Time of Test Periods Influence the Behavioral Responses of Anopheles minimus and Anopheles dirus (Diptera: Culicidae) to DEET
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.1.1. Anopheles minimus
2.1.2. Anopheles dirus
2.2. Repellent-Treated Paper
2.3. Behavioral Tests
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Malaria Information and Prophylaxis, by Country. 2020. Available online: https://www.cdc.gov/malaria/travelers/country_table/t.html (accessed on 12 July 2021).
- Sriwichai, P.; Karl, S.; Samung, Y.; Kiattibutr, K.; Sirichaisinthop, J.; Mueller, I.; Cui, L.; Sattabongkot, J. Imported Plasmodium falciparum and locally transmitted Plasmodium vivax: Cross-border malaria transmission scenario in northwestern Thailand. Malar. J. 2017, 16, 258. [Google Scholar] [CrossRef] [Green Version]
- Parker, D.M.; Carrara, V.I.; Pukrittayakamee, S.; McGready, R.; Nosten, F.H. Malaria ecology along the Thailand–Myanmar border. Malar. J. 2015, 14, 388. [Google Scholar] [CrossRef] [Green Version]
- Tainchum, K.; Kongmee, M.; Manguin, S.; Bangs, M.J.; Chareonviriyaphap, T. Anopheles species diversity and distribution of the malaria vectors of Thailand. Trends Parasitol. 2015, 31, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, H.J.; Ekbom, B.; Suwonkerd, W.; Takagi, M. Effect of landscape structure on anopheline mosquito density and diversity in northern Thailand: Implications for malaria transmission and control. Landsc. Ecol. 2003, 18, 605. [Google Scholar] [CrossRef]
- Tainchum, K.; Ritthison, W.; Chuaycharoensuk, T.; Bangs, M.J.; Manguin, S.; Chareonviriyaphap, T. Diversity of Anopheles species and trophic behavior of putative malaria vectors in two malaria endemic areas of northwestern Thailand. J. Vector Ecol. 2014, 39, 424–436. [Google Scholar] [CrossRef]
- Curtis, C.; Lines, J.D.; Ijumba, J.; Callaghan, A.; Hill, N.; Karimzad, M.A. The relative efficacy of repellents against mosquito vectors of disease. Med. Vet. Entomol. 1987, 1, 109–119. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rutledge, L.C. Role of repellents in vector control and disease prevention. Am. J. Trop. Med. Hyg. 1994, 50, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Debboun, M.; Strickman, D. Insect repellents and associated personal protection for a reduction in human disease. Med. Vet. Entomol. 2013, 27, 1–9. [Google Scholar] [CrossRef]
- Lupi, E.; Hatz, C.; Schlagenhauf, P. The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp.—A literature review. Travel. Med. Infect. Dis. 2013, 11, 374–411. [Google Scholar] [CrossRef]
- Ditzen, M.; Pellegrino, M.; Vosshall, L.B. Insect odorant receptors are molecular targets of the insect repellent DEET. Science 2008, 319, 1838–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, E.E.; Sokolove, P.G. Lactic acid-sensitive receptors on the antennae of the mosquito, Aedes aegypti. J. Comp. Physiol. A Neuroethol. 1976, 105, 43–54. [Google Scholar] [CrossRef]
- Corbel, V.; Stankiewicz, M.; Pennetier, C.; Fournier, D.; Stojan, J.; Girard, E.; Dimitrov, M.; Molgó, J.; Hougard, J.-M.; Lapied, B. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent deet. BMC Biol. 2009, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieco, J.P.; Achee, N.L.; Sardelis, M.R.; Chauhan, K.R.; Roberts, D.R. A novel high-throughput screening system to evaluate the behavioral response of adult mosquitoes to chemicals. J. Am. Mosq. Control Assoc. 2005, 21, 404–411. [Google Scholar] [CrossRef]
- Barnard, D.R.; Xue, R.-D. Laboratory evaluation of mosquito repellents against Aedes albopictus, Culex nigripalpus, and Ochlerotatus triseriatus (Diptera: Culicidae). J. Med. Entomol. 2004, 41, 726–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kongmee, M.; Prabaripai, A.; Akratanakul, P.; Bangs, M.J.; Chareonviriyaphap, T. Behavioral responses of Aedes aegypti (Diptera: Culicidae) exposed to deltamethrin and possible implications for disease control. J. Med. Entomol. 2004, 41, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Chareonviriyaphap, T.; Prabaripai, A.; Bangs, M.J. Excito-repellency of deltamethrin on the malaria vectors, Anopheles minimus, Anopheles dirus, Anopheles swadiwongporni, and Anopheles maculatus, in Thailand. J. Am. Mosq. Control Assoc. 2004, 20, 45–54. [Google Scholar] [PubMed]
- Pothikasikorn, J.; Overgaard, H.; Ketavan, C.; Visetson, S.; Bangs, M.J.; Chareonviriyaphap, T. Behavioral responses of malaria vectors, Anopheles minimus complex, to three classes of agrochemicals in Thailand. J. Med. Entomol. 2007, 44, 1032–1039. [Google Scholar] [CrossRef]
