The Identification of Boll Weevil, Anthonomus grandis grandis (Coleoptera: Curculionidae), Genes Involved in Pheromone Production and Pheromone Biosynthesis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Boll Weevil Collections and Experimental Design
2.2. RNA Extraction and Sequencing
2.3. Transcriptome Assembly and Annotation
2.4. RNA-Seq Analysis
3. Results and Discussion
3.1. Transcriptome
3.2. RNA-Seq Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paula, D.P.; Claudino, D.; Timbó, R.V.; Miranda, J.E.; Bemquerer, M.; Ribeiro, A.; Sujii, E.; Fontes, E.; Pires, C. Reproductive dormancy in boll-weevil from populations of the Midwest of Brazil. J. Econ. Ѐntomol. 2013, 106, 86–96. [Google Scholar] [CrossRef]
- Smith, J.W. Boll weevil eradication: Area-wide pest management. Ann. Ѐntomol. Soc. Am. 1998, 91, 239–247. [Google Scholar] [CrossRef]
- Carter, F.L.; Nelson, T.C.; Jordan, A.G.; Smith, J.R. US cotton declares war on the boll weevil. In Boll Weevil Eradication in the United States through 1999; The Cotton Foundation: Memphis, TN, USA, 1999; pp. 25–54. [Google Scholar]
- El-Lissy, O.A.; Grefenstette, W.J. Progress of boll weevil eradication in the U.S., 2005. In Proceedings of the Beltwide Cotton Conferences, San Antonio, TX, USA, 3–6 January 2006; pp. 1266–1276. [Google Scholar]
- Texas Boll Weevil Eradication Foundation. Trapping. Available online: https://www.txbollweevil.org/trapping.html (accessed on 7 May 2021).
- Tumlinson, J.H.; Hardee, D.D.; Gueldner, R.C.; Thompson, A.C.; Hedin, P.A.; Minyard, J.P. Sex pheromones produced by male boll weevil: Isolation, identification, and synthesis. Science 1969, 166, 1010–1012. [Google Scholar] [CrossRef]
- Hardee, D.D.; McKibben, G.H.; Gueldner, R.C.; Mitchell, E.B.; Tumlinson, J.H.; Cross, W.H. Boll weevils in nature respond to grandlure, a synthetic pheromone. J. Econ. Ѐntomol. 1972, 65, 97–100. [Google Scholar]
- Rogers, C.; Oakes, S.; Rummel, D. Evaluation of infield pheromone traps for boll weevil suppression in the Texas Rolling Plains. Res. Monogr -Tex. Agric. Exp. Station 1976, 8, 45–52. [Google Scholar]
- Rummel, D.R.; Bottrell, D.G. Seasonally related decline in response of boll weevils to pheromone traps during mid-season. Environ. Ѐntomol. 1976, 5, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Wolfenbarger, D.A.; Graham, H.M.; Parker, R.D.; Davis, J.W. Boll weevil: Seasonal patterns of response to traps baited with grandlure in the Lower Rio Grande Valley. Environ. Ѐntomol. 1976, 5, 403–408. [Google Scholar] [CrossRef]
- Suh, C.P.-C.; Westbrook, J.K. Failure of pheromone traps in detecting incipient populations of boll weevils (Coleoptera: Curculiondae): Investigation of two potential contributing factors. J. Ѐntomol. Sci. 2014, 49, 211–214. [Google Scholar] [CrossRef]
