Andean Plants Essential Oils: A Scented Alternative to Synthetic Insecticides for the Control of Blowflies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Chemical Analyses of the Essential Oils
2.3. Sensory Analysis of the Essential Oils
2.4. Rearing of Calliphora Vomitoria
2.5. Behavioural Assay
2.6. Toxicity Bioassays
2.7. Data Analysis
3. Results
3.1. Chemical Composition of the Essential Oils
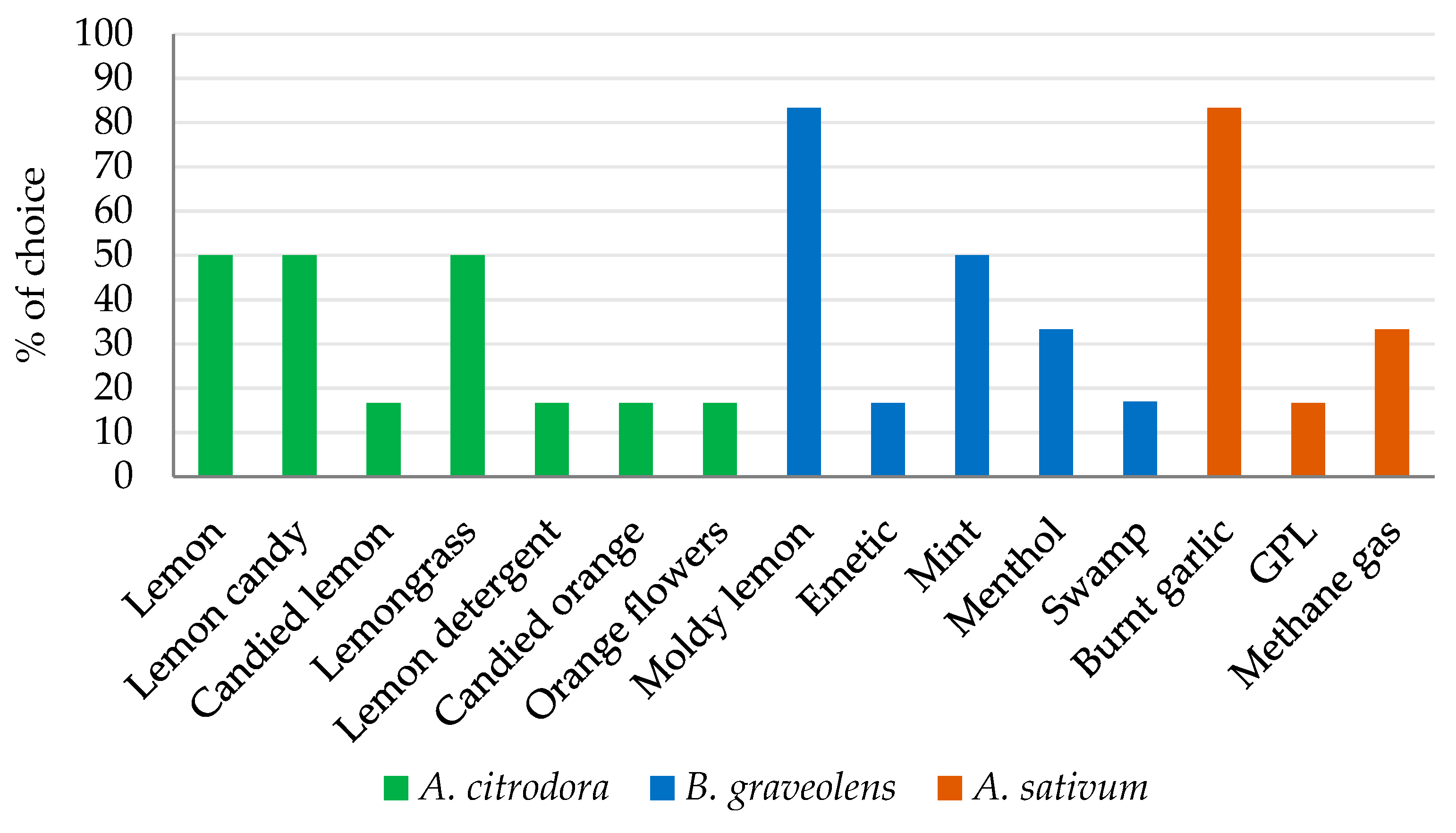
3.2. Sensory Profiles of the Essential Oils
3.3. Behavioral Response of the C. vomitoria Adults to the Essential Oils
3.4. Toxicity of the Essential Oils on C. vomitoria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. An initial study of insect succession and carrion decomposition in various forest habitats of Central Europe. Forensic Sci. Int. 2008, 180, 61–69. [Google Scholar] [CrossRef]
- Bonacci, T.; Brandmayr, P.; Greco, S.; Tersaruolo, C.; Vercillo, V.; Zetto Brandmayr, T. A preliminary investigation of insect succession on carrion in Calabria (southern Italy). Terr. Arthropod Rev. 2010, 3, 97–110. [Google Scholar] [CrossRef]
- Prado e Castro, C.; Paulo Sousa, J.; Arnaldos, M.I.; Gaspar, J.; García, M.D. Blowflies (Diptera: Calliphoridae) activity in sun exposed and shaded carrion in Portugal. Ann. Soc. Entomol. Fr. 2011, 47, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Prado e Castro, C.; Serrano, A.; Martins da Silva, P.; García, M.D. Carrion flies of forensic interest: A study of seasonal community composition and succession in Lisbon, Portugal. Med. Vet. Entomol. 2012, 26, 417–431. [Google Scholar] [CrossRef]
- Iancu, L.; Carter, D.O.; Junkins, E.N.; Purcarea, C. Using bacterial and necrophagous insect dynamics for post-mortem interval estimation during cold season: Novel case study in Romania. Forensic Sci. Int. 2015, 254, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Naqqash, M.N.; Jaleel, W.; Saeed, Q.; Ghouri, F. The effect of blow flies (Diptera: Calliphoridae) on the size and weight of mangos (Mangifera indica L.). PeerJ 2016, 4, e2076. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.F.; Voss, S.C.; Finch, J.T.D.; Rader, R.C.; Cook, J.M.; Spurr, C.J. The role of flies as pollinators of horticultural crops: An Australian case study with worldwide relevance. Insects 2020, 11, 341. [Google Scholar] [CrossRef]
- Rader, R.; Cunningham, S.A.; Howlett, B.G.; Inouye, D.W. Non-bee insects as visitors and pollinators of crops: Biology, ecology, and management. Ann. Rev. Entomol. 2020, 65, 391–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förster, M.; Klimpel, S.; Mehlhorn, H.; Sievert, K.; Messler, S.; Pfeffer, K. Pilot study on synanthropic flies (e.g., Musca, Sarcophaga, Calliphora, Fannia, Lucilia, Stomoxys) as vectors of pathogenic microorganisms. Parasitol. Res. 2007, 101, 243–246. [Google Scholar] [CrossRef] [PubMed]
- De Blackburn, C.; McClure, P.J. Foodborne Pathogens: Hazards, Risk Analysis and Control, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2009; p. 1232. [Google Scholar]