- Polsomboon, S.; Grieco, J.P.; Achee, N.L.; Chauhan, K.R.; Tanasinchayakul, S.; Pothikasikorn, J.; Chareonviriyaphap, T. Behavioral responses of catnip (Nepeta cataria) by two species of mosquitoes, Aedes aegypti and Anopheles harrisoni, in Thailand. J. Am. Mosq. Control Assoc. 2008, 24, 513–519. [Google Scholar] [CrossRef]
- Sathantriphop, S.; White, S.A.; Achee, N.L.; Sanguanpong, U.; Chareonviriyaphap, T. Behavioral responses of Aedes aegypti, Aedes albopictus, Culex quinquefasciatus, and Anopheles minimus against various synthetic and natural repellent compounds. J. Vector Ecol. 2014, 39, 328–339. [Google Scholar] [CrossRef]
- Nararak, J.; Sathantriphop, S.; Chauhan, K.; Tantakom, S.; Eiden, A.L.; Chareonviriyaphap, T. Avoidance behavior to essential oils by Anopheles minimus, a malaria vector in Thailand. J. Am. Mosq. Control Assoc. 2016, 32, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Boonyuan, W.; Sathantriphop, S.; Tainchum, K.; Muenworn, V.; Prabaripai, A.; Bangs, M.J.; Chareonviriyaphap, T. Insecticidal and behavioral avoidance responses of Anopheles minimus and Culex quinquefasciatus (Diptera: Culicidae) to three synthetic repellents. J. Med. Entomol. 2017, 54, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Tisgratog, R.; Tananchai, C.; Bangs, M.J.; Tainchum, K.; Juntarajumnong, W.; Prabaripai, A.; Chauhan, K.R.; Pothikasikorn, J.; Chareonviriyaphap, T. Chemically induced behavioral responses in Anopheles minimus and Anopheles harrisoni in Thailand. J. Vector Ecol. 2011, 36, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Tainchum, K.; Ritthison, W.; Sathantriphop, S.; Tanasilchayakul, S.; Manguin, S.; Bangs, M.J.; Chareonviriyaphap, T. Influence of time of assay on behavioral responses of laboratory and field populations Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) to DEET. J. Med. Entomol. 2014, 51, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Choomsang, I.; Nararak, J.; Bangs, M.J.; Chareonviriyaphap, T. Diurnal test periods influence behavioral responses of Aedes aegypti (Diptera: Culicidae) to repellents. J. Asia Pac. Entomol. 2018, 21, 971–983. [Google Scholar] [CrossRef]
- Gilbert, I.; Gouck, H. Evaluation of repellents against mosquitoes in Panama. Fla. Entomol. 1955, 38, 153–163. [Google Scholar] [CrossRef]
- Logan, J.G.; Stanczyk, N.M.; Hassanali, A.; Kemei, J.; Santana, A.E.; Ribeiro, K.A.; Pickett, J.A.; Mordue, A.J. Arm-in-cage testing of natural human-derived mosquito repellents. Malar. J. 2010, 9, 239. [Google Scholar] [CrossRef] [Green Version]
- Phasomkusolsil, S.; Tawong, J.; Monkanna, N.; Pantuwatana, K.; Damdangdee, N.; Khongtak, W.; Kertmanee, Y.; Evans, B.P.; Schuster, A.L. Maintenance of mosquito vectors: Effects of blood source on feeding, survival, fecundity, and egg hatching rates. J. Vector Ecol. 2013, 38, 38–45. [Google Scholar] [CrossRef]
- Lardeux, F.; Loayza, P.; Bouchité, B.; Chavez, T. Host choice and human blood index of Anopheles pseudopunctipennis in a village of the Andean valleys of Bolivia. Malar. J. 2007, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Robbins, P.J.; Cherniack, M.G. Review of the biodistribution and toxicity of the insect repellent N, N-diethyl-m-toluamide (DEET). J. Toxicol. Environ. Health A Curr. Issues 1986, 18, 503–525. [Google Scholar] [CrossRef]
- Dogan, E.B.; Rossignol, P.A. An olfactometer for discriminating between attraction, inhibition, and repellency in mosquitoes (Diptera: Culicidae). J. Med. Entomol. 1999, 36, 788–793. [Google Scholar] [CrossRef]
- Mehr, Z.; Rutledge, L.; Buescher, M.; Gupta, R.K.; Zakaria, M. Attraction of mosquitoes to diethyl methylbenzamide and ethyl hexanediol. J. Am. Mosq. Control Assoc. 1990, 6, 469–476. [Google Scholar]
- Chareonviriyaphap, T.; Prabaripai, A.; Sungvornyothrin, S. An improved excito-repellency test chamber for mosquito behavioral tests. J. Vector Ecol. 2002, 27, 250–252. [Google Scholar] [PubMed]
- Roberts, D.R.; Chareonviriyaphap, T.; Harlan, H.H.; Hshieh, P. Methods of testing and analyzing excito-repellency responses of malaria vectors to insecticides. J. Am. Mosq. Control Assoc. 1997, 13, 13–17. [Google Scholar] [PubMed]
- WHO. Guidelines for Testing Mosquito Adulticides for Indoor Residual Spraying and Treatment of Mosquito Nets. 2006. Available online: http://apps.who.int/iris/bitstream/handle/10665/69296/WHO_CDS_NTD_WHOPES_GCDPP_2006.3_eng.pdf?sequence=1 (accessed on 6 October 2020).