- Rummel, D.; Bottrell, D. Relationship of overwintered boll weevil response to pheromone traps and natural entry into cotton. Res. Monogr.-Tex. Agric. Exp. Stn. 1976, 8, 26–31. [Google Scholar]
- Hardee, D.D.; Cross, W.H.; Huddleston, P.M.; Davich, T.B. Survey and control of the boll weevil in West Texas with traps baited with males. J. Econ. Ѐntomol. 1970, 63, 1041–1048. [Google Scholar] [CrossRef]
- Boyd, F., Jr.; Brazzel, J.; Helms, W.; Moritz, R.; Edwards, R. Spring destruction of overwintered boll weevils in West Texas with wing traps. J. Econ. Entomol. 1973, 66, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Scott, W.P.; Lloyd, E.P.; Bryson, J.O.; Davich, T.B. Trap plots for suppression of low-density overwintered populations of boll weevils. J. Econ. Ѐntomol. 1974, 67, 281–283. [Google Scholar] [CrossRef]
- Cherry, E.T. Monitoring boll weevil movement with pheromone traps. Tenn. Farm Home Sci. Prog. Rep. 1974, 90, 27–29. [Google Scholar]
- Segers, J.; Urban, T.; George, D.; Benedict, J.; Walmsley, M.; Pieters, E. Seasonal numbers, sex diapause states of boll weevils captured in pheromone traps in the Lower Gulf Coast of Texas. Southwest. Entomol. 1988, 12, 311–316. [Google Scholar]
- White, J.R.; Rummel, D.R. Emergence profile of overwintered boll weevils and entry into cotton. Environ. Ѐntomol. 1978, 7, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Spurgeon, D.W. Age dependence of pheromone production by the boll weevil (Coleoptera: Curculionidae). Environ. Ѐntomol. 2003, 32, 31–38. [Google Scholar] [CrossRef]
- Spurgeon, D.W.; Suh, C.P.-C. Pheromone production by the boll weevil (Coleoptera: Curculionidae) fed cotton squares and bolls. J. Ѐntomol. Sci. 2009, 44, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Suh, C.-C.; Spurgeon, D. Continued pheromone release by boll weevils (Coleoptera: Curculionidae) following host removal. J. Ѐntomol. Sci. 2016, 51, 332–335. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B.J.B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [Green Version]
- Simão, F.A.; Waterhouse, R.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, R.; Seppey, M.; Simão, F.A.; Manni, M.; Ioannidis, P.; Klioutchnikov, G.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Applications from quality assessments to gene prediction and phylogenomics. Mol. Biol. Evol. 2018, 35, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness. Bioinformatics 2019, 1962, 227–245. [Google Scholar]
- Kriventseva, E.V.; Kuznetsov, D.; Tegenfeldt, F.; Manni, M.; Dias, R.; Simão, F.A.; Zdobnov, E.M. OrthoDB v10: Sampling the diversity of animal, plant, fungal, protist, bacterial and viral genomes for evolutionary and functional annotations of orthologs. Nucleic Acids Res. 2019, 47, D807–D811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; McCarthy, D.; Smyth, G. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Keeling, C.I.; Blomquist, G.J.; Tittiger, C. Coordinated gene expression for pheromone biosynthesis in the pine engraver beetle, Ips pini (Coleoptera: Scolytidae). Naturwissenschaften 2004, 91, 324–328. [Google Scholar] [CrossRef]