- Junqueira, A.C.M.; Ratan, A.; Acerbi, E.; Drautz-Moses, D.I.; Premkrishnan, B.N.V.; Costea, P.I.; Linz, B.; Purbojati, R.W.; Paulo, D.F.; Gaultier, N.E.; et al. The microbiomes of blowflies and houseflies as bacterial transmission reservoirs. Sci. Rep. 2017, 7, 16324. [Google Scholar] [CrossRef] [PubMed]
- Blazar, J.; Allard, M.; Lienau, E.K. Insects as vectors of foodborne pathogenic bacteria. Terr. Arthropod Rev. 2011, 4, 5–16. [Google Scholar] [CrossRef]
- Green, A. The control of blowflies infesting slaughter-houses. I. Field observations of the habits of blowflies. Ann. Appl. Biol. 1951, 38, 475–494. [Google Scholar] [CrossRef]
- Esser, J.R. Biology of Chrysomya megacephala (Diptera: Calliphoridae) and reduction of losses caused to the salted-dried fish industry in south-east Asia. Bull. Entomol. Res. 1991, 81, 33–41. [Google Scholar] [CrossRef]
- Aak, A.; Brikemoe, T.; Mehl, R. Blowfly (Diptera, Calliphoridae) damage on stockfish in northern Norway: Pest species, damage assessment and the potential of mass trapping. J. Pest. Sci. 2010, 83, 329–337. [Google Scholar] [CrossRef]
- Bedini, S.; Cosci, F.; Girardi, J.; Bocchino, R.; Conti, C. Aromatic plant essential oils for the control of blowflies in the production of dry-cured meat. In Proceedings of the Working Group “Integrated Protection of Stored Products”, Ljubljana, Slovenia, 3–5 July 2017. [Google Scholar]
- Graf, J.F. The role of insect growth regulators in arthropod control. Parasitol. Today 1993, 9, 471–474. [Google Scholar] [CrossRef]
- Bisdorff, B.; Wall, R. Sheep blowfly strike risk management in Great Britain: A survey of current practice. Med. Vet. Entomol. 2008, 22, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, B.; Schmid, H.R.; Junquera, P. Prevention of blowfly strike on lambs with the insect growth regulator dicyclanil. Vet. Rec. 2000, 47, 540–544. [Google Scholar] [CrossRef]
- Levot, G.W.; Sales, N. Insect growth regulator cross resistance studies in field-and laboratory-selected strains of the Australian blowfly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Aust. J. Entomol. 2004, 43, 374–377. [Google Scholar] [CrossRef]
- Magoc, L.; Yen, J.L.; Hill-Williams, A.; McKenzie, J.A.; Batterham, P.; Daborn, P.J. Cross-resistance to dicyclanil in cyromazine-resistant mutants of Drosophila melanogaster and Lucilia cuprina. Pestic. Biochem. Physiol. 2005, 81, 129–135. [Google Scholar] [CrossRef]
- Wright, J.E. Environmental and toxicological aspects of insect growth regulators. Environ. Health Perspect. 1976, 14, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Gajendiran, A.; Abraham, J. An overview of pyrethroid insecticides. Front. Biol. 2018, 13, 79–90. [Google Scholar] [CrossRef]