- Chareonviriyaphap, T.; Roberts, D.R.; Andre, R.G.; Harlan, H.J.; Manguin, S.; Bangs, M.J. Pesticide avoidance behavioral in Anopheles albimanus, a malaria vector in the Americas. J. Am. Most. Control Assoc. 1997, 13, 171–183. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Henderson, C.F.; Tilton, E.W. Tests with acaricides against the brow wheat mite. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]
- Sathantriphop, S.; Kongmee, M.; Tainchum, K.; Suwansirisilp, K.; Sanguanpong, U.; Bangs, M.J.; Chareonviriyaphap, T. Comparison of field and laboratory-based tests for behavioral response of Aedes aegypti (Diptera: Culicidae) to repellents. J. Econ. Entomol. 2015, 108, 2770–2778. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Takken, W.; Snellen, W.; Verhave, J.; Knols, B.; Atmosoedjono, S. Environmental Measures for Malaria Control in Indonesia—A Historical Review on Species Sanitation; Agricultural University Wageningen: Wageningen, The Netherlands, 1990; 167p. [Google Scholar]
- Ndoen, E.; Wild, C.; Dale, P.; Sipe, N.; Dale, M. Dusk to dawn activity patterns of anopheline mosquitoes in West Timor and Java, Indonesia. Southeast Asian J. Trop. Med. Public Health 2011, 42, 550–561. [Google Scholar]
- Tisgratog, R.; Tananchai, C.; Juntarajumnong, W.; Tuntakom, S.; Bangs, M.J.; Corbel, V.; Chareonviriyaphap, T. Host feeding patterns and preference of Anopheles minimus (Diptera: Culicidae) in a malaria endemic area of western Thailand: Baseline site description. Parasit. Vectors 2012, 5, 114. [Google Scholar] [CrossRef] [Green Version]
- Manguin, S.; Garros, C.; Dusfour, I.; Harbach, R.; Coosemans, M. Bionomics, taxonomy, and distribution of the major malaria vector taxa of Anopheles subgenus Cellia in Southeast Asia: An updated review. Infect. Genet. Evol. 2008, 8, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Baimai, V.; Green, C.; Andre, R.; Harrison, B.; Peyton, E. Cytogenetic studies of some species complexes of Anopheles in Thailand and Southeast Asia. Southeast Asian J. Trop. Med. Public Health 1984, 15, 536–546. [Google Scholar] [PubMed]
