A Fine-Scale Hotspot at the Edge: Epigean Arthropods from the Atacama Coast (Paposo-Taltal, Antofagasta Region, Chile)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods Used for Sampling Terrestrial Arthropods
2.3. Taxonomic Identification
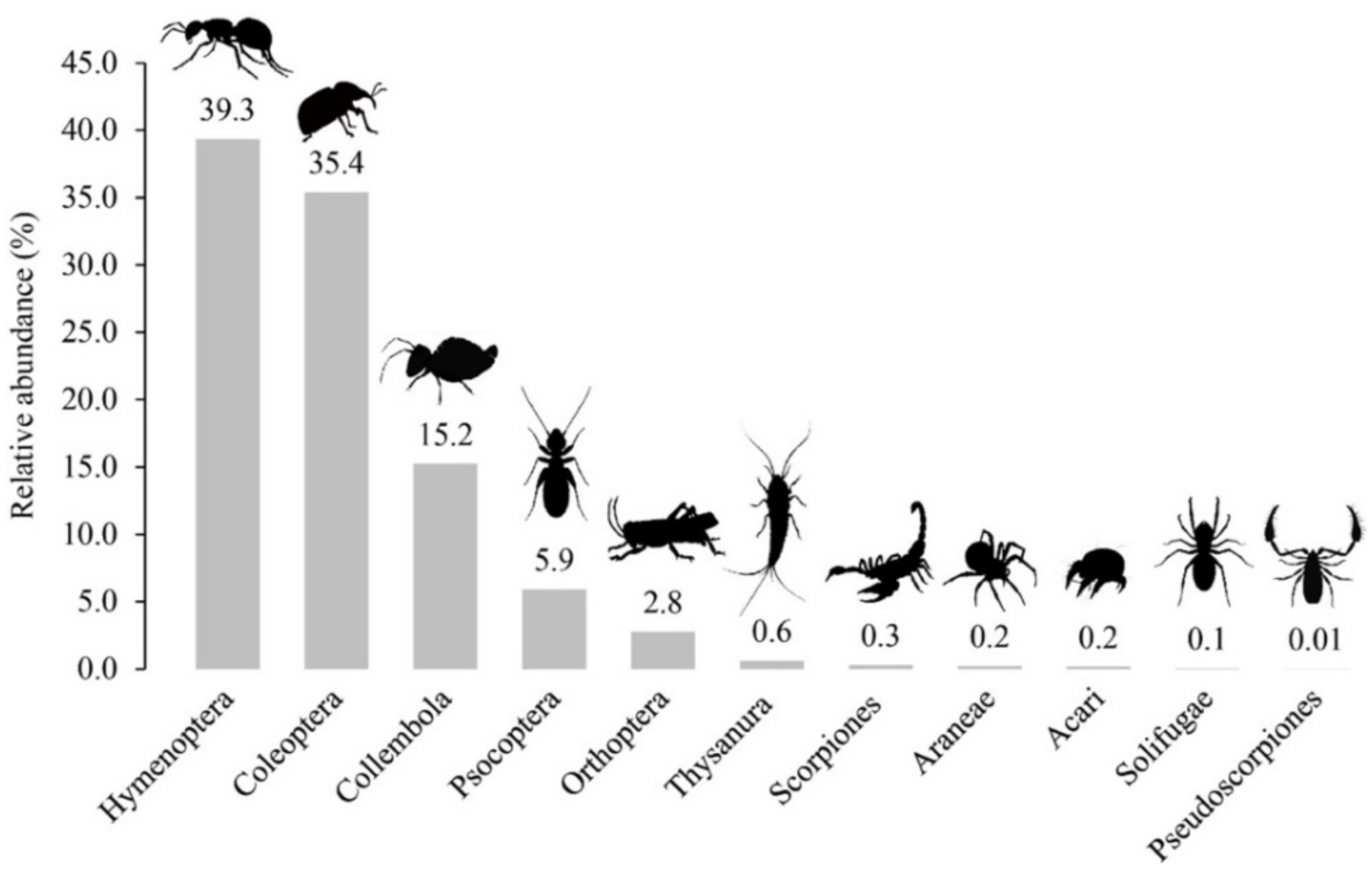
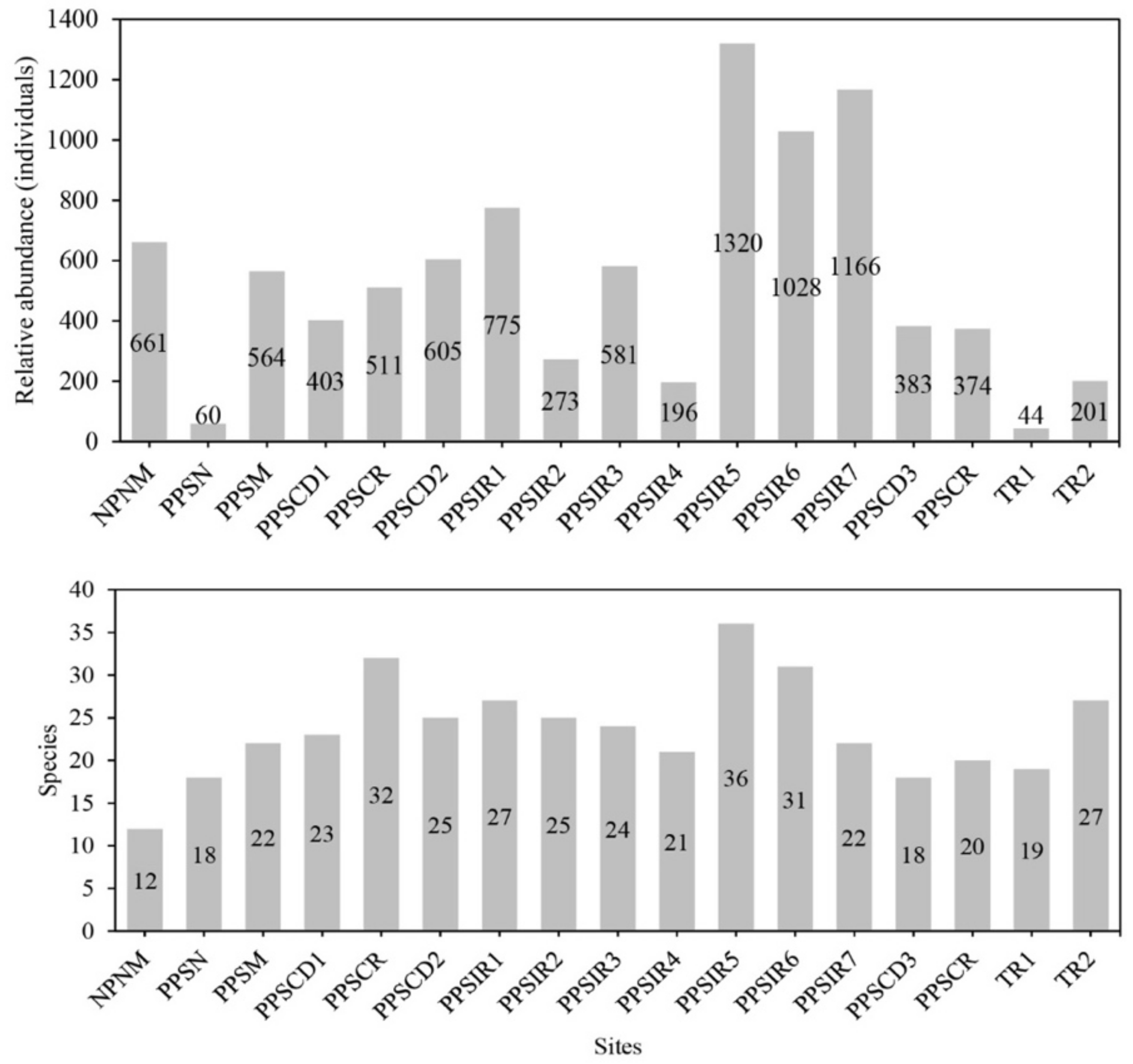
3. Results
Taxonomic Composition and Richness
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Sumser, H.; Ho, T.; Schwan, H.; Stenmans, W.; Mu, A.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Samways, M.J. Translocating fauna to foreign lands: Here comes the Homogenocene. J. Insect Conserv. 1999, 3, 65–66. [Google Scholar]
- Mclean, K.I.; Mushet, D.M.; Sweetman, J.N.; Anteau, M.J.; Wiltermuth, M.T. Invertebrate communities of prairie-pothole wetlands in the age of the aquatic Homogenocene. Hydrobiologia 2020, 847, 3773–3793. [Google Scholar] [CrossRef]
- Milić, D.; Radenković, S.; Ačanski, J.; Vujić, A. The importance of hidden diversity for insect conservation: A case study in hoverflies (the Merodon atratus complex, Syrphidae, Diptera). J. Insect Conserv. 2019, 23, 29–44. [Google Scholar] [CrossRef]
- Somavilla, A.; de Moraes Junior, R.; Oliveira, M.; Rafael, J. Biodiversity of insects in the Amazon: Survey of social wasps (Vespidae: Polistinae) in Amazon rainforest areas in Amazonas State, Brazil. Sociobiology 2020, 67, 312–321. [Google Scholar] [CrossRef]
- Niu, Y.; Ren, G.; Lin, G.; Di Biase, L.; Fattorini, S. Fine-scale vegetation characteristics drive insect ensemble structures in a desert ecosystem: The tenebrionid beetles (Coleoptera: Tenebrionidae) inhabiting the Ulan Buh Desert (Inner Mongolia, China). Insects 2020, 11, 410. [Google Scholar] [CrossRef]
- Packer, L.; Graham, L. Four new species of Isepeolini (Hymenoptera; Apidae) from northern Chile. BMC Zool. 2020, 5, 1–16. [Google Scholar] [CrossRef]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.; New, T.R. The seven impediments in invertebrate conservation and how to overcome them. Biol. Conserv. 2011, 144, 2647–2655. [Google Scholar] [CrossRef]
- Brito, D. Overcoming the Linnean shortfall: Data deficiency and biological survey priorities. Basic Appl. Ecol. 2010, 11, 709–713. [Google Scholar] [CrossRef]