- Kaur Sidhu, G.; Singh, S.; Kumar, V.; Singh Dhanjal, D.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Env. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverría, M.C.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Artemisia spp. essential oils against the disease-carrying blowfly Calliphora vomitoria. Parasite. Vector. 2017, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverría, M.C.; Gomez, E.V.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Toxicity and oviposition deterrence of essential oils of Clinopodium nubigenum and Lavandula angustifolia against the myiasis inducing blowfly Lucilia sericata. PLoS ONE 2019, 14, e0212576. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Guarino, S.; Echeverría, M.C.; Flamini, G.; Ascrizzi, R.; Loni, A.; Conti, B. Allium sativum, Rosmarinus officinalis, and Salvia officinalis essential oils: A spiced shield against blowflies. Insects 2020, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Bedini, S.; Farina, P.; Napoli, E.; Flamini, G.; Ascrizzi, R.; Verzera, A.; Conti, B.; Zappalà, L. Bioactivity of different chemotypes of Oregano essential oil against the blowfly Calliphora vomitoria vector of foodborne pathogens. Insects 2021, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Siedo, S.J. Systematics of Aloysia (Verbenaceae). Ph.D. Dissertation, The University of Texas at Austin, Austin, TX, USA, 2006; p. 309. [Google Scholar]
- Soukup, J. Vocabulario de los Nombres vulgares de la Flora Peruana y Catálogo de los Géneros; Editorial Salesiana: Lima, Peru, 1987; p. 436. [Google Scholar]
- Tene, V.; Malagón, O.; Vita Finzi, P.; Vidari, G.; Armijos, C.; Zaragoza, T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007, 111, 63–81. [Google Scholar] [CrossRef]
- Bouasla, A.; Bouasla, I. Ethnobotanical survey of medicinal plants in northeastern of Algeria. Phytomedicine 2017, 36, 68–81. [Google Scholar] [CrossRef]
- Eddouks, M.; Ajebli, M.; Hebi, M. Ethnopharmacological survey of medicinal plants used in Daraa-Tafilalet region (Province of Errachidia), Morocco. J. Ethnopharmacol. 2017, 198, 516–530. [Google Scholar] [CrossRef]
- Raghavan, S. Handbook of Spices, Seasonings, and Flavorings, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 330. [Google Scholar] [CrossRef]
- Da Cunha, A.P.; Roque, O.R.; Nogueira, M.T. Plantas Aromáticas e Óleos Essenciais, Composição e Aplicações; Edições Fundação Calouste Gulbenkian: Lisbon, Portugal, 2012; p. 678. [Google Scholar]
- Raz, L.; Agudelo Zamora, H. Bursera graveolens (Kunth) Triana & Planch. In Catálogo de Plantas y Líquenes de Colombia; Bernal, R., Gradstein, S.R., Celis, M., Eds.; Universidad Nacional de Colombia: Bogotà, Colombia, 2020. [Google Scholar]
- Yukawa, C.; Imayoshi, Y.; Iwabuchi, H.; Komemushi, S.; Sawabe, A. Chemical composition of three extracts of Bursera graveolens. Flavour Frag. J. 2006, 21, 234–238. [Google Scholar] [CrossRef]
- Sotelo, A.H.; Figueroa, C.G.; Césare, M.F.; Alegría, M.C. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Bursera graveolens (Burseraceae) from Perú. Indian J. Pharm. Educ. Res. 2017, 51, 429–436. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Standards and Technology. NIST/EPA/NIH Mass Spectral Library, NIST Standard Reference Database Number 69; The NIST Mass Spectrometry Data Center: Gaithersburg, Maryland, 2014.