- Baimai, V. Population cytogenetics of the malaria vector Anopheles leucosphyrus group. Southeast Asian J. Trop. Med. Public Health 1988, 19, 667–680. [Google Scholar]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Chareonviriyaphap, T.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Elyazar, I.R.; Kabaria, C.W.; Harbach, R.E. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic précis. Parasit. Vectors 2011, 4, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tananchai, C.; Tisgratog, R.; Juntarajumnong, W.; Grieco, J.P.; Manguin, S.; Prabaripai, A.; Chareonviriyaphap, T. Species diversity and biting activity of Anopheles dirus and Anopheles baimaii (Diptera: Culicidae) in a malaria prone area of western Thailand. Parasit. Vectors 2012, 5, 211. [Google Scholar] [CrossRef] [Green Version]
- Rund, S.S.; Lee, S.J.; Bush, B.R.; Duffield, G.E. Strain-and sex-specific differences in daily flight activity and the circadian clock of Anopheles gambiae mosquitoes. J. Insect Physiol. 2012, 58, 1609–1619. [Google Scholar] [CrossRef]
- Rund, S.S.C.; Hou, T.Y.; Ward, S.M.; Collins, F.H.; Duffield, G.E. Genome-wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2011, 108, E421–E430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
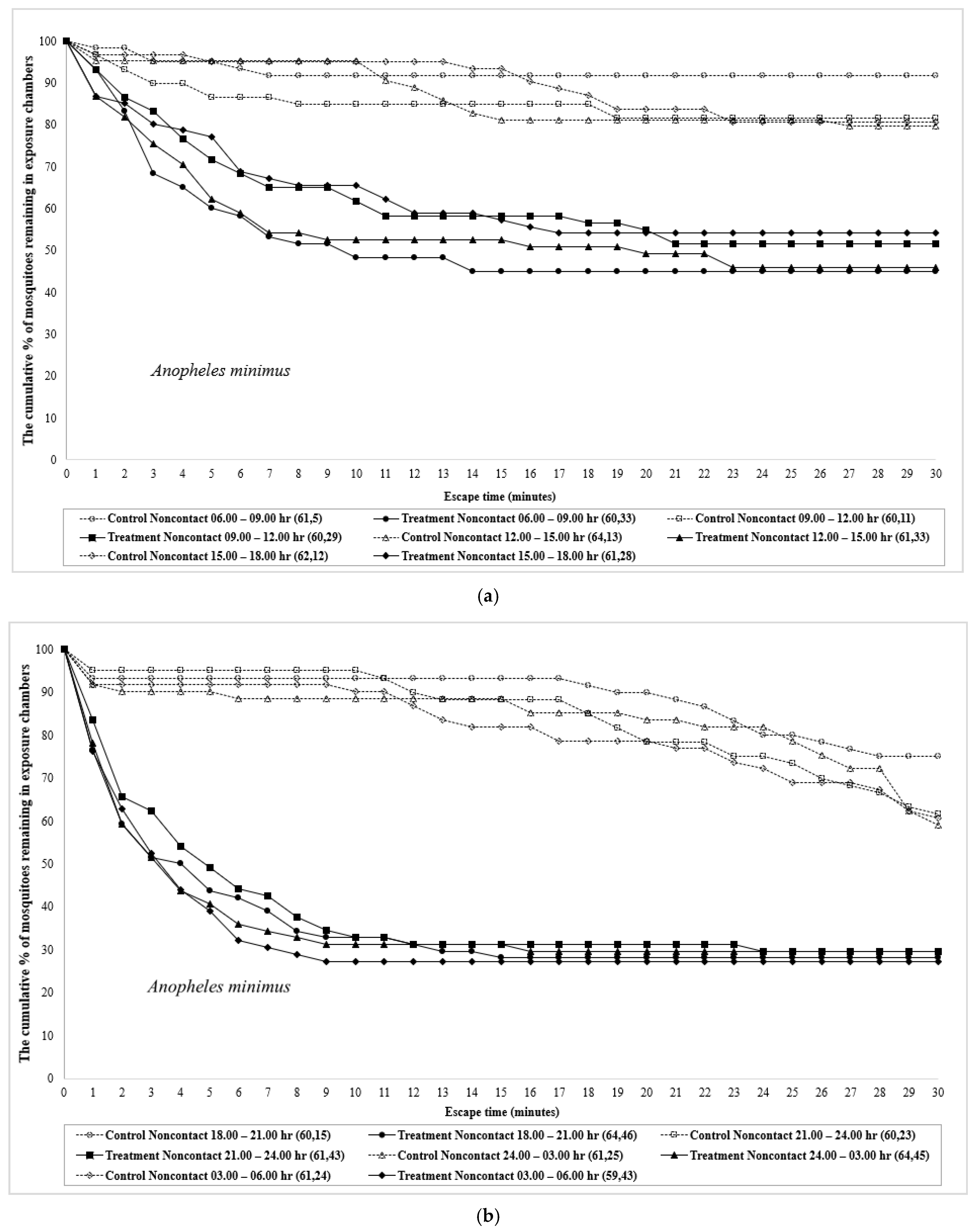
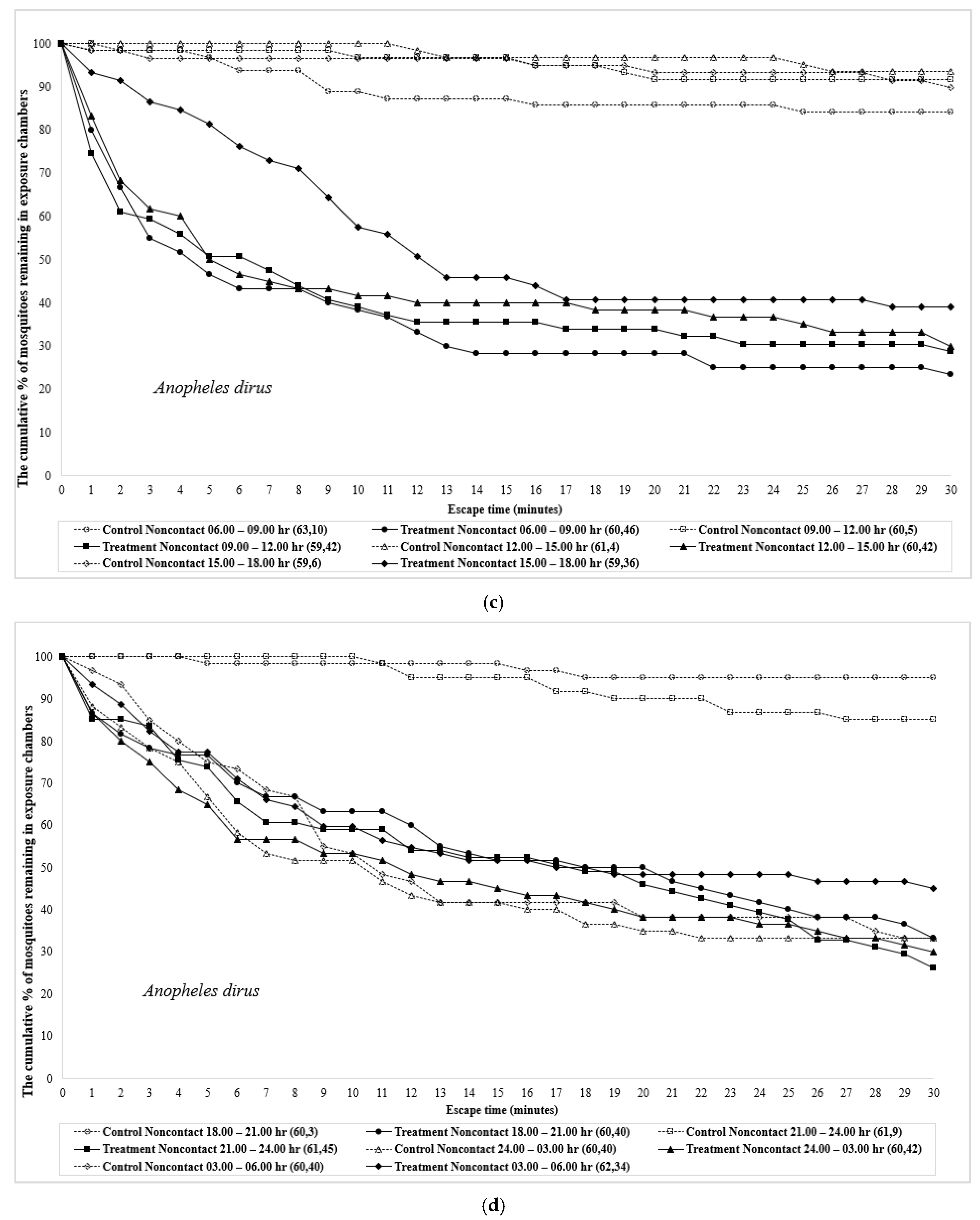
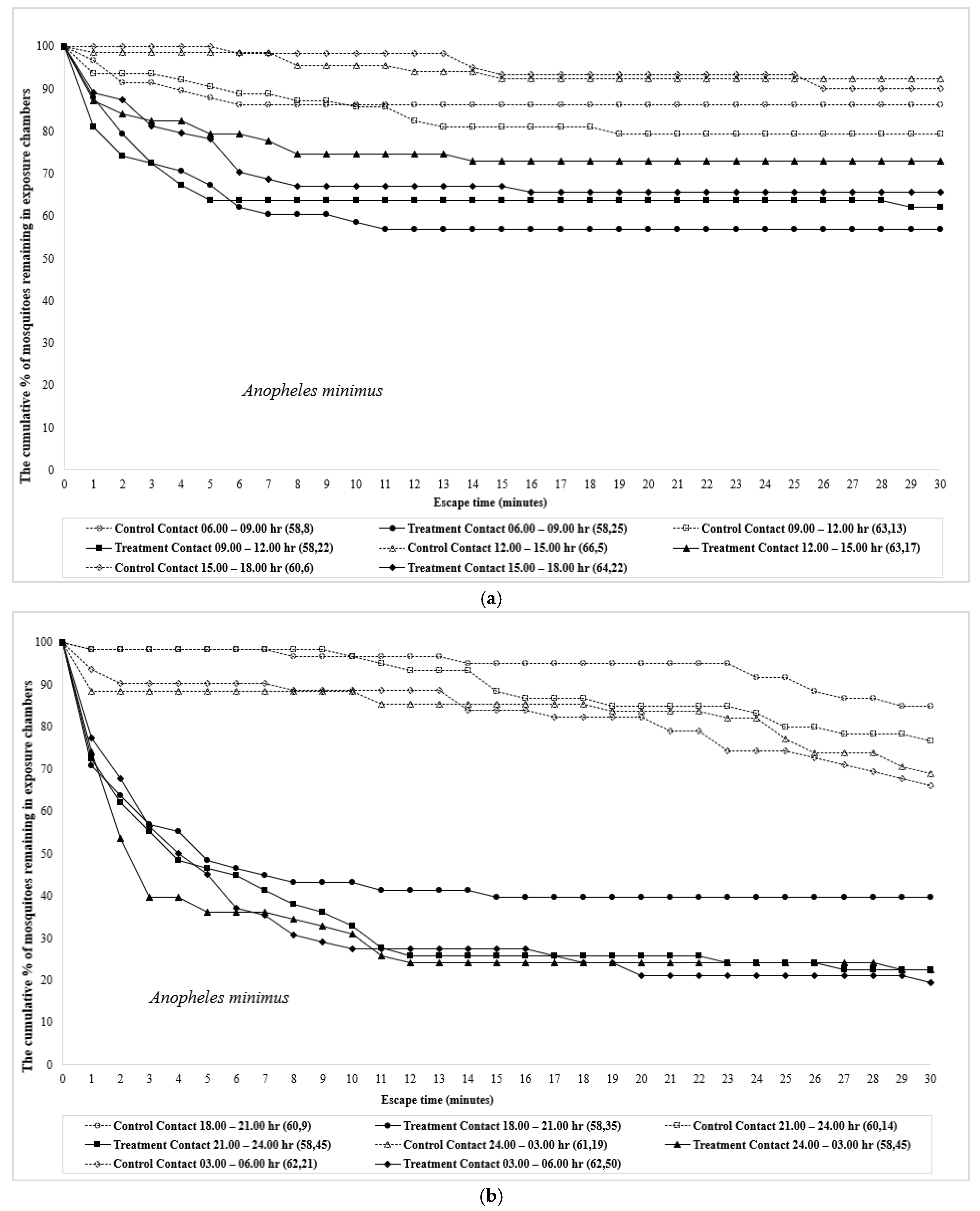
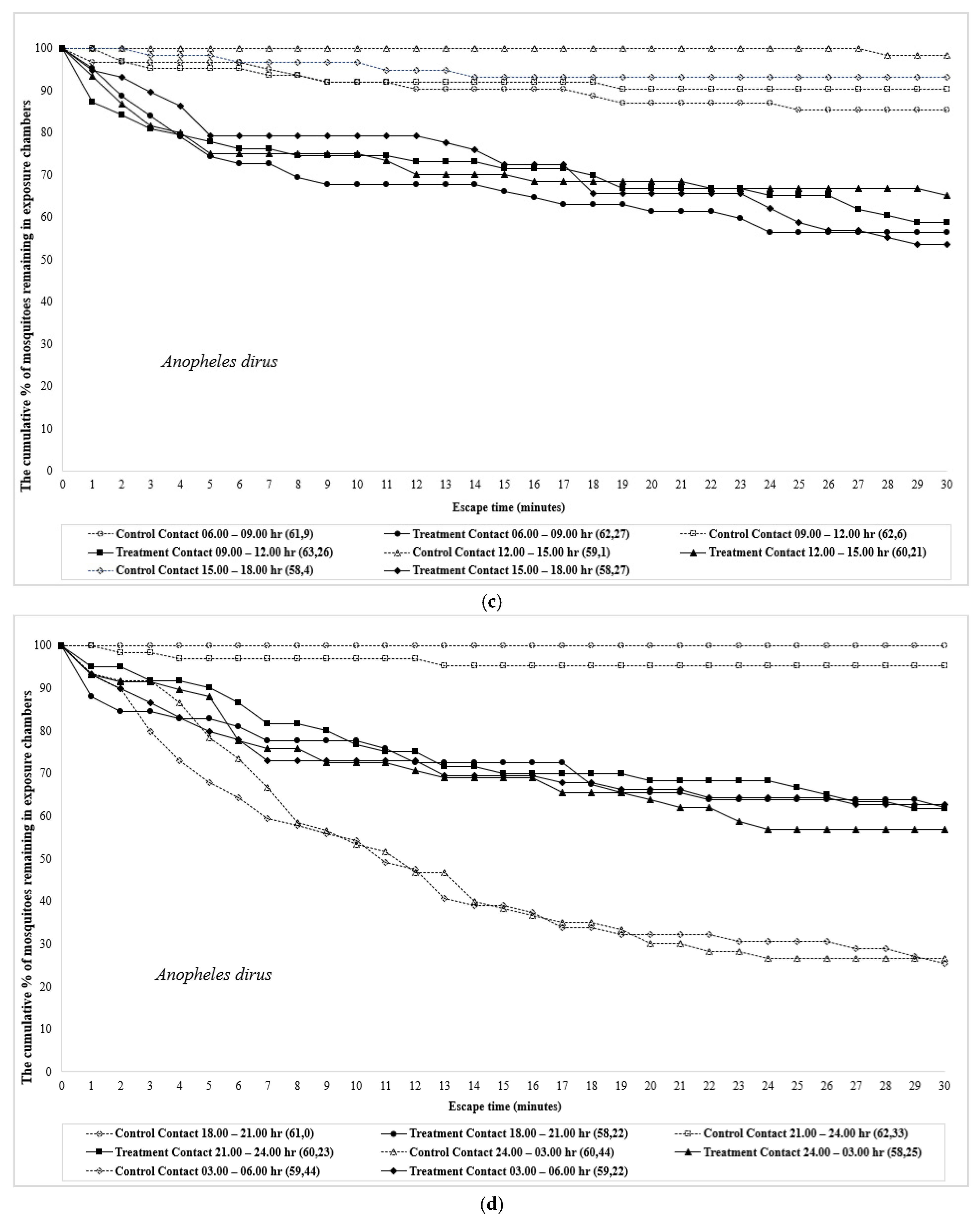
| Time | Species | Test Design | No. Mosquitoes | Percent Escape (Mean % Escape ± SE) [a] | Corrected % Escape b | % Knockdown | % Mortality | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DEET | Control | DEET | Control | E | R | E | R | ||||
| Day | Anopheles minimus | Noncontact | 61 | 62 | 52.46 (13.12 ± 0.24) | 16.13 | 43.56 | 6.56 | 39.34 | 6.56 | 13.11 |
| Contact | 62 | 64 | 22.58 (5.65 ± 0.26) [0] a | 15.63 | 8.24 | 9.68 | 77.42 | 1.61 | 53.22 | ||