- Bellés, X.; Martín, D.; Piulachs, M.-D. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu. Rev. Ѐntomol. 2005, 50, 181–199. [Google Scholar] [CrossRef] [Green Version]
- González-Caballero, N.; Rodríguez-Vega, A.; Dias-Lopes, G.; Valenzuela, J.G.; Ribeiro, J.M.; Carvalho, P.C.; Valente, R.H.; Brazil, R.P.; Cuervo, P. Expression of the mevalonate pathway enzymes in the Lutzomyia longipalpis (Diptera: Psychodidae) sex pheromone gland demonstrated by an integrated proteomic approach. J. Proteom. 2014, 96, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Beedle, A.; Walton, M.; Goodwin, T. Isoprenoid biosynthesis in aseptic larvae of Calliphora erythrocephala. Insect Biochem. 1975, 5, 465–472. [Google Scholar] [CrossRef]
- Tittiger, C.; Blomquist, G. Pheromone production in pine bark beetles. Adv. Insect Physiol. 2016, 50, 235–263. [Google Scholar]
- Tillman, J.A.; Holbrook, G.L.; Dallara, P.L.; Schal, C.; Wood, D.L.; Blomquist, G.J.; Seybold, S.J. Endocrine regulation of de novo aggregation pheromone biosynthesis in the pine engraver, Ips pini (Say) (Coleoptera: Scolytidae). Insect Biochem. Mol. Biol. 1998, 28, 705–715. [Google Scholar] [CrossRef]
- Stroumbakis, N.D.; Li, Z.; Tolias, P.P. A homolog of human transcription factor NF-X1 encoded by the Drosophila shuttle craft gene is required in the embryonic central nervous system. Mol. Cell. Biol. 1996, 16, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Goodman, C.S. The likeness of being: Phylogenetically conserved molecular mechanisms of growth cone guidance. Cell 1994, 78, 353–356. [Google Scholar] [CrossRef]
- Yamaguchi, M. The transcriptional regulation of regucalcin gene expression. Mol. Cell. Biochem. 2011, 346, 147–171. [Google Scholar] [CrossRef]
- Barrett, A.; Rawlings, N. Families and Clans of Serine Peptidases. Arch. Biochem. Biophys. 1995, 318, 247–250. [Google Scholar] [CrossRef]
- Rao, M.B.; Tanksale, A.M.; Ghatge, M.S.; Deshpande, V.V. Molecular and biotechnological aspects of microbial proteases. Microbiol. Mol. Biol. Rev. 1998, 62, 597–635. [Google Scholar] [CrossRef] [Green Version]
- Perkin, L.; Elpidina, E.; Oppert, B. RNA interference and dietary inhibitors induce a similar compensation response in Tribolium castaneum larvae. Insect Mol. Biol. 2017, 26, 35–45. [Google Scholar] [CrossRef]