- Adams, R.P. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Bedini, S.; Cosci, F.; Tani, C.; Pierattini, E.C.; Venturi, F.; Lucchi, A.; Ioriatti, C.; Ascrizzi, R.; Flamini, G.; Ferroni, G.; et al. Essential oils as post-harvest crop protectants against the Fruit Fly Drosophila suzukii: Bioactivity and organoleptic profile. Insects 2020, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Tonacci, A.; Billeci, L.; Di Mambro, I.; Marangoni, R.; Sanmartin, C.; Venturi, F. Wearable sensors for assessing the role of olfactory training on the autonomic response to olfactory stimulation. Sensors 2021, 21, 770. [Google Scholar] [CrossRef] [PubMed]
- Ujvari, B.; Wallman, J.F.; Madsen, T.; Whelan, M.; Hulbert, A.J. Experimental studies of blowfly (Calliphora stygia) longevity: A little dietary fat is beneficial but too much is detrimental. Comp. Biochem. Phys. A 2009, 154, 383–388. [Google Scholar] [CrossRef]
- Kelly, M.A.; Zieba, A.P.; Buttemer, W.A.; Hulbert, A.J. Effect of temperature on the rate of ageing: An experimental study of the blowfly Calliphora stygia. PLoS ONE 2013, 8, e73781. [Google Scholar] [CrossRef]
- Abbott, W.J. A method of computing effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 256–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971; p. 333. [Google Scholar] [CrossRef]
- Bedini, S.; Muniz, E.R.; Tani, C.; Conti, B.; Ruiu, L. Insecticidal potential of Brevibacillus laterosporus against dipteran pest species in a wide ecological range. J. Invertebr. Pathol. 2020, 177, 107493. [Google Scholar] [CrossRef]
- Gil, A.; Van Baren, C.M.; Di Leo Lira, P.M.; Bandoni, A.L. Identification of the genotype from the content and composition of the essential oil of lemon verbena (Aloysia citriodora Palau). J. Agric. Food Chem. 2007, 55, 8664–8669. [Google Scholar] [CrossRef]
- Abuhamdah, S.; Abuhamdah, R.; Howes, M.-J.R.; Al-Olimat, S.; Ennaceur, A.; Chazot, P.L. Pharmacological and neuroprotective profile of an essential oil derived from leaves of Aloysia citrodora Palau. J. Pharm. Pharmacol. 2015, 67, 1306–1315. [Google Scholar] [CrossRef] [Green Version]
- Young, D.G.; Chao, S.; Casablanca, H.; Bertrand, M.-C.; Minga, D. Essential Oil of Bursera graveolens (Kunth) Triana et Planch from Ecuador. J. Essent. Oil Res. 2007, 19, 525–526. [Google Scholar] [CrossRef]