- Moreira-Muñoz, A. Plant Geography of Chile; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Despland, E. Butterflies of the high-altitude Atacama Desert: Habitat use and conservation. Front. Genet. 2014, 5, 1–8. [Google Scholar] [CrossRef][Green Version]
- Böhnert, T.; Weigend, M.; Merklinger, F.; Quandt, D.; Luebert, F. Historical assembly of Zygophyllaceae in the Atacama Desert. Front. Biogeogr. 2020, 12, e45197. [Google Scholar] [CrossRef]
- Moreira-Muñoz, A. Central Chile Ecoregion. In Endemism in Vascular Plants; Hobohm, C., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 221–233. [Google Scholar]
- Klausmeyer, K.R.; Shaw, M.R. Climate change, habitat loss, protected areas and the climate adaptation potential of species in mediterranean ecosystems worldwide. PLoS ONE 2009, 4, 1–9. [Google Scholar] [CrossRef]
- Rundel, P.; Dillon, M.O.; Palma, B.; Mooney, H.; Gulmon, S.; Ehleringer, J. The phytogeography and ecology of the coastal Atacama and Peruvian deserts. Aliso 1991, 13, 1–49. [Google Scholar] [CrossRef]
- Tovar, C.; Sánchez Infantas, E.; Texeira Roth, V. Plant community dynamics of lomas fog oasis of Central Peru after the extreme precipitation caused by the 1997–98 El Niño event. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ruhm, J.; Böhnert, T.; Weigend, M.; Merklinger, F.F.; Stoll, A.; Quandt, D.; Luebert, F. Plant life at the dry limit—Spatial patterns of floristic diversity and composition around the hyperarid core of the Atacama Desert. PLoS ONE 2020, 15, e0233729. [Google Scholar] [CrossRef] [PubMed]
- del Río, C.; Garcia, J.; Osses, P.; Zanetta, N.; Lambert, F.; Rivera, D.; Siegmund, A.; Wolf, N.; Cereceda, P.; Larraín, H.; et al. ENSO influence on coastal fog-water yield in the Atacama Desert, Chile. Aerosol Air Qual. Res. 2018, 18, 127–144. [Google Scholar] [CrossRef]
- Cepeda-Pizarro, J.; Pizarro-Araya, J.; Vásquez, H. Composición y abundancia de artrópodos del Parque Nacional Llanos de Challe: Impactos del ENOS de 1997 y efectos del hábitat pedológico. Rev. Chil. Hist. Nat. 2005, 78, 635–650. [Google Scholar] [CrossRef][Green Version]
- Cepeda-Pizarro, J.; Pizarro-Araya, J.; Vásquez, H. Variación en la abundancia de Arthropoda en un transecto latitudinal del desierto costero transicional de Chile, con énfasis en los tenebriónidos epígeos. Rev. Chil. Hist. Nat. 2005, 78, 651–663. [Google Scholar] [CrossRef]
