Molecular and Functional Characterization of Pyrokinin-Like Peptides in the Western Tarnished Plant Bug Lygus hesperus (Hemiptera: Miridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
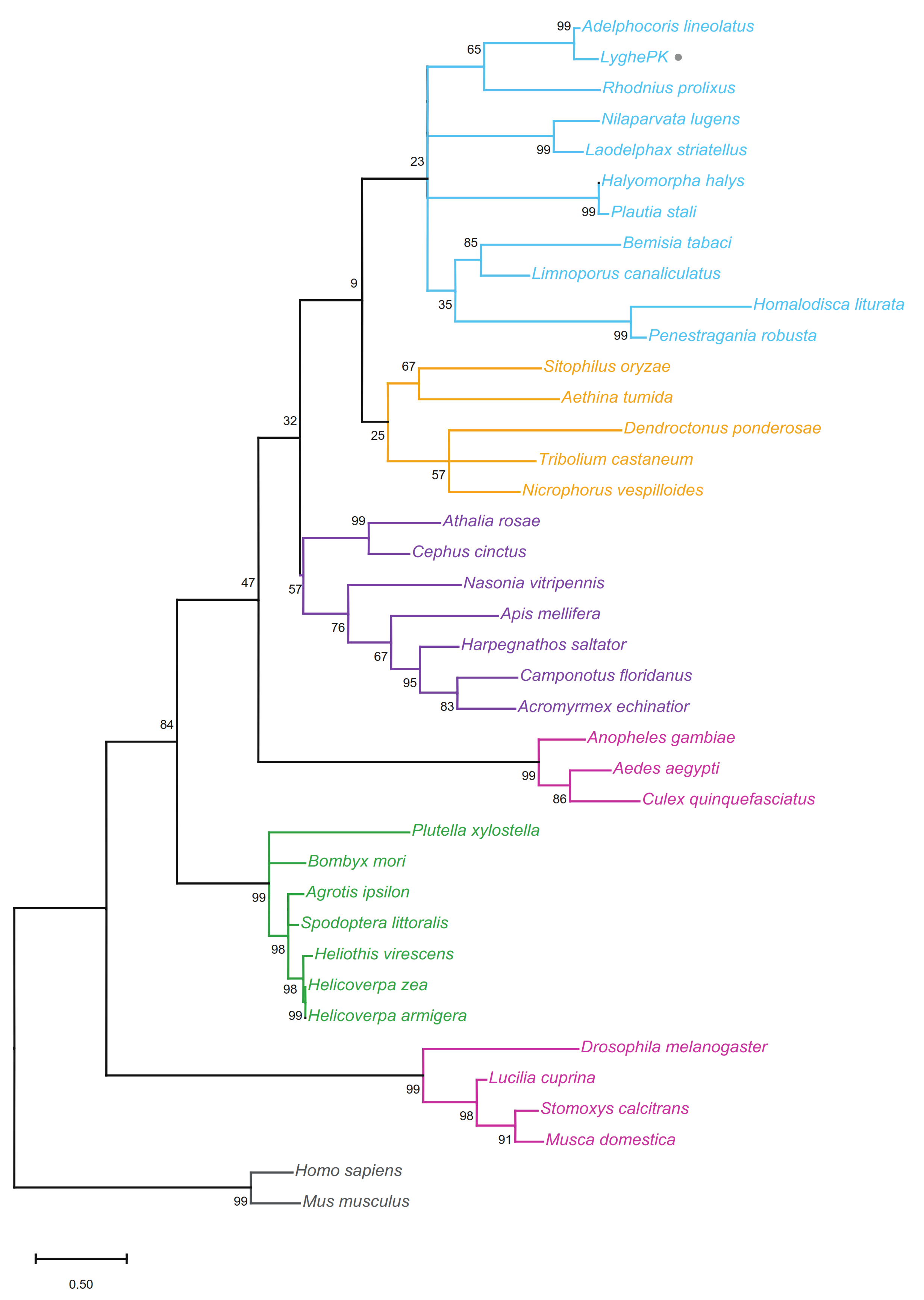
2.2. Multiple Sequence Alignments and Phylogenetics
2.3. RT-PCR-Based Expression Profiling
2.4. Immunohistochemistry
2.5. Insect Cell Culture-Based Characterization of Heterologously Expressed PK Receptor Activation
2.6. In Vivo Characterization of Pheromonotropic Activity
2.6.1. Injection of Synthetic Neuropeptides and Preparation of Pheromone Gland Extracts
2.6.2. Gas Chromatography-Mass Spectrometry Analysis
3. Results
3.1. L. hesperus PK Prepropeptide Transcript
3.2. RT-PCR Expression Profiling
3.3. Localization of FXPRLamide-like Immunoreactivity in the CNS
3.4. Heterologous PK2 Receptor Activation
3.5. In Vivo Pheromonotropic Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nässel, D.R.; Pauls, D.; Huetteroth, W. Neuropeptides in modulation of Drosophila behavior: How to get a grip on their pleiotropic actions. Curr. Opin. Insect Sci. 2019, 36, 1–8. [Google Scholar] [CrossRef]
- Altstein, M.; Nässel, D.R. Neuropeptide signaling in insects. Adv. Exp. Med. Biol. 2010, 692, 155–165. [Google Scholar]
- Bendena, W. Neuropeptide physiology in insects. In Neuropeptide Systems as Targets for Parasite and Pest Control; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2010; Volume 692, pp. 166–191. [Google Scholar]
- Schoofs, L.; De Loof, A.; Van Hiel, M.B. Neuropeptides as regulators of behavior in insects. Annu. Rev. Entomol. 2017, 62, 35–52. [Google Scholar] [CrossRef]
- Predel, R.; Nachman, R.J. The FXPRLamide (pyrokinin/PBAN) peptide family. In Handbook of Biologically Active Peptides; Academic Press: Cambridge, MA, USA, 2006; pp. 207–212. [Google Scholar]
- Jurenka, R. The PRXamide neuropeptide signalling system: Conserved in animals. Adv. Insect Physiol. 2015, 49, 123–170. [Google Scholar]
- Jurenka, R.; Nusawardani, T. The pyrokinin/ pheromone biosynthesis-activating neuropeptide (PBAN) family of peptides and their receptors in Insecta: Evolutionary trace indicates potential receptor ligand-binding domains. Insect Mol. Biol. 2011, 20, 323–334. [Google Scholar] [CrossRef]
- Yaginuma, T.; Niimi, T. FXPRLamide Peptide Family. In Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research; Academic Press: Cambridge, MA, USA, 2015; p. 395. [Google Scholar]
- Holman, G.M.; Cook, B.J.; Nachman, R.J. Primary structure and synthesis of a blocked myotropic neuropeptide isolated from the cockroach, Leucophaea maderae. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1986, 85, 219–224. [Google Scholar] [CrossRef]
- Raina, A.; Jaffe, H.; Kempe, T.; Keim, P.; Blacher, R. Identification of a neuropeptide hormone that regulates sex pheromone production in female moths. Science 1989, 244, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Nagasawa, H.; Kataoka, H.; Inoue, T.; Matsumoto, S.; Ando, T.; Suzuki, A. Amino acid sequence of pheromone-biosynthesis-activating neuropeptide (PBAN) of the silkworm, Bombyx mori. Biochem. Biophys. Res. Commun. 1989, 163, 520–526. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kitamura, A.; Nagasawa, H.; Kataoka, H.; Orikasa, C.; Mitsui, T.; Suzuki, A. Functional diversity of a neurohormone produced by the suboesophageal ganglion: Molecular identity of melanization and reddish colouration hormone and pheromone biosynthesis activating neuropeptide. J. Insect Physiol. 