Aposematic Coloration of Moths Decreases Strongly along an Elevational Gradient in the Andes
Abstract
:Simple Summary
Abstract
1. Introduction
- In line with an expected decrease in the predation pressure exerted by visually hunting insectivores, the overall prevalence of aposematic coloration continually decreases from low to high elevations.
- Similarly, the prevalence of hymenopteran and beetle mimicry decreases towards higher elevations.
- This elevational pattern is largely concordant between all types of warning colorations.
- Body size of tiger moths increases with elevation.
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrams, P.A. The evolution of predator-prey interactions: Theory and evidence. Annu. Rev. Ecol. Syst. 2000, 31, 79–105. [Google Scholar] [CrossRef]
- Ter Hofstede, H.M.; Ratcliffe, J.M. Evolutionary escalation: The bat–moth arms race. J. Exp. Biol. 2016, 219, 1589–1602. [Google Scholar] [CrossRef] [Green Version]
- Winters, A.E.; Lommi, J.; Kirvesoja, J.; Nokelainen, O.; Mappes, J. Multimodal aposematic defenses through the predation sequence. Front. Ecol. Evol. 2021, 9, 657740. [Google Scholar] [CrossRef]
- Colwell, R.K.; Brehm, G.; Cardelús, C.L.; Gilman, A.C.; Longino, J.T. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brehm, G.; Strutzenberger, P.; Fiedler, K. Phylogenetic diversity of geometrid moths decreases with elevation in the tropical Andes. Ecography 2013, 36, 1247–1253. [Google Scholar] [CrossRef]
- Romero, G.Q.; Gonçalves-Souza, T.; Kratina, P.; Marino, N.A.C.; Petry, W.K.; Sobral-Souza, T.; Roslin, T. Global predation pressure redistribution under future climate change. Nat. Clim. Chang. 2018, 8, 1087–1091. [Google Scholar] [CrossRef]
- Roslin, T.; Hardwick, B.; Novotny, V.; Petry, W.K.; Andrew, N.R.; Asmus, A.; Barrio, I.C.; Yves Basset, Y.; Boesing, A.L.; Bonebrake, T.C.; et al. Higher predation risk for insect prey at low latitudes and elevations. Science 2017, 356, 742–744. [Google Scholar] [CrossRef] [Green Version]
- Hillyer, R.; Silman, M. Changes in species interactions across a 2.5 km elevation gradient: Effects on plant migration in response to climate change. Glob. Chang. Biol. 2010, 16, 3205–3214. [Google Scholar] [CrossRef]
- Simmons, R. Adaptive coloration and mimicry. In Tiger Moths and Woolly Bears: Behavior, Ecology, and Evolution of the Arctiidae; Conner, W.E., Ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2009; pp. 115–126. [Google Scholar]
- Brehm, G. Patterns of arctiid diversity. In Tiger Moths and Woolly Bears: Behavior, Ecology, and Evolution of the Arctiidae; Conner, W.E., Ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2009; pp. 223–232. [Google Scholar]
- Bowers, M.D. Chemical defenses in woolly bears: Sequestration and efficacy against predators and parasitoids. In Tiger Moths and Woolly Bears: Behavior, Ecology, and Evolution of the Arctiidae; Conner, W.E., Ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2009; pp. 83–102. [Google Scholar]
- Brehm, G.; Hartmann, T.; Willmott, K.R. Pyrrolizidine alkaloids and pharmacophagous Lepidoptera visitors of Prestonia amabilis (Apocynaceae) in a montane rain forest in Ecuador. Ann. Mo. Bot. Gard. 2007, 94, 465–475. [Google Scholar] [CrossRef]
- Zaspel, J.M.; Weller, S.J.; Wardwell, C.T.; Zahiri, R.; Wahlberg, N. Phylogeny and evolution of pharmacophagy in tiger moths (Lepidoptera: Erebidae: Arctiinae). PLoS ONE 2014, 9, e101975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hristov, N.I.; Conner, W.E. Sound strategy: Acoustic aposematism in the bat–tiger moth arms race. Naturwissenschaften 2005, 92, 164–169. [Google Scholar] [CrossRef]
- Simmons, R.B.; Weller, S.J. What kind of signals do mimetic tiger moths send? A phylogenetic test of wasp mimicry systems (Lepidoptera: Arctiidae: Euchromiini). Proc. R. Soc. Lond. B 2002, 269, 983–990. [Google Scholar] [CrossRef] [Green Version]
- Linsley, E.G.; Eisner, T.; Klots, A.B. Mimetic assemblages of sibling species of lycid beetles. Evolution 1961, 15, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.K. A new lichen moth record for the United States: Lycomorphodes sordida (Arctiidae: Lithosiinae) from south Texas. J. Lepid. Soc. 1992, 46, 160–161. Available online: https://images.peabody.yale.edu/lepsoc/jls/1990s/1992/1992-46(2)160-Adams.pdf (accessed on 29 September 2021).