| Anopheles dirus | Noncontact | 57 | 62 | 84.21 (21.05 ± 0.78) | 11.29 | 82.20 | 3.51 | 15.79 | 7.02 | 1.75 | |
| Contact | 60 | 61 | 28.33 (7.08 ± 0.57) [0] a | 6.56 | 23.30 | 5.00 | 38.33 | 1.67 | 45.00 | ||
| Night | Anopheles minimus | Noncontact | 68 | 60 | 69.12 (17.28 ± 0.37) | 38.33 | 49.93 | 5.88 | 14.70 | 1.47 | 7.35 |
| Contact | 57 | 60 | 66.67 (16.67 ± 0.48) [0] a | 26.67 | 54.55 | 0 | 10.53 | 0 | 8.77 | ||
| Anopheles dirus | Noncontact | 63 | 61 | 63.49 (15.87 ± 0.33) | 21.31 | 53.60 | 7.93 | 11.11 | 1.59 | 1.59 | |
| Contact | 58 | 59 | 39.66 (9.92 ± 0.28) [0] a | 35.59 | 6.32 | 10.34 | 44.82 | 8.62 | 25.86 | ||
| Time | Test Design | Time Period | No. Mosquitoes | Percent Escape (Mean % Escape ± SE) [a] | Corrected % Escape b | % Knockdown | % Mortality | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DEET | Control | DEET | Control | E | R | E | R | ||||
| Day | Noncontact | 06.00–09.00 | 60 | 61 | 55.00 (13.75 ± 0.27) | 8.20 | 50.98 | 11.67 | 41.67 | 1.67 | 6.67 |
| 09.00–12.00 | 60 | 60 | 48.33 (12.08 ± 0.51) | 18.33 | 36.73 | 3.33 | 35.00 | 6.67 | 8.33 | ||
| 12.00–15.00 | 61 | 64 | 54.10 (13.53 ± 0.20) | 20.31 | 42.40 | 11.47 | 40.98 | 0 | 16.39 | ||
| 15.00–18.00 | 61 | 62 | 45.90 (11.48 ± 0.45) | 19.35 | 32.92 | 11.47 | 45.90 | 0 | 14.75 | ||
| Contact | 06.00–09.00 | 58 | 58 | 43.10 (10.78 ± 0.39) [0] a | 13.79 | 34.00 | 13.79 | 56.90 | 6.89 | 50.00 | |
| 09.00–12.00 | 58 | 63 | 37.93 (9.48 ± 0.57) [0] a | 20.63 | 21.80 | 27.58 | 62.07 | 22.41 | 39.65 | ||
| 12.00–15.00 | 63 | 66 | 26.98 (6.75 ± 0.25) [0] a | 7.58 | 20.99 | 22.22 | 73.01 | 3.17 | 73.01 | ||
| 15.00–18.00 | 64 | 60 | 34.38 (8.60 ± 0.52) [0] a | 10.00 | 27.09 | 21.87 | 65.62 | 18.75 | 53.13 | ||
| Night | Noncontact | 18:00–21:00 | 64 | 60 | 71.88 (17.97 ± 0.41) | 25.00 | 62.51 | 5.68 | 25 | 0 | 18.75 |
| 21:00–24:00 | 61 | 60 | 70.49 (17.62 ± 0.36) | 38.33 | 52.15 | 0 | 0 | 0 | 9.83 | ||
| 24:00–03:00 | 64 | 61 | 70.31 (17.58 ± 0.70) | 40.98 | 49.70 | 7.81 | 17.19 | 3.12 | 17.18 | ||