1990, 36, 427–432. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yamashita, O.; Fónagy, A.; Kurihara, M.; Uchiumi, K.; Nagamine, T.; Mitsui, T. Functional diversity of a pheromonotropic neuropeptide: Induction of cuticular melanization and embryonic diapause in lepidopteran insects by Pseudaletia pheromotropin. J. Insect Physiol. 1992, 38, 847–851. [Google Scholar] [CrossRef]
- Imai, K.; Konno, T.; Nakazawa, Y.; Komiya, T.; Isobe, M.; Koga, K.; Goto, T.; Yaginuma, T.; Sakakibara, K.; Hasegawa, K.; et al. Isolation and structure of diapause hormone of the silkworm, Bombyx mori. Proc. Jpn. Acad. B Phys. Biol. Sci. 1991, 67, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Denlinger, D.L. Dynamics of diapause hormone and prothoracicotropic hormone transcript expression at diapause termination in pupae of the corn earworm, Helicoverpa zea. Peptides 2012, 34, 120–126. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Sun, J.-S.; Zhang, L.-B.; Shen, J.-L.; Xu, W.-H. Cloning and expression of the cDNA encoding the FXPRL family of peptides and a functional analysis of their effect on breaking pupal diapause in Helicoverpa armigera. J. Insect Physiol. 2004, 50, 25–33. [Google Scholar] [CrossRef]
- Xu, W.-H.; Denlinger, D.L. Molecular characterization of prothoracicotropic hormone and diapause hormone in Heliothis virescens during diapause, and a new role for diapause hormone. Insect Mol. Biol. 2003, 12, 509–516. [Google Scholar] [CrossRef]
- Uehara, H.; Senoh, Y.; Yoneda, K.; Kato, Y.; Shiomi, K. An FXPRLamide neuropeptide induces seasonal reproductive polyphenism underlying a life-history tradeoff in the tussock moth. PLoS ONE 2011, 6, e24213. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Hull, J.J.; Niimi, T.; Imai, K.; Matsumoto, S.; Yaginuma, T.; Kataoka, H. FXPRL-amide peptides induce ecdysteroidogenesis through a G-protein coupled receptor expressed in the prothoracic gland of Bombyx mori. Mol. Cell. Endocrinol. 2007, 273, 51–58. [Google Scholar] [CrossRef]
- Schoofs, L.; Vanden Broeck, J.; De Loof, A. The myotropic peptides of Locusta migratoria: Structures, distribution, functions and receptors. Insect Biochem. Mol. Biol. 1993, 23, 859–881. [Google Scholar] [CrossRef]
- Zdárek, J.; Verleyen, P.; Mares, M.; Dolecková, L.; Nachman, R.J. Comparison of the effects of pyrokinins and related peptides identified from arthropods on pupariation behaviour in flesh fly (Sarcophaga bullata) larvae (Diptera: Sarcophagidae). J. Insect Physiol. 2004, 50, 233–239. [Google Scholar] [CrossRef]
- Zdárek, J.; Nachman, R.J.; Hayes, T.K. Structure-activity relationships of insect neuropeptides of the pyrokinin/PBAN family and their selective action on pupariation in fleshfly (Neobelleria bullata) larvae (Diptera: Sarcophagidae). Eur. J. Entomol. 1998, 95, 9–16. [Google Scholar]
- Zhao, W.; Li, L.; Zhang, Y.; Liu, X.; Wei, J.; Xie, Y.; Du, M.; An, S. Calcineurin is required for male sex pheromone biosynthesis and female acceptance. Insect Mol. Biol. 2018, 27, 373–382. [Google Scholar] [CrossRef]