- Balsbaugh, E.U.; Fauske, G. Possible Müllerian mimicry of Galerucinae with Criocerinae (both Coleoptera: Chrysomelidae) and with Maepha opulenta (Lepidoptera: Arctiidae). Coleopt. Bull. 1991, 45, 227–231. Available online: http://www.jstor.com/stable/4008833 (accessed on 1 September 2021).
- do Nascimento, E.A. Estudos do Mimetismo em Lycidae (Insecta: Coleoptera). Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2009. [Google Scholar]
- Brehm, G.; Zeuss, D.; Colwell, R.K. Moth body size increases with elevation along a complete tropical elevational gradient for two hyperdiverse clades. Ecography 2019, 42, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Süßenbach, D. Diversität von Nachtfaltergemeinschaften Entlang Eines Höhengradienten in Südecuador (Lepidoptera: Pyraloidea, Arctiidae). Ph.D. Thesis, University of Bayreuth, Bayreuth, Germany, 2003. Available online: https://epub.uni-bayreuth.de/992 (accessed on 17 September 2021).
- Fiedler, K.; Brehm, G.; Hilt, N.; Süssenbach, D.; Onore, G.; Bartsch, D.; Häuser, C.H. Moths (Lepidoptera: Arctiidae, Geometridae, Hedylidae, Pyraloidea, Sphingidae, Uraniidae) In Liede-Schumann, S., Breckle, S.W., Eds., Provisional checklist of flora and fauna of the San Francisco valley and its surroundings (Reserva Biológica San Francisco, Province Zamora-Chinchipe, southern Ecuador). Ecotropical Monogr. 2008, 4, 155–214. [Google Scholar]
- Fiedler, K.; Brehm, G.; Hilt, N.; Süßenbach, D.; Häuser, C.L. Variation of diversity patterns across moth families along a tropical elevational gradient. In Gradients in a Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2008; Volume 198, pp. 167–179. [Google Scholar] [CrossRef]
- Brehm, G.; Hebert, P.D.N.; Colwell, R.K.; Adams, M.O.; Bodner, F.; Friedemann, K.; Möckel, L.; Fiedler, K. Turning up the heat at a hotspot: DNA barcodes reveal 80% more species of geometrid moths along an Andean elevational gradient. PLoS ONE 2016, 11, e0150327. [Google Scholar] [CrossRef] [PubMed]
- Homeier, J.; Werner, F.A.; Gradstein, S.R.; Breckle, S.W.; Richter, M. Potential vegetation and floristic composition of Andean forests in south Ecuador, with a focus on RBSF. In Gradients in a Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2008; Volume 198, pp. 87–100. [Google Scholar] [CrossRef]
- Mandl, N.; Lehnert, M.; Gradstein, S.R.; Kessler, M.; Abiy, M.; Richter, M. The unique Purdiaea nutans forest of southern Ecuador—Abiotic characteristics and cryptogamic diversity. In Gradients in a Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2008; Volume 198, pp. 275–280. [Google Scholar] [CrossRef]
- Homeier, J.; Breckle, S.W.; Günter, S.; Rollenbeck, R.T.; Leuschner, C. Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species-rich Ecuadorian montane rain forest. Biotropica 2010, 42, 140–148. [Google Scholar] [CrossRef]
- Richter, M.; Diertl, K.-H.; Peters, T.; Bussmann, R.W. Vegetation structures and ecological features of the upper timberline ecotone. In Gradients in a Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2008; Volume 198, pp. 123–135. [Google Scholar] [CrossRef]
- Beck, E.; Bendix, J.; Kottke, I.; Makeschin, F.; Mosandl, R. (Eds.) Gradients in a Tropical Mountain Ecosystem of Ecuador; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2008; Volume 198. [Google Scholar]
- Brehm, G. Diversity of Geometrid Moths in a Montane Rainforest in Ecuador. Ph.D. Thesis, University of Bayreuth, Bayreuth, Germany, 2002. Available online: https://epub.uni-bayreuth.de/1012 (accessed on 17 September 2021).