- Oliveira-Neto, O.B.; Batista, J.A.; Rigden, D.J.; Fragoso, R.R.; Silva, R.O.; Gomes, E.A.; Franco, O.L.; Dias, S.C.; Cordeiro, C.M.; Monnerat, R.G.; et al. A diverse family of serine proteinase genes expressed in cotton boll weevil (Anthonomus grandis): Implications for the design of pest-resistant transgenic cotton plants. Insect Biochem. Mol. Biol. 2004, 34, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Li, C.-F.; He, Z.; Lu, Y.; Liu, X.-S.; Wang, Y.-F.; Ip, Y.T.; Strand, M.R.; Yu, X.-Q. Toll family members bind multiple Spätzle proteins and activate antimicrobial peptide gene expression in Drosophila. J. Biol. Chem. 2019, 294, 10172–10181. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Swevers, L.; Iatrou, K.; Huvenne, H.; Smagghe, G. Bombyx mori DNA/RNA non-specific nuclease: Expression of isoforms in insect culture cells, subcellular localization and functional assays. J. Insect Physiol. 2012, 58, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.G.; Jones, T.M. The role of chemical communication in mate choice. Biol. Rev. 2007, 82, 265–289. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Jokinen, I.; Kortet, R.; Vainikka, A.; Suhonen, J. Do pheromones reveal male immunocompetence? Proc. Biol. Sci. 2002, 269, 1681–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugumaran, M. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment. Cell Res. 2002, 15, 2–9. [Google Scholar] [CrossRef]
- Stoehr, A. Costly melanin ornaments: The importance of taxon? Func. Ecol. 2006, 20, 276–281. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S.; Beeman, R.W.; Kanost, M.R.; Kramer, K.J. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc. Natl. Acad. Sci. USA 2005, 102, 11337–11342. [Google Scholar] [CrossRef] [Green Version]
- Arakane, Y.; Lomakin, J.; Beeman, R.W.; Muthukrishnan, S.; Gehrke, S.H.; Kanost, M.R.; Kramer, K.J. Molecular and functional analyses of amino acid decarboxylases involved in cuticle tanning in Tribolium castaneum. J. Biol. Chem. 2009, 284, 16584–16594. [Google Scholar] [CrossRef] [Green Version]
- Lukacsovich, T.; Yuge, K.; Awano, W.; Asztalos, Z.; Kondo, S.; Juni, N.; Yamamoto, D. The ken and barbie gene encoding a putative transcription factor with a BTB domain and three zinc finger motifs functions in terminalia development of Drosophila. Arch. Insect Biochem. Physiol. 2003, 54, 77–94. [Google Scholar] [CrossRef] [PubMed]
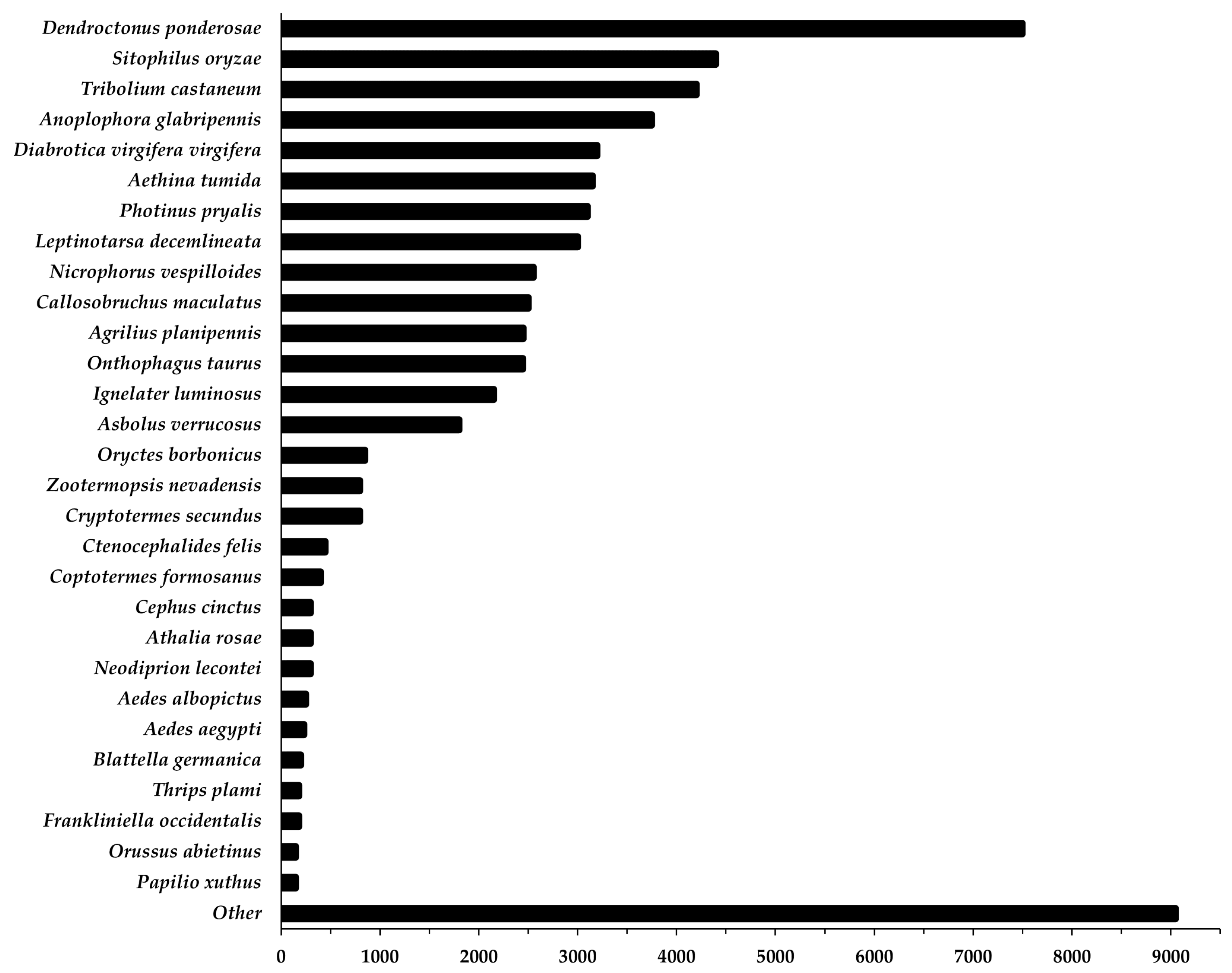



| Raw sequences | 352,189,343 |
| Total transcripts | 195,128 |
| Average sequence length | 922.89 bp |
| N50 | 2040 bp |
| BUSCO | |
| Complete | 99.27% |
| Complete single copy | 97.81% |
| Complete duplicated | 1.46% |
| Fragmented | 0.59% |
| Missing | 0.15% |
| Sequence Name | Sequence Description | BLAST Top Hit | Fold Change | UP/DOWN | p-Value | FDR |
|---|---|---|---|---|---|---|
| Mevalonate pathway or associated with pheromone production and biosynthesis | ||||||
| TRINITY_DN11108 | hydroxymethylglutaryl-CoA synthase 1 (HMG-S) | D. ponderosae | 23.08 | ↑ | 1.45 × 10−37 | 2.67 × 10−34 |
| TRINITY_DN13668 | dolichyl pyrophosphate Man9GlcNAc2 α-1,3-glucosyltransferase | D. ponderosae | 2.01 | ↑ | 4.44 × 10−05 | 1.82 × 10−03 |