- Bober, R.; Rafaeli, A. Gene-silencing reveals the functional significance of pheromone biosynthesis activating neuropeptide receptor (PBAN-R) in a male moth. Proc. Natl. Acad. Sci. USA 2010, 107, 16858–16862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.-Y.; Vander Meer, R.K. Ant trail pheromone biosynthesis is triggered by a neuropeptide hormone. PLoS ONE 2012, 7, e50400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lajevardi, A.; Paluzzi, J.-P.V. Receptor characterization and functional activity of pyrokinins on the hindgut in the adult mosquito, Aedes aegypti. Front. Physiol. 2020, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Melcher, C.; Pankratz, M.J. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 2005, 3, e305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.-Y.; Köhler, R.; Vander Meer, R.K.; Neupert, S.; Predel, R. Identification and expression of capa gene in the fire ant, Solenopsis invicta. PLoS ONE 2014, 9, e94274. [Google Scholar] [CrossRef]
- Xu, W.-H.; Sato, Y.; Ikeda, M.; Yamashita, O. Molecular characterization of the gene encoding the precursor protein of diapause hormone and pheromone biosynthesis activating neuropeptide (DH-PBAN) of the silkworm, Bombyx mori and its distribution in some insects. Biochim. Biophys. Acta 1995, 1261, 83–89. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Sun, J.-S.; Liu, W.-Y.; Kang, L.; Shen, J.-L.; Xu, W.-H. Structural characterization and transcriptional regulation of the gene encoding diapause hormone and pheromone biosynthesis activating neuropeptide in the cotton bollworm, Helicoverpa armigera. Biochim. Biophys. Acta 2005, 1728, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-C.; Ramasamy, S. Identification and expression analysis of diapause hormone and pheromone biosynthesis activating neuropeptide (DH-PBAN) in the legume pod borer, Maruca vitrata Fabricius. PLoS ONE 2014, 9, e84916. [Google Scholar] [CrossRef]
- Fodor, J.; Köblös, G.; Kákai, Á.; Kárpáti, Z.; Molnár, B.P.; Dankó, T.; Bozsik, G.; Bognár, C.; Szőcs, G.; Fónagy, A. Molecular cloning, mRNA expression and biological activity of the pheromone biosynthesis activating neuropeptide (PBAN) from the European corn borer, Ostrinia nubilalis. Insect Mol. Biol. 2017, 31, 355–417. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Wahlström, G.; Immonen, T.; Kolmer, M.; Tirronen, M.; Predel, R.; Kalkkinen, N.; Heino, T.I.; Sariola, H.; Roos, C. The Drosophila hugin gene codes for myostimulatory and ecdysis-modifying neuropeptides. Mech. Dev. 2002, 117, 5–13. [Google Scholar] [CrossRef]
- Derst, C.; Dircksen, H.; Meusemann, K.; Zhou, X.; Liu, S.; Predel, R. Evolution of neuropeptides in non-pterygote hexapods. BMC Evol. Biol. 2016, 16, 51. [Google Scholar] [CrossRef] [Green Version]
- Paluzzi, J.-P.; Park, Y.; Nachman, R.J.; Orchard, I. Isolation, expression analysis, and functional characterization of the first antidiuretic hormone receptor in insects. Proc. Natl. Acad. Sci. USA 2010, 107, 10290–10295. [Google Scholar] [CrossRef] [Green Version]
- Paluzzi, J.-P.; O’Donnell, M.J. Identification, spatial expression analysis and functional characterization of a pyrokinin-1 receptor in the Chagas’ disease vector, Rhodnius prolixus. Mol. Cell. Endocrinol. 2012, 363, 36–45. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Corcoran, J.A.; Vander Meer, R.K.; Choi, M.-Y. Identification and characterization of GPCRs for pyrokinin and CAPA peptides in the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Front. Physiol. 2020, 11, 14. [Google Scholar] [CrossRef]