- Weller, S.J.; Simmons, R.B.; Boada, R.; Conner, W.E. Abdominal modifications occurring in wasp mimics of the ctenuchine-euchromiine clade (Lepidoptera: Arctiidae). Ann. Entomol. Soc. Am. 2000, 93, 920–928. [Google Scholar] [CrossRef]
- Zenker, M.M.; Wahlberg, N.; Brehm, G.; Teston, J.A.; Przybylowicz, L.; Pie, M.R.; Freitas, A.V. Systematics and origin of moths in the subfamily Arctiinae (Lepidoptera, Erebidae) in the Neotropical region. Zool. Scr. 2017, 46, 348–362. [Google Scholar] [CrossRef]
- Rabl, D.; Alonso-Rodríguez, A.M.; Brehm, G.; Fiedler, K. Trait variation in moths mirrors small-scaled ecological gradients in a tropical forest landscape. Insects 2020, 11, 612. [Google Scholar] [CrossRef]
- Guariento, E.; Strutzenberger, P.; Truxa, C.; Fiedler, K. The trinity of ecological contrasts: A case study on rich insect assemblages by means of species, functional and phylogenetic diversity measures. BMC Ecol. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: https://www.nhm.uio.no/english/research/infrastructure/past/ (accessed on 29 September 2021).
- Sam, K.; Koane, B.; Bardos, D.C.; Jeppy, S.; Novotny, V. Species richness of birds along a complete rain forest elevational gradient in the tropics: Habitat complexity and food resources matter. J. Biogeogr. 2019, 46, 279–290. [Google Scholar] [CrossRef]
- Paulsch, D.; Müller-Hohenstein, K. Bird species distribution along an altitudinal gradient in southern Ecuador and its functional relationships with vegetation structure. In Gradients in a Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 198, pp. 149–156. [Google Scholar] [CrossRef]