| 03:00–06:00 | 59 | 61 | 72.88 (18.22 ± 0.87) | 39.34 | 55.29 | 3.39 | 23.73 | 0 | 8.47 | ||
| Contact | 18:00–21:00 | 58 | 60 | 60.34 (15.09 ± 0.47) [0] a | 15.00 | 53.34 | 5.17 | 27.59 | 1.72 | 18.95 | |
| 21:00–24:00 | 58 | 60 | 77.59 (19.40 ± 0.34) [7.09] a | 23.33 | 70.77 | 0 | 0 | 0 | 6.77 | ||
| 24:00–03:00 | 58 | 61 | 77.59 (19.40 ± 0.60) [7.27] a | 31.15 | 67.45 | 6.90 | 17.24 | 6.89 | 10.34 | ||
| 03:00–06:00 | 62 | 62 | 80.65 (20.16 ± 0.52) [7.76] a | 33.87 | 70.74 | 1.61 | 16.13 | 0 | 11.29 | ||
| Time | Test Design | Time Period | No. Mosquitoes | Percent Escape (Mean % Escape ± SE) [a] | Corrected % Escape b | % Knockdown | % Mortality | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DEET | Control | DEET | Control | E | R | E | R | ||||
| Day | Noncontact | 06.00–09.00 | 60 | 63 | 76.67 (19.17 ± 0.37) | 15.87 | 72.27 | 0 | 1.67 | 6.67 | 0 |
| 09.00–12.00 | 59 | 60 | 71.19 (17.80 ± 0.38) | 8.33 | 68.57 | 0 | 1.67 | 0 | 1.67 | ||
| 12.00–15.00 | 60 | 61 | 70.00 (17.50 ± 0.57) | 6.56 | 67.89 | 0 | 5.00 | 0 | 1.67 | ||
| 15.00–18.00 | 59 | 59 | 61.02 (15.26 ± 0.36) | 10.17 | 56.61 | 3.39 | 38.98 | 0 | 3.39 | ||
| Contact | 06.00–09.00 | 62 | 61 | 43.55 (10.89 ± 0.68) [0] a | 14.75 | 33.78 | 6.45 | 22.58 | 0 | 24.19 | |
| 09.00–12.00 | 63 | 62 | 41.27 (10.32 ± 0.84) [0] a | 9.68 | 34.98 | 7.93 | 42.86 | 1.58 | 26.98 | ||
| 12.00–15.00 | 60 | 59 | 35.00 (8.75 ± 0.32) [0] a | 1.69 | 33.88 | 0 | 30.00 | 0 | 8.33 | ||
| 15.00–18.00 | 58 | 58 | 46.55 (11.64 ± 0.72) [0] a | 6.90 | 42.59 | 13.79 | 41.38 | 0 | 22.41 | ||
| Night | Noncontact | 18:00–21:00 | 60 | 60 | 66.67 (16.67 ± 0.63) | 5.00 | 64.92 | 11.67 | 11.67 | 1.67 | 11.67 |
| 21:00–24:00 | 61 | 61 | 73.77 (18.44 ± 0.63) | 14.75 | 69.23 | 0 | 9.83 | 0 | 1.64 | ||
| 24:00–03:00 | 60 | 60 | 70.00 (17.50 ± 0.51) | 66.67 | 9.99 | 10.00 | 23.33 | 3.33 | 11.67 | ||
| 03:00–06:00 | 62 | 60 | 54.84 (13.71 ± 0.70) | 66.67 | N/A | 11.29 | 4.84 | 0 | 0 | ||