- Christie, A.E.; Hull, J.J.; Richer, J.A.; Geib, S.M.; Tassone, E.E. Prediction of a peptidome for the western tarnished plant bug Lygus hesperus. Gen. Comp. Endocrinol. 2017, 243, 22–38. [Google Scholar] [CrossRef] [Green Version]
- Hull, J.J.; Gross, R.J.; Brent, C.S.; Christie, A.E. Filling in the gaps: A reevaluation of the Lygus hesperus peptidome using an expanded de novo assembled transcriptome and molecular cloning. Gen. Comp. Endocrinol. 2021, 303, 113708. [Google Scholar] [CrossRef]
- Tanaka, Y.; Suetsugu, Y.; Yamamoto, K.; Noda, H.; Shinoda, T. Transcriptome analysis of neuropeptides and G-protein coupled receptors (GPCRs) for neuropeptides in the brown planthopper Nilaparvata lugens. Peptides 2014, 53, 125–133. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Choi, M.-Y. Identification and characterization of capa and pyrokinin genes in the brown marmorated stink bug, Halyomorpha halys (Hemiptera): Gene structure, immunocytochemistry, and differential expression. Arch. Insect Biochem. Physiol. 2018, 99, e21500. [Google Scholar] [CrossRef]
- Ons, S.; Sterkel, M.; Diambra, L.; Urlaub, H.; Rivera-Pomar, R. Neuropeptide precursor gene discovery in the Chagas disease vector Rhodnius prolixus. Insect Mol. Biol. 2010, 20, 29–44. [Google Scholar] [CrossRef]
- Li, J.J.; Shi, Y.; Lin, G.L.; Yang, C.H.; Liu, T.-X. Genome-wide identification of neuropeptides and their receptor genes in Bemisia tabaci and their transcript accumulation change in response to temperature stresses. Insect Sci. 2020, 83, 409–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavore, A.; Perez-Gianmarco, L.; Esponda-Behrens, N.; Palacio, V.; Catalano, M.I.; Rivera-Pomar, R.; Ons, S. Nezara viridula (Hemiptera: Pentatomidae) transcriptomic analysis and neuropeptidomics. Sci. Rep. 2018, 8, 17244–17315. [Google Scholar] [CrossRef] [PubMed]
- Predel, R.; Russell, W.K.; Russell, D.H.; Lopez, J.; Esquivel, J.; Nachman, R.J. Comparative peptidomics of four related hemipteran species: Pyrokinins, myosuppressin, corazonin, adipokinetic hormone, sNPF, and periviscerokinins. Peptides 2008, 29, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Predel, R.; Russell, W.K.; Russell, D.H.; Suh, C.P.-C.; Nachman, R.J. Neuropeptides of the cotton fleahopper, Pseudatomoscelis seriatus (Reuter). Peptides 2012, 34, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Predel, R.; Neupert, S.; Derst, C.; Reinhardt, K.; Wegener, C. Neuropeptidomics of the bed bug Cimex lectularius. J. Proteome Res. 2018, 17, 440–454. [Google Scholar] [CrossRef]
- Ons, S.; Richter, F.; Urlaub, H.; Pomar, R.R. The neuropeptidome of Rhodnius prolixus brain. Proteomics 2009, 9, 788–792. [Google Scholar] [CrossRef]
- Neupert, S.; Russell, W.K.; Russell, D.H.; Predel, R. Two capa-genes are expressed in the neuroendocrine system of Rhodnius prolixus. Peptides 2010, 31, 408–411. [Google Scholar] [CrossRef]
- Fleites, L.A.; Johnson, R.; Kruse, A.R.; Nachman, R.J.; Hall, D.G.; MacCoss, M.; Heck, M.L. Peptidomics approaches for the identification of bioactive molecules from Diaphorina citri. J. Proteome Res. 2020, 19, 1392–1408. [Google Scholar] [CrossRef]
- Scott, D.R. An annotated listing of host plants of Lygus hesperus Knight. Entomo. Soc. Am. Bull. 1977, 23, 19–22. [Google Scholar] [CrossRef]