- Fon-Fay, F.M.; Pino, J.A.; Hernández, I.; Rodeiro, I.; Fernández, M.D. Chemical composition and antioxidant activity of Bursera graveolens (Kunth) Triana et Planch essential oil from Manabí, Ecuador. J. Essent. Oil Res. 2019, 31, 211–216. [Google Scholar] [CrossRef]
- Carmona, R.; Quijano-Celís, C.E.; Pino, J.A. Leaf oil composition of Bursera graveolens (Kunth) Triana et Planch. J. Essent. Oil Res. 2009, 21, 387–389. [Google Scholar] [CrossRef]
- Monzote, L.; Hill, G.M.; Cuellar, A.; Scull, R.; Setzer, W.N. Chemical composition and anti-proliferative properties of Bursera graveolens essential oil. Nat. Prod. Commun. 2012, 7, 1934578X1200701. [Google Scholar] [CrossRef] [Green Version]
- Werdin González, J.O.; Gutiérrez, M.M.; Murray, A.P.; Ferrero, A.A. Biological activity of essential oils from Aloysia polystachya and Aloysia citriodora (Verbenaceae) against the soybean pest Nezara viridula (Hemiptera: Pentatomidae). Nat. Prod. Commun. 2010, 5, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Mehlhorn, H. Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol. Res. 2006, 99, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nthan, S.; Mehlhorn, H.; Maggi, F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Leyva, M.; del Carmen Marquetti, M.; Montada, D.; Payroll, J.; Scull, R.; Morejón, G.; Pino, O. Essential oils of Eucalyptus globulus (Labill) and Bursera graveolens (Kunth) Triana & Planch for the control of mosquitoes of medical importance. Biol. (Lima) 2020, 18, 239–250. [Google Scholar] [CrossRef]
- Benzi, V.S.; Stefanazzi, N.; Murray, A.P.; Werdin González, J.O.; Ferrero, A.A. Composition, repellent, and insecticidal activities of two South American plants against the Stored Grain Pests Tribolium castaneum and Tribolium confusum (Coleoptera: Tenebrionidae). ISRN Entomol. 2014, 55, 175827. [Google Scholar] [CrossRef] [Green Version]
- Khani, A.; Basavand, F.; Rakhshani, E. Chemical composition and insecticide activity of lemon verbena essential oil. J. Crop. Prot. 2012, 1, 313–320. [Google Scholar]
- Buentello-Wong, S.; Galán-Wong, L.; Arévalo-Nino, K.; Almaguer-Cantú, V.; Rojas-Verde, G. Toxicity of some essential oil formulations against the Mexican fruitfly Anastrepha ludens (Loew) (Diptera: Tephritidae). Ind. Crops Prod. 2016, 85, 58–62. [Google Scholar] [CrossRef]