| Contact | 18:00–21:00 | 58 | 61 | 37.93 (9.48 ± 0.54) [0] a | 0 | 37.93 | 12.07 | 48.26 | 3.45 | 10.34 | |
| 21:00–24:00 | 60 | 62 | 38.33 (9.58 ± 0.27) [0] a | 4.84 | 35.19 | 15.00 | 40.00 | 8.33 | 31.67 | ||
| 24:00–03:00 | 58 | 60 | 43.10 (10.78 ± 0.47) [0] a | 73.33 | N/A | 12.07 | 55.17 | 15.52 | 31.03 | ||
| 03:00–06:00 | 59 | 59 | 37.29 (9.32 ± 0.64) [0] a | 74.58 | N/A | 6.78 | 28.81 | 1.69 | 6.78 | ||
| Mosq. Species | Test Conditions | p Value of Time Period Comparisons (Chi-Square Value) | |||||
|---|---|---|---|---|---|---|---|
| D1 vs. D2 | D1 vs. D3 | D1 vs. N4 | D2 vs. D3 | D2 vs. D4 | D3 vs. D4 | ||
| Anopheles minimus | noncontact control | 0.1018 (2.6778) | 0.0662 (3.3747) | 0.0888 (2.8966) | 0.8507 (0.0354) | 0.9746 (0.0010) | 0.8015 (0.0632) |
| noncontact treatment | 0.3317 (0.9421) | 0.8850 (0.0209) | 0.2504 (1.3213) | 0.4253 (0.6356) | 0.8296 (0.0463) | 0.3168 (1.0019) | |
| contact control | 0.3620 (0.8310) | 0.2360 (1.4045) | 0.4552 (0.5577) | 0.0307* (4.6702) | 0.0815 (3.0353) | 0.6638 (0.1890) | |
| contact treatment | 0.7113 (0.1370) | 0.0698 (3.2866) | 0.2873 (1.1320) | 0.1777 (1.8164) | 0.5369 (0.3813) | 0.4033 (0.6984) | |
| Anopheles dirus | noncontact control | 0.1907 (1.7121) | 0.0894 (2.8846) | <0.0001 * (28.3614) | 0.6939 (0.1549) | <0.0001 * (48.0177) | <0.0001 * (61.6943) |
| noncontact treatment | 0.6513 (0.2043) | 0.3907 (0.7368) | 0.0046 * (8.0413) | 0.7344 (0.1151) | 0.0278 * (4.8387) | 0.0519 (3.7785) | |
| contact control | 0.4073 (0.1084) | 0.0093 * (4.8862) | 0.1771 (0.7715) | 0.0587 (3.5724) | 0.5717 (0.3198) | 0.1612 (1.9626) | |
| contact treatment | 0.7834 (0.0756) | 0.4031 (0.6990) | 0.9979 (0.0000) | 0.5305 (0.3935) | 0.6979 (0.1510) | 0.3618 (0.8318) | |
| Mosq. Species | Test Conditions | p Value of Time Period Comparisons (Chi-Square Value) | |||||
|---|---|---|---|---|---|---|---|
| N1 vs. N2 | N1 vs. N3 | N1 vs. N4 | N2 vs. N3 | N2 vs. N4 | N3 vs. N4 | ||