- Muñoz-Schick, M.; Pinto, R.; Mesa, A.; Moreira-Muñoz, A. Fog oases during the El Niño Southern Oscillation 1997-1998, in the coastal hills south of Iquique, Tarapacá Region, Chile. Rev. Chil. Hist. Nat. 2001, 74, 1–15. [Google Scholar]
- Chávez, R.O.; Moreira-Muñoz, A.; Galleguillos, M.; Olea, M.; Aguayo, J.; Latín, A. GIMMS NDVI time series reveal the extent, duration, and intensity of “blooming desert” events in the hyper-arid Atacama Desert, Northern Chile. Int. J. Appl. Earth. Obs. Geoinf. 2019, 76, 193–203. [Google Scholar] [CrossRef]
- Holmgren, M.; Stapp, P.; Dickman, C.R.; Gracia, C.; Graham, S.; Guti, J.R. A synthesis of ENSO effects on drylands in Australia, North America and South America. Adv. Geosci. 2006, 6, 69–72. [Google Scholar] [CrossRef]
- Block, M.; Richter, M. Impacts of heavy rainfalls in El Niño 1997/98 on the vegetation of Sechura desert in Northern Peru. Phytocoenologia 2000, 30, 491–517. [Google Scholar] [CrossRef]
- Packer, L. Penapis larraini Packer, a new species of rophitine bee (Hymenoptera: Halictidae) from a fog oasis in Northern Chile. Zootaxa 2012, 3408, 54–58. [Google Scholar] [CrossRef]
- Vargas, H.A.; Hundsdoerfer, A.K. Two new native larval host plants of Hyles annei (Guérin-Méneville, 1839) (Lepidoptera, Sphingidae) in the Atacama Desert of Northern Chile following exceptional summer rainfall. Nota Lepidopterol. 2019, 42, 151–156. [Google Scholar] [CrossRef]
- Schulz, N.; Aceituno, P.; Richter, M. Phytogeographic divisions, climate change and plant dieback along the coastal desert of Northern Chile. Erdkunde 2011, 65, 169–187. [Google Scholar] [CrossRef]
- Barrett, B.S.; Campos, D.A.; Veloso, J.V.; Rondanelli, R. Extreme temperature and precipitation events in march 2015 in Central and Northern Chile. J. Geophys. Res. Atmos. 2016, 121, 1–18. [Google Scholar] [CrossRef]
- Bozkurt, D.; Rondanelli, R.; Garreaud, R.; Arriagada, A. Impact of warmer eastern tropical Pacific SST on the march 2015 Atacama Floods. Mon. Weather Rev. 2016, 144, 4441–4460. [Google Scholar] [CrossRef]
- Philippi, R.A. Viaje al Desierto de Atacama Hecho de Orden del Gobierno Chileno en el Verano de 1853–54. Halle en Sajonia: Santiago, Chile, 1860. [Google Scholar]
- Pizarro-Araya, J.; Jerez, V. Distribución geográfica del género Gyriosomus Guérin-Méneville, 1834 (Coleoptera: Tenebrionidae): Una aproximación biogeográfica. Rev. Chil. Hist. Nat. 2004, 77, 491–500. [Google Scholar] [CrossRef]