- Benelli, G.; Flamini, G.; Canale, A.; Cioni, P.L.; Conti, B. Toxicity of some essential oil formulations against the Mediterranean fruitfly Ceratitis capitata (Wiedemann) (Diptera Tephritidae). Crop. Prot. 2012, 42, 223–229. [Google Scholar] [CrossRef]

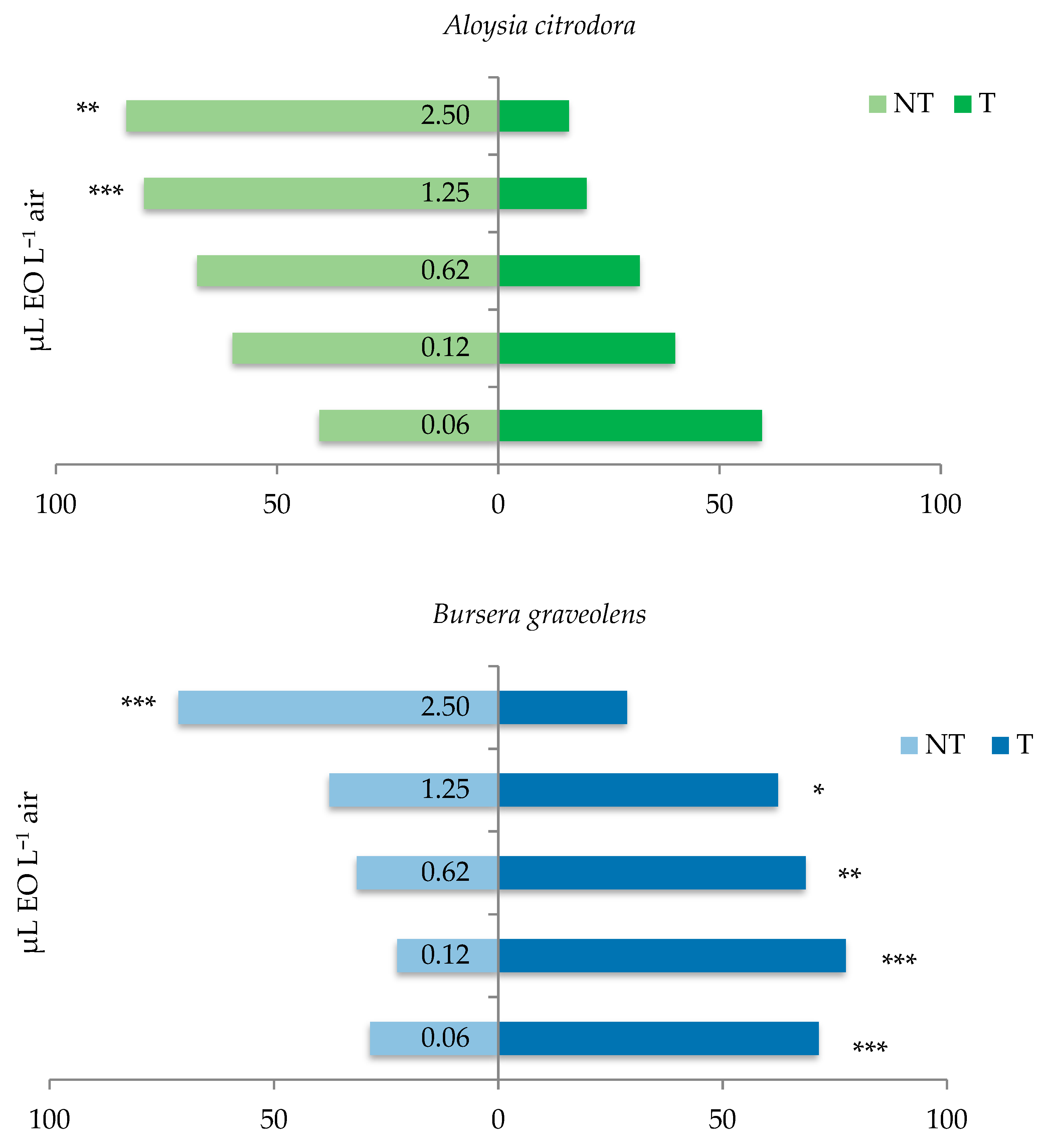

| Compound | l.r.i. a | Relative Abundance (%) | ||
|---|---|---|---|---|
| Allium sativum * | Aloysia citrodora | Bursera graveolens | ||
| diallyl sulfide | 866 | 5.5 | -b | - |
| 2,3-dimethyl thiophene | 901 | 0.3 | - | - |
| methyl-2-propenyl disulfide | 920 | 3.6 | - | - |
| (Z)-methylpropenyl disulfide | 932 | 0.2 | - | - |
| (E)-methylpropenyl disulfide | 940 | 0.2 | - | - |
| α-pinene | 941 | - | 0.3 | - |
| dimethyl trisulfide | 975 | 0.8 | - | - |
| sabinene | 978 | - | 0.9 | - |
| 1-octen-3-ol | 980 | - | 0.2 | - |
| 6-methyl-5-hepten-2-one | 986 | - | 0.6 | - |
| myrcene | 991 | - | 0.2 | 3.0 |
| 3- octanol | 994 | - | 0.1 | - |
| p-cymene | 1028 | - | - | 1.1 |
| limonene | 1032 | - | 7.2 | 46.2 |
| 1,8- cineole | 1033 | - | 2.2 | - |
| (Z)-β-ocimene | 1042 | - | 0.1 | - |
| (E)-β-ocimene | 1052 | - | 2.4 | - |
| cis-sabinene hydrate | 1070 | - | 0.2 | - |
| diallyl disulfide | 1082 | 16.1 | - | - |
| linalool | 1101 | - | 0.2 | - |
| (E)-1-allyl-2-(prop-1-en-1-yl) disulfane | 1103 | 0.7 | - | - |
| (Z)-1-allyl-2-(prop-1-en-1-yl) disulfane | 1107 | 0.6 | - | - |
| trans-p-mentha-2,8-dien-1-ol | 1121 | - | - | 0.5 |
| cis-p-mentha-2,8-dien-1-ol | 1135 | - | - | 0.4 |
| trans-limonene oxide | 1139 | - | - | 0.5 |
| methyl allyl trisulfide | 1142 | 9.5 | - | - |
| β-terpineol | 1153 | - | - | 0.4 |
| menthone | 1148 | - | - | 1.0 |
| 4-methyl-1,2,3-trithiolane | 1154 | 0.9 | - | - |
| β-pinene oxide | 1155 | - | 0.6 | - |
| citronellal | 1156 | - | 0.2 | - |
| menthofurane | 1165 | - | - | 3.4 |
| isoneral | 1170 | - | 0.6 | - |
| isogeranial | 1184 | - | 0.9 | - |
| α-terpineol | 1190 | - | 0.6 | 17.8 |
| cis-dihydrocarvone | 1194 | - | - | 0.7 |
| cis-piperitol | 1195 | - | - | 0.7 |
| 2-vinyl-4H-1,3-dithiine | 1206 | 0.6 | - | - |
| dimethyl tetrasulfide | 1210 | 0.8 | - | - |
| trans-carveol | 1220 | - | - | 2.1 |
| cis-carveol | 1228 | - | - | 5.0 |
| nerol | 1230 | - | 0.6 | - |
| pulegone | 1239 | - | - | 0.8 |
| neral | 1240 | - | 21.0 | - |
| carvone | 1244 | - | - | 1.3 |
| geraniol | 1257 | - | 0.4 | - |
| geranial | 1271 | - | 26.8 | - |
| diallyl trisulfide | 1297 | 23.1 | - | - |