| Anopheles minimus | noncontact control | 0.1258 (2.3436) | 0.0946 (2.7945) | 0.0924 (2.8317) | 0.8914 (0.0186) | 0.8515 (0.0350) | 0.9809 (0.0006) |
| noncontact treatment | 0.6623 (0.1907) | 0.9821 (0.0005) | 0.7903 (0.0707) | 0.6444 (0.2131) | 0.4541 (0.5603) | 0.8174 (0.0533) | |
| contact control | 0.2312 (1.4337) | 0.0320 * (4.5962) | 0.0128 * (6.1964) | 0.3364 (0.9239) | 0.1919 (1.7029) | 0. 7393 (0.1108) | |
| contact treatment | 0.1346 (2.2382) | 0.0835 (2.9961) | 0.0855 (2.9568) | 0.7596 (0.0937) | 0.7730 (0.0832) | 0.8484 (0.0365) | |
| Anopheles dirus | noncontact control | 0.0793 (3.9578) | <0.0001 * (102.4690) | <0.0001 * (101.6812) | <0.0001 * (41.3270) | <0.0001 * (40.0028) | 0.6367 (0.2215) |
| noncontact treatment | 0.4934 (0.4691) | 0.4759 (0.5083) | 0.3346 (0.9311) | 0.9248 (0.0089) | 0.1002 (2.7031) | 0.0986 (0.7285) | |
| contact control | 0.0832 (3.0004) | <0.0001 * (74.5956) | <0.0001 * (76.9401) | <0.0001 * (62.2740) | <0.0001 * (64.9718) | 0.7145 (0.1338) | |
| contact treatment | 0.2099 (0.0051) | 0.0672 (0.2218) | 0.2139 (0.0019) | 0.5440 (0.3682) | 0.9291 (0.0079) | 0.6470 (0.2097) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tisgratog, R.; Sukkanon, C.; Sugiharto, V.A.; Bangs, M.J.; Chareonviriyaphap, T. Time of Test Periods Influence the Behavioral Responses of Anopheles minimus and Anopheles dirus (Diptera: Culicidae) to DEET. Insects 2021, 12, 867. https://doi.org/10.3390/insects12100867
Tisgratog R, Sukkanon C, Sugiharto VA, Bangs MJ, Chareonviriyaphap T. Time of Test Periods Influence the Behavioral Responses of Anopheles minimus and Anopheles dirus (Diptera: Culicidae) to DEET. Insects. 2021; 12(10):867. https://doi.org/10.3390/insects12100867
Chicago/Turabian StyleTisgratog, Rungarun, Chutipong Sukkanon, Victor Arief Sugiharto, Michael J. Bangs, and Theeraphap Chareonviriyaphap. 2021. "Time of Test Periods Influence the Behavioral Responses of Anopheles minimus and Anopheles dirus (Diptera: Culicidae) to DEET" Insects 12, no. 10: 867. https://doi.org/10.3390/insects12100867