- Johnston, I.M. Papers on the flora of Northern Chile. Contrib. Gray Herb. 1929, 4, 1–172. [Google Scholar]
- Finger, K.; Teillier, S. Contribución al Conocimiento de la flora endémica de Taltal y Paposo, Región de Antofagasta (II), Chile. Chloris Chil. 2010, 2, 1–12. [Google Scholar]
- Moat, J.; Orellana-Garcia, A.; Tovar, C.; Arakaki, M.; Arana, C.; Cano, A.; Faundez, L.; Gardner, M.; Hechenleitner, P.; Hepp, J.; et al. Seeing through the clouds—Mapping desert fog oasis ecosystems using 20 years of MODIS imagery over Peru and Chile. Int. J. Appl. Earth Obs. Geoinf. 2021, 103, 1–14. [Google Scholar] [CrossRef]
- Sharifi, N.M.I.R.; Graham, L.; Packer, L. Fifteen new species of Liphanthus Reed (Hymenoptera: Andrenidae) with two bubmarginal cells. Zootaxa 2019, 4645, 1–80. [Google Scholar] [CrossRef] [PubMed]
- Dumesh, S.; Packer, L. Three new species of Neofidelia (Hymenoptera: Apoidea: Megachilidae) from Northern Chile. Zootaxa 2013, 3609, 471–483. [Google Scholar] [CrossRef]
- Mondaca, J.; Pizarro-Araya, J.; Alfaro, F.M. Revision of the genus Luispenaia Martínez (Coleoptera: Scarabaeidae: Melolonthinae: Tanyproctini), with description of three new species from the Atacama Desert, Chile. Zootaxa 2019, 4615, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.E. Catálogo de los Tenebrionidae (Coleoptera) de Chile. Entomol. Arb. aus dem Museum Georg. Frey 1966, 17, 397–453. [Google Scholar]
- Peña, L.E. Revisión del género Nycterinus Eschscholtz, 1829 (Coleoptera-Tenebrionidae). Bol. Mus. Nac. Hist. Nat. 1971, 32, 129–158. [Google Scholar]
- Ferrú, M.A.; Elgueta, M. Lista de coleópteros (Insecta: Coleoptera) de las regiones de Arica y Parinacota y de Tarapacá, Chile. Bol. Mus. Nac. Hist. Nat. 2011, 60, 9–61. [Google Scholar]
- Silvestro, V.A. Revision of the chilean genus Diastoleus Solier (Coleoptera: Tenebrionidae), with a preliminary phylogenetic analysis of the tribe Scotobiini. Ann. Zool. 2019, 69, 113–131. [Google Scholar]
- Zuñiga-Reinoso, A.; Pinto, P.; Predel, R. A new species of Gyriosomus Guérin-Méneville (Coleoptera: Tenebrionidae) from the chilean Atacama Desert. Ann. Zool. 2019, 69, 105–112. [Google Scholar]
- Dajoz, R. Contribution a l’etude des coleopteres Lathridiidae du Chili. Biol. l’Amerique Australe 1967, 3, 587–609. [Google Scholar]