- Homeier, J.; Werner, F.A.; Gawlik, J.; Peters, T.; Diertl, K.-H.J.; Richter, M. Plant diversity and its relevance for the provision of ecosystem services. In Ecosystem Services, Biodiversity and Environmental Change in a Tropical Mountain Ecosystem of South Ecuador; Bendix, J., Beck, E., Bräuning, A., Makeschin, F., Mosandl, R., Scheu, S., Wilcke, W., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2013; Volume 221, pp. 93–106. [Google Scholar] [CrossRef]
- Homeier, J.; Seeler, T.; Pierick, K.; Leuschner, C. Leaf trait variation in species-rich tropical Andean forests. Sci. Rep. 2021, 11, 9993. [Google Scholar] [CrossRef] [PubMed]
- Descombes, P.; Marchon, J.; Pradervand, J.N.; Bilat, J.; Guisan, A.; Rasmann, S.; Pellissier, L. Community-level plant palatability increases with elevation as insect herbivore abundance declines. J. Ecol. 2017, 105, 142–151. [Google Scholar] [CrossRef]
- Fernandez-Conradi, P.; Defossez, E.; Delavallade, A.; Descombes, P.; Pitteloud, C.; Glauser, G.; Rasmann, S. The effect of community-wide phytochemical diversity on herbivory reverses from low to high elevation. J. Ecol. 2021. [Google Scholar] [CrossRef]
- Dowdy, N.J.; Conner, W.E. Characteristics of tiger moth (Erebidae: Arctiinae) anti-bat sounds can be predicted from tymbal morphology. Front. Zool. 2019, 16, 1–11. [Google Scholar] [CrossRef]
- Terborgh, J. Bird species diversity on an Andean elevational gradient. Ecology 1977, 58, 1007–1019. [Google Scholar] [CrossRef]
- Jankowski, J.E.; Merkord, C.L.; Rios, W.F.; Cabrera, K.G.; Revilla, N.S.; Silman, M.R. The relationship of tropical bird communities to tree species composition and vegetation structure along an Andean elevational gradient. J. Biogeogr. 2013, 40, 950–962. [Google Scholar] [CrossRef]