- Slosser, J.E.; Boring, E.P., 3rd; Parajulee, M.N. A survey of Lygus spp. occurring in cotton, alfalfa, and roadside weeds in the northern Texas rolling plains. Southwestern Entomol. 2006, 31, 2. [Google Scholar]
- Jackson, C.G.; Debolt, J.W.; Ellington, J.J. Lygus bugs. Biological Control in the Western United States. In Accomplishments and Benefits of Regional Research Project W-84, 1964–1989; UCANR Publications: Davis, CA, USA, 1995; Volume 3361, pp. 87–90. [Google Scholar]
- Parys, K.; Luttrell, R.; Snodgrass, G.; Portilla, M.; Copes, J. Longitudinal measurements of tarnished plant bug (Hemiptera: Miridae) susceptibility to insecticides in Arkansas, Louisiana, and Mississippi: Associations with insecticide use and insect control recommendations. Insects 2017, 8, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Nachman, R.J. Peptidomics applied: A new strategy for development of selective antagonists/agonists of insect pyrokinin (FXPRLamide) family using a novel conformational-mimetic motif. EuPA Open Proteom. 2014, 3, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Wei, Z.; Nachman, R.J.; Kaczmarek, K.; Zabrocki, J.; Park, Y. Functional characterization of five different PRXamide receptors of the red flour beetle Tribolium castaneum with peptidomimetics and identification of agonists and antagonists. Peptides 2015, 68, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Alford, L.; Marley, R.; Dornan, A.; Dow, J.A.T.; Nachman, R.J.; Davies, S.-A. Desiccation, thermal stress and associated mortality in Drosophila fruit flies induced by neuropeptide analogue treatment. J. Pest Sci. 2019, 92, 1123–1137. [Google Scholar] [CrossRef] [Green Version]
- Alford, L.; Marley, R.; Dornan, A.; Pierre, J.S.; Dow, J.A.; Nachman, R.J.; Davies, S.-A. Assessment of neuropeptide binding sites and the impact of biostable kinin and CAP2b analogue treatment on aphid (Myzus persicae and Macrosiphum rosae) stress tolerance. Pest. Manag. Sci. 2019, 75, 1750–1759. [Google Scholar] [CrossRef] [Green Version]
- Debolt, J.W. Meridic diet for rearing successive generations of Lygus hesperus. Ann. Entomol. Soc. Am. 1982, 75, 119–122. [Google Scholar] [CrossRef]
- Patana, R. Disposable diet packet for feeding and oviposition of Lygus hesperus (Hemiptera: Miridae). J. Econ. Entomol. 1982, 75, 668–669. [Google Scholar] [CrossRef]
- Nagy, B. Rearing of the European corn borer (Ostrinia nubilalis Hbn.) on a simplified artificial diet. Acta Phytopathol. Acad. Sci. Hun. 1970, 5, 73–79. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.-J.; Adams, M.E. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc. Natl. Acad. Sci. USA 2002, 99, 11423–11428. [Google Scholar] [CrossRef] [Green Version]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Hejne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Southey, B.R.; Amare, A.; Zimmerman, T.A.; Rodriguez-Zas, S.L.; Sweedler, J.V. NeuroPred: A tool to predict cleavage sites in neuropeptide precursors and provide the masses of the resulting peptides. Nucl. Acids Res. 2006, 34, W267–W272. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-Y.; Rafaeli, A.; Jurenka, R.A. Pyrokinin/PBAN-like peptides in the central nervous system of Drosophila melanogaster. Cell Tissue Res. 2001, 306, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-Y.; Raina, A.; Vander Meer, R.K. PBAN/pyrokinin peptides in the central nervous system of the fire ant, Solenopsis invicta. Cell Tissue Res. 2009, 335, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.W.K.; Roelofs, W.L. Sites of synthesis and release of PBAN-like factor in the female European corn borer, Ostrinia nubilalis. J. Insect Physiol. 1995, 41, 339–350. [Google Scholar] [CrossRef]