- Moore, T. Ectinogonia barrigai nov. sp.: Primera especie de bupréstido del Monumento Natural Paposo Norte, Región de Antofagasta, Chile (Coleoptera: Buprestidae). Rev. Chil. Entomol. 2017, 42, 5–10. [Google Scholar]
- Huber, B.A. New world pholcid spiders (Araneae: Pholcidae): A revision at generic level. Bull. Am. Museum Nat. Hist. 2000, 254, 4–348. [Google Scholar] [CrossRef]
- Platnick, N.I.; Shadab, M.U.; Sorkin, L.N. On the chilean spiders of the family Prodidomidae (Araneae, Gnaphosoidea) with a revision of the genus Moreno Mello-Leitão. Am. Museum Novit. 2005, 3499, 1–31. [Google Scholar] [CrossRef]
- Laborda, A.; Ramírez, M.J.; Pizarro-Araya, J. New species of the spider genera Aysenia and Aysenoides from Chile and Argentina: Description and phylogenetic relationships (Araneae: Anyphaenidae, Amaurobioidinae). Zootaxa 2013, 3731, 133–152. [Google Scholar] [CrossRef]
- Brescovit, A.D.; Sánchez-Ruiz, A. Descriptions of two new genera of the spider family Caponiidae (Arachnida, Araneae) and an update of Tisentnops and Taintnops from Brazil and Chile. Zookeys 2016, 622, 47–84. [Google Scholar] [CrossRef]
- Covarrubias, R. Oribatides (Acarina) of Chili: Licnodamaeolus travei n. gen., n. sp. Acarologia 1998, 39, 157–164. [Google Scholar]
- Noodt, W. Estudios sobre crustáceos chilenos de aguas subterráneas II. nueva Ingolfiella de aguas subterráneas límnicas de Las Lomas de Paposo en el Norte de Chile. Investig. Zoológicas Chil. 1961, 7, 7–16. [Google Scholar]
- Ojanguren-Affilastro, A.A.; Pizarro-Araya, J. Two new scorpion species from Paposo, in the coastal desert of Taltal, Chile (Scorpiones, Bothriuridae, Brachistosternus). Zootaxa 2014, 3785, 400–418. [Google Scholar] [CrossRef] [PubMed]
- Ojanguren-Affilastro, A.A.; Pizarro-Araya, J.; Ochoa-Cámara, J.A. Five new scorpion species of genus Brachistosternus (Scorpiones: Bothriuridae) from the deserts of Chile and Peru, with comments about some poorly studied diagnostic characters of the genus. Zootaxa 2018, 4531, 151–194. [Google Scholar] [CrossRef] [PubMed]
- Jerez, V. Diversidad y patrones de distribución geográfica de insectos coleópteros en ecosistemas desérticos de la Región de Antofagasta, Chile. Rev. Chil. Hist. Nat. 2000, 73, 79–92. [Google Scholar] [CrossRef]
- Giraldo-Mendoza, A.E. Distribución de las especies de Epitragini (Coleoptera: Tenebrionidae) en Perú. Rev. Chil. Entomol. 2020, 46, 563–575. [Google Scholar]
