ♀ | * | POLAND, Bieszczady 49°06′ N; 22°35′ E |
| Cacopsylla fraudatrix | CFR_f7 | MZ684133 | ♀ | * | POLAND, Bieszczady 49°06′ N; 22°35′ E |
| Cacopsylla fraudatrix | CFR_f8 | MZ684134 | ♀ | wFr01 wFr02 | POLAND, Bieszczady 49°06′ N; 22°35′ E |
| Cacopsylla fraudatrix | CFR_f9 | MZ684135 | ♀ | * | POLAND, Bieszczady 49°06′ N; 22°35′ E |
| Cacopsylla borealis | CBST_f3 | MZ684111 | ♀ | wMyr01 | FINLAND, Tuntsantie, 67°18′11″ N; 29°16′18″ E |
| Cacopsylla borealis | CBST_f4 | MZ684112 | ♀ | wMyr01 | FINLAND, Tuntsantie, 67°18′11″ N; 29°16′18″ E |
| Cacopsylla borealis | CBST_f5 | MZ684113 | ♀ | wMyr01 | FINLAND, Tuntsantie, 67°18′11″ N; 29°16′18″ E |
| Cacopsylla borealis | CV_f11 | MZ684114 | ♀ | wMyr02 | RUSSIA, Vorkuta 67°27′34″ N; 63°59′01″ E |
| Cacopsylla borealis | CV_f1 | MZ684115 | ♀ | wLed | RUSSIA, Vorkuta 67°27′34″ N; 63°59′01″ E |
| Cacopsylla ledi | CacoS1_f1D | MZ684119 | ♀ | wMyr01 | FINLAND, Sevettijärvi, 69°12′58″ N; 27°52′14″ E |
| Cacopsylla ledi | CacoS1_f16D | MZ684120 | ♀ | wMyr01 | FINLAND, Sevettijärvi, 69°12′58″ N; 27°52′14″ E |
| Cacopsylla ledi | CacoS1_f19T | MZ684121 | ♀ | wMyr01 | FINLAND, Sevettijärvi, 69°12′58″ N; 27°52′14″ E |
| Cacopsylla ledi | CacoS1_f23T | MZ684122 | ♀ | wMyr01 | FINLAND, Sevettijärvi, 69°12′58″ N; 27°52′14″ E |
| Cacopsylla ledi | CacoS1_m2 | MZ684123 | ♂ | wMyr01 | FINLAND, Sevettijärvi, 69°12′58″ N; 27°52′14″ E |
| Cacopsylla ledi | CacoS1_m3 | MZ684124 | ♂ | wMyr01 | FINLAND, Sevettijärvi, 69°12′58″ N; 27°52′14″ E |
| Cacopsylla ledi | CLKH_f2D | MZ684125 | ♀ | wMyr01 | FINLAND, Kuhmo, 64°11′40″ N; 29°57′49″ E |
| Cacopsylla ledi | CLKH_f3D | MZ684126 | ♀ | wMyr01 | FINLAND, Kuhmo, 64°11′40″ N; 29°57′49″ E |
| Cacopsylla ledi | CLKH_m30 | MZ684127 | ♂ | wMyr01 | FINLAND, Kuhmo, 64°11′40″ N; 29°57′49″ E |
| Cacopsylla ledi | CLKH_m34 | MZ684128 | ♂ | wMyr01 | FINLAND, Kuhmo, 64°11′40″ N; 29°57′49″ E |
| Cacopsylla ledi | CLV_f2 | MZ684129 | ♀ | wLed | RUSSIA, Vorkuta 67°27′34″ N; 63°59′01″ E |
| Cacopsylla ledi | CLV_f3 | MZ684130 | ♀ | wLed | RUSSIA, Vorkuta 67°27′34″ N; 63°59′01″ E |
| Cacopsylla ledi | CLV_f6 | MZ684131 | ♀ | wLed | RUSSIA, Vorkuta 67°27′34″ N; 63°59′01″ E |
| Cacopsylla myrtilli | CMFIJUO_f08 | MZ684102 | ♀ | wMyr01 | NORWAY, Suoššjavri, 69°22′11″ N; 24°18′20″ E |
| Cacopsylla myrtilli | CMFI_f14 | MZ684103 | ♀ | wMyr01 | NORWAY, Suoššjavri, 69°22′11″ N; 24°18′20″ E |
| Cacopsylla myrtilli | CMPAL_m16 | MZ684104 | ♂ | wMyr01 | FINLAND, Paltamo, 64°33′28″ N; 27°43′41″ E |
| Cacopsylla myrtilli | CMST_f8 | MZ684105 | ♀ | wMyr01 | SWEDEN, Storuman, 65°05′16″ N; 17°06′51″ E |
| Cacopsylla myrtilli | CMST_f11 | MZ684106 | ♀ | wMyr01 | SWEDEN, Storuman, 65°05′16″ N; 17°06′51″ E |
| Cacopsylla myrtilli | CMJO_f22 | MZ684107 | ♀ | wMyr01 | SWEDEN, Jokkmok, 66°35′36″ N; 19°49′20″ E |
| Cacopsylla myrtilli | CMV_f22 | MZ684108 | ♀ | wMyr01 | RUSSIA, Vorkuta 67°27′34″ N; 63°59′01″ E |
| Cacopsylla myrtilli | CM6.10 | MZ684109 | ♀ | wMyr01 | KAZAKHSTAN, Zyryanovsk, 50°00′05″ N; 84°13′32″ E |
| Cacopsylla myrtilli | CMMAG_f10 | MZ684110 | ♀ | wMyr02 | RUSSIA, Magadan 59°34′24″ N; 150°46′04′′ E |
| Allele | Nucleotide Position | |||
|---|---|---|---|---|
| 360 | 495 | 498 | 499 | |
| wMyr01 | C | C | A | G |
| wMyr02 | C | C | G | G |
| wLed | C | C | A | C |
| wFr01 | C | A | A | G |
| wFr02 | A | A | A | G |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapoval, N.A.; Nokkala, S.; Nokkala, C.; Kuftina, G.N.; Kuznetsova, V.G. The Incidence of Wolbachia Bacterial Endosymbiont in Bisexual and Parthenogenetic Populations of the Psyllid Genus Cacopsylla (Hemiptera, Psylloidea). Insects 2021, 12, 853. https://doi.org/10.3390/insects12100853
Shapoval NA, Nokkala S, Nokkala C, Kuftina GN, Kuznetsova VG. The Incidence of Wolbachia Bacterial Endosymbiont in Bisexual and Parthenogenetic Populations of the Psyllid Genus Cacopsylla (Hemiptera, Psylloidea). Insects. 2021; 12(10):853. https://doi.org/10.3390/insects12100853
Chicago/Turabian StyleShapoval, Nazar A., Seppo Nokkala, Christina Nokkala, Galina N. Kuftina, and Valentina G. Kuznetsova. 2021. "The Incidence of Wolbachia Bacterial Endosymbiont in Bisexual and Parthenogenetic Populations of the Psyllid Genus Cacopsylla (Hemiptera, Psylloidea)" Insects 12, no. 10: 853. https://doi.org/10.3390/insects12100853
APA StyleShapoval, N. A., Nokkala, S., Nokkala, C., Kuftina, G. N., & Kuznetsova, V. G. (2021). The Incidence of Wolbachia Bacterial Endosymbiont in Bisexual and Parthenogenetic Populations of the Psyllid Genus Cacopsylla (Hemiptera, Psylloidea). Insects, 12(10), 853. https://doi.org/10.3390/insects12100853