- Thormann, B.; Ahrens, D.; Espinosa, C.I.; Armijos, D.M.; Wagner, T.; Wägele, J.W.; Peters, M.K. Small-scale topography modulates elevational α-, β-and γ-diversity of Andean leaf beetles. Oecologia 2018, 187, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Brehm, G.; Homeier, J.; Fiedler, K.; Kottke, I.; Illig, J.; Nöske, N.M.; Werner, F.; Breckle, S.-W. Mountain rain forests in southern Ecuador as a hotspot of biodiversity—Limited knowledge and diverging patterns. In Gradients in a Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2008; Volume 198, pp. 15–24. [Google Scholar] [CrossRef]
- Tapia-Armijos, M.F.; Homeier, J.; Espinosa, C.I.; Leuschner, C.; de la Cruz, M. Deforestation and forest fragmentation in South Ecuador since the 1970s—Losing a hotspot of biodiversity. PLoS ONE 2015, 10, e0133701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, I.C.; Shiu, H.-J.; Benedick, S.; Holloway, J.D.; Chey, V.K.; Barlow, H.S.; Hill, J.K.; Thomas, C.D. Elevation increases in moth assemblages over 42 years on a tropical mountain. Proc. Natl. Acad. Sci. USA 2009, 106, 1479–1483. [Google Scholar] [CrossRef] [Green Version]
- Maicher, V.; Sáfián, S.; Murkwe, M.; Delabye, S.; Przybyłowicz, Ł.; Potocký, P.; Kobe, I.N.; Janeček, Š.; Mertens, J.E.J.; Fokam, E.B.; et al. Seasonal shifts of biodiversity patterns and species’ elevation ranges of butterflies and moths along a complete rainforest elevational gradient on Mount Cameroon. J. Biogeogr. 2020, 47, 342–354. [Google Scholar] [CrossRef]