- Ito, K.; Shinomiya, K.; Ito, M.; Armstrong, J.D.; Boyan, G.; Hartenstein, V.; Harzsch, S.; Heisenberg, M.; Homberg, U.; Jenett, A.; et al. Insect Brain Name Working Group: A systematic nomenclature for the insect brain. Neuron 2014, 81, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.-Y.; Fuerst, E.-J.; Rafaeli, A.; Jurenka, R. Role of extracellular domains in PBAN/pyrokinin GPCRs from insects using chimera receptors. Insect Biochem. Mol. Biol. 2007, 37, 296–306. [Google Scholar] [CrossRef]
- Hull, J.J.; Ohnishi, A.; Moto, K.; Kawasaki, Y.; Kurata, R.; Suzuki, M.G.; Matsumoto, S. Cloning and characterization of the pheromone biosynthesis activating neuropeptide receptor from the silkmoth, Bombyx mori. Significance of the carboxyl terminus in receptor internalization. J. Biol. Chem. 2004, 279, 51500–51507. [Google Scholar] [CrossRef] [Green Version]
- Fodor, J.; Hull, J.J.; Köblös, G.; Jacquin-Joly, E.; Szlanka, T.; Fónagy, A. Identification and functional characterization of the pheromone biosynthesis activating neuropeptide receptor isoforms from Mamestra brassicae. Gen. Comp. Endocrinol. 2018, 258, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nature Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Hull, J.J.; Fónagy, A. Molecular Basis of Pheromonogenesis Regulation in Moths. In Olfactory Concepts of Insect Control Alternative to Insecticides; Springer International Publishing: Cham, Switzerland, 2019; Volume 1, pp. 151–202. [Google Scholar]
- Jurenka, R. Regulation of pheromone biosynthesis in moths. Curr. Opin. Insect Sci. 2017, 24, 29–35. [Google Scholar] [CrossRef]
- Köblös, G.; Dankó, T.; Sipos, K.; Geiger, Á.; Szlanka, T.; Fodor, J.; Fónagy, A. The regulation of Δ11-desaturase gene expression in the pheromone gland of Mamestra brassicae (Lepidoptera; Noctuidae) during pheromonogenesis. Gen. Comp. Endocrinol. 2015, 221, 217–227. [Google Scholar] [CrossRef]
- Moustafa, M.; Kákai, Á.; Awad, M.; Fónagy, A. Sublethal effects of spinosad and emamectin benzoate on larval development and reproductive activities of the cabbage moth, Mamestra brassicae L. (Lepidoptera: Noctuidae). Crop Prot. 2016, 90, 197–204. [Google Scholar] [CrossRef]
- Moustafa, M.; Fouad, E.A.; Abdel-Mobdy, Y.; Hamow, K.Á.; Mikó, Z.; Molnár, B.P.; Fónagy, A. Toxicity and sublethal effects of chlorantraniliprole and indoxacarb on Spodoptera littoralis (Lepidoptera: Noctuidae). App. Entomol. Zoo. 2021, 56, 115–124. [Google Scholar] [CrossRef]
- Choi, M.-Y.; Sanscrainte, N.D.; Estep, A.S.; Vander Meer, R.K.; Becnel, J.J. Identification and expression of a new member of the pyrokinin/pban gene family in the sand fly Phlebotomus papatasi. J. Insect Physiol. 2015, 79, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Hull, J.J.; Kawai, T.; Tsuneizumi, K.; Kurihara, M.; Tanokura, M.; Nagata, K.; Nagasawa, H.; Matsumoto, S. Establishment of Sf9 transformants constitutively expressing PBAN receptor variants: Application to functional evaluation. Front. Endocrinol. 2012, 3, 56. [Google Scholar] [CrossRef] [Green Version]