| (Z)-1-allyl-3-(prop-1-en-1-yl) trisulfane | 1329 | 0.2 | - | - |
| (E)-1-allyl-3-(prop-1-en-1-yl) trisulfane | 1346 | 0.6 | - | - |
| S-methyl-1,2,3,4-tetrathiane | 1364 | 1.0 | - | - |
| α-copaene | 1377 | - | 0.4 | - |
| geranyl acetate | 1385 | - | 2.4 | - |
| S-propylpropane thiosulfonate | 1388 | 6.7 | - | - |
| α-cedrene | 1409 | - | 0.2 | - |
| β-caryophyllene | 1419 | - | 2.7 | - |
| 1-(1-(methylthio)propyl)-2-propyl disulfane | 1431 | 0.5 | - | - |
| dimethyl pentasulfide | 1450 | 0.3 | - | - |
| α-humulene | 1455 | - | 0.1 | - |
| alloaromadendrene | 1462 | - | 0.4 | - |
| γ-muurolene | 1477 | - | - | 0.8 |
| geranyl propionate | 1478 | - | 0.3 | - |
| germancrene D | 1482 | - | 3.1 | - |
| ar-curcumene | 1483 | - | 3.1 | - |
| bicyclogermancrene | 1496 | - | 6.8 | - |
| mint lactone | 1499 | - | - | 1.3 |
| β- curcumene | 1513 | - | 1.1 | - |
| cubebol | 1515 | - | 0.8 | - |
| δ- cadinene | 1524 | - | 0.2 | - |
| diallyl tetrasulfide | 1540 | 17.4 | - | - |
| (E)-nerolidol | 1564 | - | 2.0 | - |
| spathulenol | 1572 | - | 4.4 | 0.7 |
| germancrene D-4 ol | 1575 | - | 1.1 | - |
| 1-propyl-2-(4-thiohept-2-en-5-yl) disulfide | 1580 | 0.2 | - | - |
| caryophyllene oxide | 1581 | - | 2.0 | - |
| 6-methyl-4,5,8-trithia-1,10-undecadiene | 1597 | 0.9 | - | - |
| α-epi-7-epi-5-eudesmol | 1617 | - | - | 5.0 |
| 3-amino-tert-butyl benzoate | 1620 | 0.9 | - | - |
| isospathulenol | 1639 | - | 0.5 | - |
| τ-cadinol | 1641 | - | 0.7 | 0.8 |
| α-cadinol | 1653 | - | - | 1.3 |
| α-bisabolol | 1684 | - | - | 0.7 |
| 1-allyl-3-(2-(allylthio)propyl) trisulfane | 1818 | 2.0 | - | - |
| cyclic octaatomic sulfur | 2030 | 0.2 | - | - |
| 1-allyl-3-(2-(allyldisulfanyl)propyl) trisulfane | 2066 | 1.2 | - | - |
| Monoterpene hydrocarbons | - | 11.1 | 50.3 | |
| Oxygenated monoterpenes | - | 57.0 | 35.4 | |
| Sesquiterpene hydrocarbons | - | 18.1 | 0.8 | |
| Oxygenated sesquiterpenes | - | 11.5 | 9.2 | |
| Nitrogen compounds | 0.9 | - | - | |
| Sulfur compounds | 94.0 | - | - | |
| Other non-terpene derivatives | - | 0.9 | - | |
| Total identified (%) | 94.8 | 98.6 | 95.7 | |
| EO | LC50 (95% FL) | Intercept ± SE | p |
|---|---|---|---|
| A. citrodora | 0.034 (0.024–0.049) | 3.811 ± 0.139 | <0.001 |
| B. graveolens | 0.024 (0.017–0.034) | 4.185 ± 0.145 | <0.001 |
| A. sativum | 0.037 (0.022–0.060) | 3.722 ± 0.131 | <0.001 |
| EO (X) | A. citrodora | B. graveolens | |