- Giraldo-Mendoza, A.; Arellano Cruz, G. Resiliencia de la comunidad epígea de Coleoptera en Las Lomas de Lachay despueés del evento El Ninño 1997-98. Ecol. Apl. 2003, 2, 59–68. [Google Scholar] [CrossRef][Green Version]
- Fora Quispe, O. Diversidad y Distribución de la Artropofauna en la Quebrada de Las Brujas, Distrito de Sama—Tacna. Bachelor thesis, Universidad Nacional Jorge Basadre Grohmann, Tacna, Peru, 2017. [Google Scholar]
- Perla Gutiérrez, D. Diversidad y Distribución de la Familia Coccinellidae (Coleoptera: Cucujoidea), en un Gradiente Altitudinal, en la Cuenca del Río Cañete, Perú (2009–2010); Universidad Ricardo Palma: Lima, Peru, 2018. [Google Scholar]
- Aguilar, P. Fauna desértico-costera peruana—I Invertebrados más frecuentes en Las Lomas. Rev. Peru. Entomol. 1976, 19, 67–70. [Google Scholar]
- Gajardo, R. La Vegetación Natural de Chile. Clasificación y Distribución Geográfica; Editorial Universitaria: Santiago de Chile, Chile, 1994. [Google Scholar]
- Rundel, P.W.; Dillon, M.O. Ecological patterns in the Bromeliaceae of the Lomas formation of coastal Chile and Peru. Plant Syst. Evol. 1998, 212, 261–278. [Google Scholar] [CrossRef]
- Hartley, A.J.; Chong, G.; Houston, J.; Mather, A.E. 150 million years of climatic stability: Evidence from the Atacama Desert, Northern Chile. J. Geol. Soc. London. 2005, 162, 421–424. [Google Scholar] [CrossRef]
- Rundel, P.W.; Villagra, P.E.; Dillon, M.O.; Roig-Juñent, S.; Debandi, G. Deserts and semi-desert environments. In The Physical Geography of South America; Oxford Regional Environment Series: Oxford, UK, 2007; pp. 158–183. [Google Scholar]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile, 2nd ed.; Editorial Universitaria: Santiago de Chile, Chile, 2017. [Google Scholar]
- Juliá, C.; Montecinos, S.; Maldonado, A. Características climáticas de la Región de Atacama. In Libro Rojo de la Flora Nativa de los Sitios Prioritarios para su Conservación: Región de Atacama; Squeo, F.A., Arancio, G., Gutiérrez, J.R., Eds.; Ediciones Universidad de La Serena: La Serena, Chile, 2008; pp. 25–42. [Google Scholar]
- Sarricolea, P.; Herrera-Ossandon, M.; Meseguer-Ruiz, O. Climatic regionalisation of continental Chile. J. Maps 2016, 13, 66–73. [Google Scholar] [CrossRef]