- Fónagy, A.; Teal, P.; Meredith, J.; Körmendy, C.; Tumlinson, J. Partial identification of a new pheromonotropic peptide from Mamestra brassicaea. Ann. N. Y. Acad. Sci. 1998, 839, 488–490. [Google Scholar] [CrossRef]
- Kochansky, J.; Cardé, R.T.; Liehberr, J.; Roelofs, W.L. Sex pheromone of the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae), in New York. J. Chem. Ecol. 1975, 1, 225–231. [Google Scholar] [CrossRef]
- Nesbitt, B.F.; Beevor, P.S.; Cole, R.A.; Lester, R.; Poppi, R.G. Sex pheromones of two noctuid moths. Nat. New Biol. 1973, 244, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Yushima, T. Sex pheromone of the cotton leafworm, Spodoptera littoralis. J. Insect Physiol. 1974, 20, 1005–1014. [Google Scholar] [CrossRef]
- Ragionieri, L.; Predel, R. The neuropeptidome of Carabus (Coleoptera, Adephaga: Carabidae). Insect Biochem. Mol. Biol. 2019, 118, 103309. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-Y.; Ahn, S.-J.; Kim, A.Y.; Koh, Y. Identification and characterization of pyrokinin and CAPA peptides, and corresponding GPCRs from spotted wing drosophila, Drosophila suzukii. Gen. Comp. Endocrinol. 2017, 246, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.-Y.; Estep, A.; Sanscrainte, N.; Becnel, J.; Vander Meer, R.K. Identification and expression of PBAN/diapause hormone and GPCRs from Aedes aegypti. Mol. Cell. Endocrinol. 2013, 375, 113–120. [Google Scholar] [CrossRef]
- Neupert, S.; Marciniak, P.; Köhler, R.; Nachman, R.J.; Suh, C.P.-C.; Predel, R. Different processing of CAPA and pyrokinin precursors in the giant mealworm beetle Zophobas atratus (Tenebrionidae) and the boll weevil Anthonomus grandis grandis (Curculionidae). Gen. Comp. Endocrinol. 2018, 258, 53–59. [Google Scholar] [CrossRef]
- Wegener, C.; Reinl, T.; Jänsch, L.; Predel, R. Direct mass spectrometric peptide profiling and fragmentation of larval peptide hormone release sites in Drosophila melanogaster reveals tagma-specific peptide expression and differential processing. J. Neurochem. 2006, 96, 1362–1374. [Google Scholar] [CrossRef]
- Veenstra, J.A. Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch. Insect Biochem. Physiol. 2000, 43, 49–63. [Google Scholar] [CrossRef]
- Amare, A.; Sweedler, J.V. Neuropeptide precursors in Tribolium castaneum. Peptides 2007, 28, 1282–1291. [Google Scholar] [CrossRef] [Green Version]
- Predel, R.; Eckert, M.; Pollák, E.; Molnár, L.; Scheibner, O.; Neupert, S. Peptidomics of identified neurons demonstrates a highly differentiated expression pattern of FXPRLamides in the neuroendocrine system of an insect. J. Comp. Neurol. 2007, 500, 498–512. [Google Scholar] [CrossRef]
- Choi, M.-Y.; Meer, R.K.V.; Shoemaker, D.; Valles, S.M. PBAN gene architecture and expression in the fire ant, Solenopsis invicta. J. Insect Physiol. 2011, 57, 161–165. [Google Scholar] [CrossRef]
- Kuniyoshi, H.; Nagasawa, H.; Ando, T.; Suzuki, A.; Nachman, R.J.; Holman, G.M. Cross-activity between pheromone biosynthesis activating neuropeptide (PBAN) and myotropic pyrokinin insect peptides. Biosci. Biotechnol. Biochem. 1992, 56, 167–168. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, H.; Kuniyoshi, H.; Arima, R.; Kawano, T.; Ando, T.; Suzuki, A. Structure and activity of Bombyx PBAN. Arch. Insect Biochem. Physiol. 1994, 25, 261–270. [Google Scholar] [CrossRef]