| TRINITY_DN5050 | farnesyl pyrophosphate synthase-like | I. pini | 11.77 | ↑ | 1.30 × 10−04 | 4.59 × 10−03 |
| TRINITY_DN5422 | phosphomevalonate kinase | D. ponderosae | 3.05 | ↑ | 9.80 × 10−12 | 1.51 × 10−09 |
| TRINITY_DN8104 | farnesyl pyrophosphate synthase | I. pini | 34.05 | ↑ | 1.10 × 10−05 | 5.33 × 10−04 |
| TRINITY_DN8499 | isopentenyl-diphosphate Delta-isomerase 1 | A. glabripennis | 37.64 | ↑ | 1.57 × 10−09 | 1.69 × 10−07 |
| TRINITY_DN873 | 3-hydroxy-3-methylglutaryl-coenzyme A reductase-like (HMG-R) | I. paraconfusus | 16.57 | ↑ | 9.18 × 10−29 | 9.18 × 10−26 |
| TRINITY_DN4352 | ATP-citrate synthase | D. ponderosae | 6.66 | ↑ | 1.89 × 10−25 | 3.07 × 10−32 |
| Juvenile hormone | ||||||
| TRINITY_DN11992 | juvenile hormone inducible protein | D. ponderosae | 7.75 | ↑ | 7.40 × 10−13 | 1.39 × 10−10 |
| TRINITY_DN4315 | juvenile hormone esterase-like | D. ponderosae | 2.98 | ↑ | 5.14 × 10−11 | 7.24 × 10−09 |
| TRINITY_DN874 | juvenile hormone esterase-like | D. ponderosae | 2.71 | ↑ | 1.99 × 10−08 | 1.79 × 10−06 |
| TRINITY_DN4184 | juvenile hormone binding protein | R. ferrugineus | 5.64 | ↑ | 2.69 × 10−08 | 2.34 × 10−06 |
| TRINITY_DN9052 | juvenile hormone inducible protein | D. ponderosae | 4.53 | ↑ | 7.72 × 10−07 | 4.94 × 10−05 |
| TRINITY_DN115965 | putative juvenile hormone inducible protein | D. ponderosae | 3.26 | ↑ | 1.10 × 10−05 | 5.33 × 10−04 |
| TRINITY_DN11903 | juvenile hormone acid O-methyltransferase | D. ponderosae | 2.89 | ↑ | 5.87 × 10−05 | 2.31 × 10−03 |
| Fatty acid metabolism | ||||||
| TRINITY_DN58 | fatty acid synthase | D. ponderosae | 4.92 | ↑ | 2.20 × 10−26 | 1.74 × 10−23 |
| TRINITY_DN5986 | glycerol-3-phosphate phosphatase-like | S. oryzae | 7.50 | ↑ | 3.68 × 10−26 | 2.89 × 10−23 |
| Neurological and hormonal regulation | ||||||
| TRINITY_DN4411 | protein shuttle craft like | D. ponderosae | 5.38 | ↑ | 3.60 × 10−29 | 3.64 × 10−26 |
| TRINITY_DN72610 | regucalcin-like | D. ponderosae | 5.28 | ↑ | 1.45 × 10−25 | 1.11 × 10−22 |
| Sequence Name | Sequence Description | Blast Top Hit | Fold Change | UP/DOWN | p-Value | FDR |
|---|---|---|---|---|---|---|
| Peptidase activity | ||||||
| TRINITY_DN62502 | transmembrane protease serine 9-like | Z. cucurbitae | 27.18 | ↑ | 1.17 × 10−53 | 7.28 × 10−50 |
| TRINITY_DN35065 | cathepsin L1-like | R. ferrugineus | 14.42 | ↑ | 3.42 × 10−51 | 1.73 × 10−47 |
| TRINITY_DN2409 | carboxypeptidase B-like | D. ponderosae | 12.74 | ↑ | 9.29 × 10−41 | 2.28 × 10−37 |
| TRINITY_DN5051 | venom serine carboxypeptidase-like | S. oryzae | 26.91 | ↑ | 2.87 × 10−31 | 3.46 × 10−28 |
| TRINITY_DN13263 | trypsin-like serine prtoease | A. grandis | 15.38 | ↑ | 4.09 × 10−26 | 3.18 × 10−23 |
| TRINITY_DN4893 | venom serine protease-like | D. ponderosae | 10.49 | ↑ | 2.70 × 10−22 | 1.45 × 10−19 |