|---|---|---|---|
| EO (Y) | |||
| B. graveolens | 1.394 (0.876–2.458) | - | |
| A. sativum | 0.924 (0.465–1.662) | 0.663 (0.300–1.196) | |
| EO | LC50/LD50 (95% FL) | Intercept ± SE | p |
|---|---|---|---|
| Fumigation | |||
| A. citrodora | 23.657 (18.698–30.706) | −4.161 ± 0.369 | <0.001 |
| B. graveolens | 25.303 (19.975–33.190) | −4.250 ± 0.372 | <0.001 |
| A. sativum | 1.860 (1.250–2.760) | −0.816 ± 0.161 | <0.001 |
| Contact | |||
| A. citrodora | 0.268 (0.189–0.367) | 1.168 ± 0.148 | <0.001 |
| B. graveolens | 0.958 (0.712–1.430) | 0.038 ± 0.107 | 0.723 |
| A. sativum | 0.462 (0.291–0.750) | 0.686 ± 0.167 | <0.001 |
| Ingestion | |||
| A. citrodora | 35.645 (23.449–52.870) | −4.068 ± 0.402 | <0.001 |
| B. graveolens | 44.975 (30.019–68.448) | −4.333 ± 0.412 | <0.001 |
| A. sativum | 8.094 (5.322–12.182) | −2.380 ± 0.252 | <0.001 |
| EO (X) | A. citrodora | B. graveolens | |
|---|---|---|---|
| EO (Y) | |||
| Fumigation | |||
| B. graveolens | 0.935 (0.653–1.313) | - | |
| A. sativum | 12.721 (3.703–124.904) | 13.606 (3.880–139.0.38) | |
| Contact | |||
| B. graveolens | 0.280 (0.108–0.498) | - | |
| A. sativum | 0.581 (0.286–1.008) | 2.076 (1.189–4.503) | |
| Ingestion | |||
| B. graveolens | 0.793 (0.418–1.367) | - | |
| A. sativum | 4.404 (1.753–26.592) | 5.557 (2.050–39.806) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, P.; Venturi, F.; Ascrizzi, R.; Flamini, G.; Chiriboga Ortega, R.D.; Echeverría, M.C.; Ortega, S.; Zinnai, A.; Bedini, S.; Conti, B. Andean Plants Essential Oils: A Scented Alternative to Synthetic Insecticides for the Control of Blowflies. Insects 2021, 12, 894. https://doi.org/10.3390/insects12100894
Farina P, Venturi F, Ascrizzi R, Flamini G, Chiriboga Ortega RD, Echeverría MC, Ortega S, Zinnai A, Bedini S, Conti B. Andean Plants Essential Oils: A Scented Alternative to Synthetic Insecticides for the Control of Blowflies. Insects. 2021; 12(10):894. https://doi.org/10.3390/insects12100894
Chicago/Turabian StyleFarina, Priscilla, Francesca Venturi, Roberta Ascrizzi, Guido Flamini, Rodrigo Daniel Chiriboga Ortega, Maria Cristina Echeverría, Sania Ortega, Angela Zinnai, Stefano Bedini, and Barbara Conti. 2021. "Andean Plants Essential Oils: A Scented Alternative to Synthetic Insecticides for the Control of Blowflies" Insects 12, no. 10: 894. https://doi.org/10.3390/insects12100894