- Zheng, L.; Lytle, C.; Njauw, C.-N.; Altstein, M.; Martins-Green, M. Cloning and characterization of the pheromone biosynthesis activating neuropeptide receptor gene in Spodoptera littoralis larvae. Gene 2007, 393, 20–30. [Google Scholar] [CrossRef]
- Abernathy, R.L.; Nachman, R.J.; Teal, P.E.A.; Yamashita, O.; Tumlinson, J.H. Pheromonotropic activity of naturally occurring pyrokinin insect neuropeptides (FXPRLamide) in Helicoverpa zea. Peptides 1995, 16, 215–219. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Nachman, R.; Aimanova, K.; Gill, S.; Adams, M. The pheromone biosynthesis activating neuropeptide (PBAN) receptor of Heliothis virescens: Identification, functional expression, and structure-activity relationships of ligand analogs. Peptides 2008, 29, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Nachman, R.J.; Hamshou, M.; Kaczmarek, K.; Zabrocki, J.; Smagghe, G. Biostable and PEG polymer-conjugated insect pyrokinin analogs demonstrate antifeedant activity and induce high mortality in the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidae). Peptides 2012, 34, 266–273. [Google Scholar] [CrossRef]
- Gui, S.-H.; Taning, C.N.T.; De Schutter, K.; Yang, Q.; Chen, P.Y.; Hamshou, M.; Nachman, R.J.; Pandit, A.A.; Dow, J.A.T.; Davies, S.-A.; et al. Assessment of insecticidal effects and selectivity of CAPA-PK peptide analogues against the peach-potato aphid and four beneficial insects following topical exposure. Pest. Manag. Sci. 2020, 76, 3451–3458. [Google Scholar] [CrossRef]






| Treatment | Z-9-14:Ac | E-11-14:Ac | Z-11-14:Ac | (Z,E)-9,12–14:Ac | (Z,E)-9,11–14:Ac |
|---|---|---|---|---|---|
| control | 1.55 ± 0.18 | 1.11 ± 0.49 | 0.54 ± 0.20 | 3.09 ± 2.08 | 3.25 ± 0.67 |
| Mambr-PT (5 pmol) | 5.14 ± 0.55 | 3.13 ± 0.58 | 1.59 ± 0.33 | 4.09 ± 0.4 | 9.10 ± 0.46 |
| water-injected | 0.25 ± 0.10 | 0.22 ± 0.06 | 0.02 ± 0.02 | 3.19 ± 1.16 | 0.41 ± 0.14 |
| LyghePKb (20 pmol) | 2.4 ± 0.75 | 1.90 ± 0.70 | 0.98 ± 0.39 | 5.30 ± 1.77 | 3.97 ± 0.3 |
| LyghePKb (5 pmol) | 1.92 ± 0.52 | 1.83 ± 0.1 | 1.11 ± 0.55 | 3.77 ± 2.99 | 4.56 ± 1.18 |
| LyghePKb (1.25 pmol) | 0.49 ± 0.25 | 0.42 ± 0.16 | 0.70 ± 0.49 | 3.75 ± 0.65 | 1.83 ± 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hull, J.J.; Brent, C.S.; Choi, M.-Y.; Mikó, Z.; Fodor, J.; Fónagy, A. Molecular and Functional Characterization of Pyrokinin-Like Peptides in the Western Tarnished Plant Bug Lygus hesperus (Hemiptera: Miridae). Insects 2021, 12, 914. https://doi.org/10.3390/insects12100914
Hull JJ, Brent CS, Choi M-Y, Mikó Z, Fodor J, Fónagy A. Molecular and Functional Characterization of Pyrokinin-Like Peptides in the Western Tarnished Plant Bug Lygus hesperus (Hemiptera: Miridae). Insects. 2021; 12(10):914. https://doi.org/10.3390/insects12100914
Chicago/Turabian StyleHull, J. Joe, Colin S. Brent, Man-Yeon Choi, Zsanett Mikó, József Fodor, and Adrien Fónagy. 2021. "Molecular and Functional Characterization of Pyrokinin-Like Peptides in the Western Tarnished Plant Bug Lygus hesperus (Hemiptera: Miridae)" Insects 12, no. 10: 914. https://doi.org/10.3390/insects12100914
APA StyleHull, J. J., Brent, C. S., Choi, M.-Y., Mikó, Z., Fodor, J., & Fónagy, A. (2021). Molecular and Functional Characterization of Pyrokinin-Like Peptides in the Western Tarnished Plant Bug Lygus hesperus (Hemiptera: Miridae). Insects, 12(10), 914. https://doi.org/10.3390/insects12100914