| TRINITY_DN34699 | trypsin alpha 3-like (Agser2p)—induced by eating | A. grandis | 3.83 | ↑ | 6.99 × 10−15 | 1.76 × 10−12 |
| TRINITY_DN35978 | brachyurin-like (Agser9p) | A. grandis | 3.36 | ↑ | 9.32 × 10−12 | 1.46 × 10−09 |
| TRINITY_DN4825 | brachyurin-like (Agser5p)—induced by eating | A. grandis | 2.26 | ↑ | 5.60 × 10−06 | 2.93 × 10−04 |
| TRINITY_DN49721 | brachyurin-like (Agser9p) | A. grandis | 3.56 | ↑ | 9.43 × 10−13 | 1.74 × 10−10 |
| TRINITY_DN63273 | trypsin alpha-3-like (Agser2p)—induced by feeding | A. grandis | 3.57 | ↑ | 2.63 × 10−13 | 5.21 × 10−11 |
| TRINITY_DN69718 | serine protease (Agser12p) | A. grandis | 2.27 | ↑ | 2.86 × 10−05 | 1.24 × 10−03 |
| Immune response | ||||||
| TRINITY_DN4819 | protein spaetzle 3 | D. ponderosae | 25.78 | ↑ | 3.30 × 10−45 | 1.11 × 10−41 |
| TRINITY_DN3567 | DNA/RNA non-specific nuclease 1 | A. grandis | 13.54 | ↑ | 3.13 × 10−39 | 6.34 × 10−36 |
| Sexual maturity | ||||||
| TRINITY_DN10824 | transcription factor Ken | 7.86 | ↓ | 1.35 × 10−44 | 4.39 × 10−41 | |
| Cuticle tanning and sclerotization | ||||||
| TRINITY_DN16013 | pupal cuticle protein 36-like | D. ponderosae | 89.53 | ↓ | 6.46 × 10−89 | 2.61 × 10−84 |
| TRINITY_DN11117 | cuticular protein 141 | D. ponderosae | 13.54 | ↓ | 1.68 × 10−68 | 2.72 × 10−64 |
| TRINITY_DN3947 | chitin deacetylase 4 | D. ponderosae | 20.48 | ↓ | 1.14 × 10−63 | 1.16 × 10−59 |
| TRINITY_DN4706 | chitin synthase 1 | A. grandis | 5.84 | ↓ | 5.48 × 10−35 | 8.37 × 10−32 |
| TRINITY_DN1999 | cuticle protein 19.8-like | S. oryzae | 15.15 | ↓ | 1.49 × 10−32 | 1.89 × 10−29 |
| TRINITY_DN4303 | cuticular protein analogous to peritrophins | D. ponderosae | 5.84 | ↓ | 1.53 × 10−32 | 1.91 × 10−29 |
| TRINITY_DN2312 | laccase 2 | A. eugenii | 18.71 | ↓ | 9.87 × 10−83 | 2.66 × 10−78 |
| TRINITY_DN22343 | dopamine D2-like receptor | S. oryzae | 4.85 | ↓ | 4.95 × 10−09 | 4.90 × 10−07 |
| TRINITY_DN6738 | dopamine N-acetyltransferase | D. ponderosae | 2.03 | ↓ | 4.40 × 10−04 | 1.30 × 10−02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perkin, L.C.; Perez, J.L.; Suh, C.P.-C. The Identification of Boll Weevil, Anthonomus grandis grandis (Coleoptera: Curculionidae), Genes Involved in Pheromone Production and Pheromone Biosynthesis. Insects 2021, 12, 893. https://doi.org/10.3390/insects12100893
Perkin LC, Perez JL, Suh CP-C. The Identification of Boll Weevil, Anthonomus grandis grandis (Coleoptera: Curculionidae), Genes Involved in Pheromone Production and Pheromone Biosynthesis. Insects. 2021; 12(10):893. https://doi.org/10.3390/insects12100893
Chicago/Turabian StylePerkin, Lindsey C., Jose L. Perez, and Charles P.-C. Suh. 2021. "The Identification of Boll Weevil, Anthonomus grandis grandis (Coleoptera: Curculionidae), Genes Involved in Pheromone Production and Pheromone Biosynthesis" Insects 12, no. 10: 893. https://doi.org/10.3390/insects12100893