- Laurance, W.F.; Useche, D.C.; Shoo, L.P.; Herzog, S.K.; Kessler, M.; Escobar, F.; Brehm, G.; Axmacher, J.C.; Chen, I.C.; Gámez, L.A.; et al. Global warming, elevational ranges and the vulnerability of tropical biota. Biol. Conserv. 2011, 144, 548–557. [Google Scholar] [CrossRef]

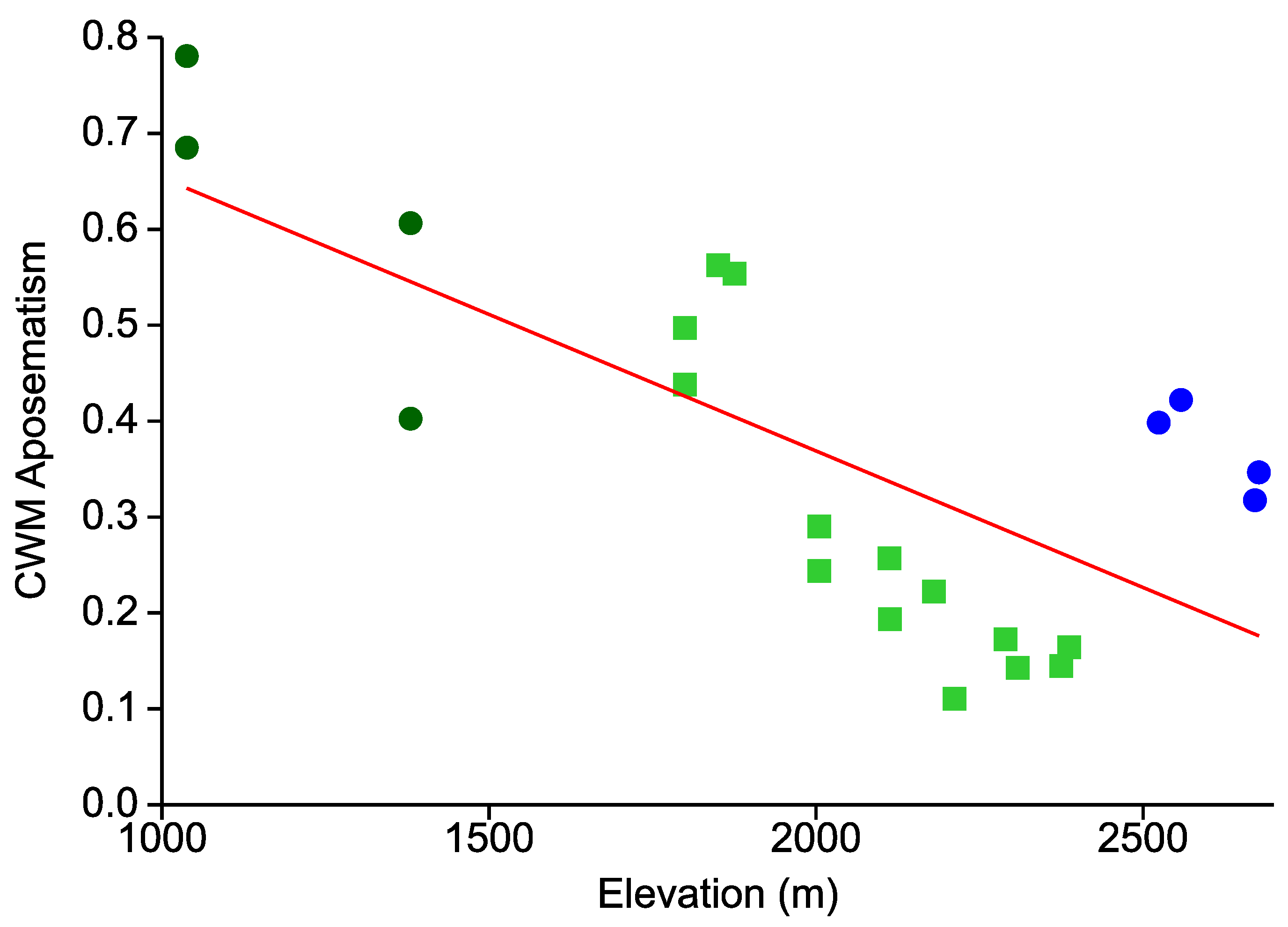
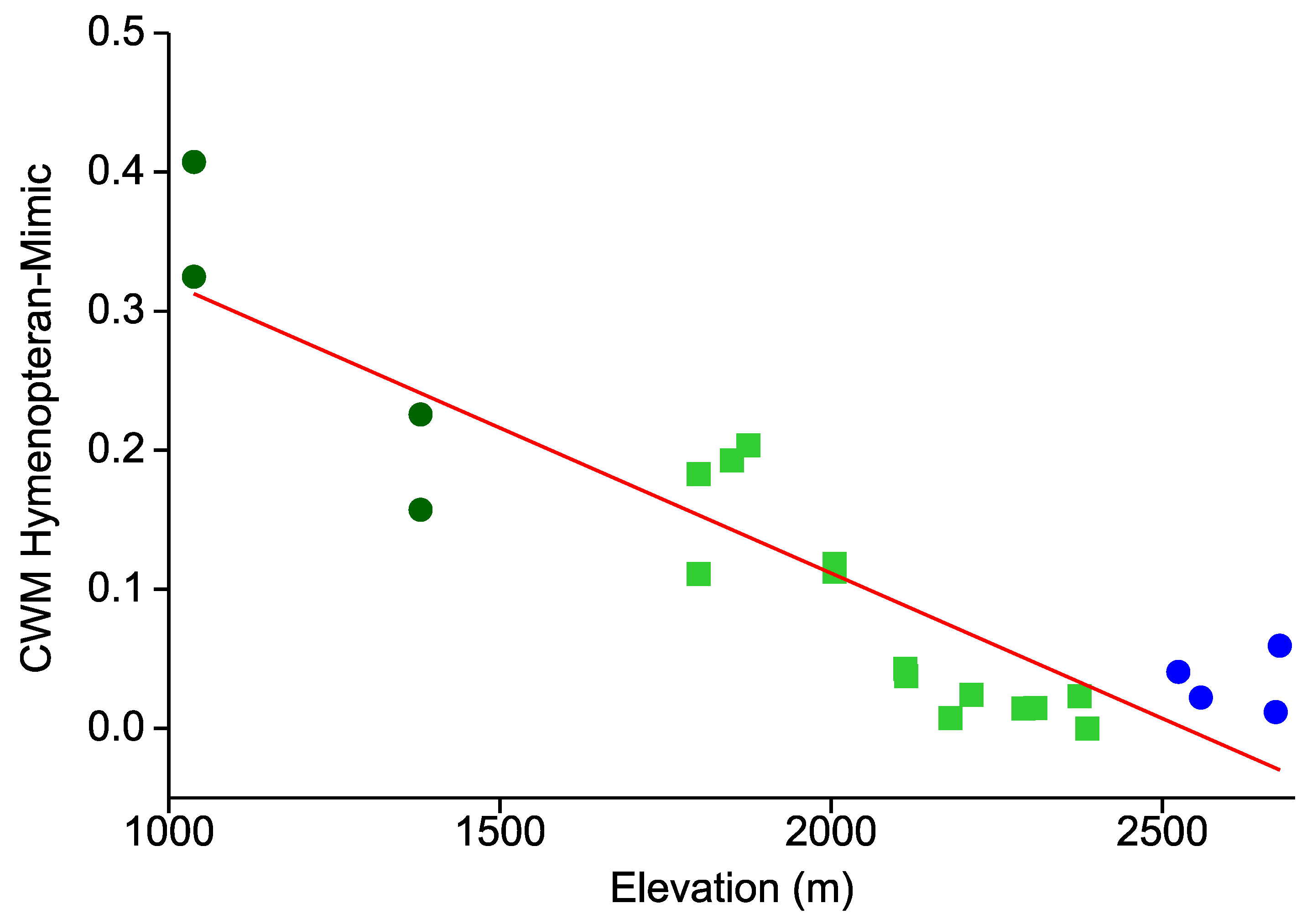


| Taxon | Species | Cryptic | Aposematic | ||
|---|---|---|---|---|---|
| Wasp Mimics | Beetle Mimics | ||||
| Lithosiini | 66 | 55 (83%) | 11 (17%) | 0 | 4 |
| Arctiini | |||||
| Arctiina | 7 | 1 (14%) | 6 (86%) | 0 | 0 |
| Ctenuchina | 71 | 13 (18%) | 58 (82%) | 7 | 6 |
| Euchromiina | 35 | 0 | 35 (100%) | 35 | 0 |
| Pericopina | 13 | 0 | 13 (100%) | 0 | 0 |
| Phaegopterina | 161 | 72 (45%) | 89 (55%) | 1 | 4 |
| Sum Arctiinae | 353 | 141 (40%) | 212 (60%) | 43 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedler, K.; Brehm, G. Aposematic Coloration of Moths Decreases Strongly along an Elevational Gradient in the Andes. Insects 2021, 12, 903. https://doi.org/10.3390/insects12100903
Fiedler K, Brehm G. Aposematic Coloration of Moths Decreases Strongly along an Elevational Gradient in the Andes. Insects. 2021; 12(10):903. https://doi.org/10.3390/insects12100903
Chicago/Turabian StyleFiedler, Konrad, and Gunnar Brehm. 2021. "Aposematic Coloration of Moths Decreases Strongly along an Elevational Gradient in the Andes" Insects 12, no. 10: 903. https://doi.org/10.3390/insects12100903
APA StyleFiedler, K., & Brehm, G. (2021). Aposematic Coloration of Moths Decreases Strongly along an Elevational Gradient in the Andes. Insects, 12(10), 903. https://doi.org/10.3390/insects12100903