- Flores, G.E.; Lagos, S.J.; Roig-Juñent, S. Artrópodos epígeos que viven bajo la copa del algarrobo (Prosopis flexuosa) en la Reserva Telteca (Mendoza, Argentina). Multequina 2004, 13, 71–90. [Google Scholar]
- Pizarro-Araya, J.; Alfaro, F.M.; Castillo, J.P.; Ojanguren-Affilastro, A.A.; Agusto, P.; Cepeda-Pizarro, J. Assemblage of arthropods in the Quebrada del Morel Private Protected Area (Atacama Region, Chile). Pan Pacific Entomol. 2012, 88, 1–14. [Google Scholar] [CrossRef]
- Torres-Contreras, H. Antecedentes biológicos de hormigas presentes en Chile publicados en revistas científicas nacionales y extranjeras durante el siglo XX. Rev. Chil. Hist. Nat. 2001, 74, 653–668. [Google Scholar] [CrossRef]
- Ceccarelli, F.S.; Pizarro-Araya, J.; Ojanguren-Affilastro, A.A. Phylogeography and population structure of two Brachistosternus species (Scorpiones: Bothriuridae) from the chilean coastal desert—The perils of coastal living. Biol. J. Linn. Soc. 2016, 120, 75–89. [Google Scholar]
- Marcuzzi, G. New species of Neotropical Tenebrionidae (Coleoptera). Ann. Hist. Musei Natl. Hungarici 1976, 68, 117–140. [Google Scholar]
- Lourenço, W.R. Considerations sur la morphologie, écologie et biogéographie de Caraboctonus keyserlingi Pocock (Scorpiones, Iuridae). Bol. Soc. Biol. Concepc. 1995, 66, 63–69. [Google Scholar]
- Ojanguren-Affilastro, A.A.; Mattoni, C.I.; Ochoa, J.A.; Ramírez, M.J.; Ceccarelli, F.S.; Prendini, L. Phylogeny, species delimitation and convergence in the South American bothriurid scorpion genus Brachistosternus Pocock 1893: Integrating morphology, nuclear and mitochondrial DNA. Mol. Phylogenet. Evol. 2016, 94, 159–170. [Google Scholar] [CrossRef]
- Squeo, F.A.; Cavieres, L.A.; Arancio, G.; Novoa, J.E.; Matthei, O.; Marticorena, C.; Rodríguez, R.; Arroyo, M.T.K.; Muñoz-Schick, M. Biodiversidad de la flora vascular en la Región de Antofagasta, Chile. Rev. Chil. Hist. Nat. 1998, 71, 571–591. [Google Scholar]
- Estrada, P.; Solervicens, J. Revisión taxonómica de las especies chilenas del género Arthrobrachus Solier, 1849 (Coleoptera: Melyridae). Acta Entomol. Chil. 1999, 23, 41–81. [Google Scholar]
- Cepeda-Pizarro, J.; Whitford, W.G. The relationships between abiotic factors and the abundance patterns of soil microarthropods on a desert watershed. Pedobiologia 1989, 33, 79–86. [Google Scholar]
- Cepeda-Pizarro, J.; Gutiérrez, J.R.; Valderrama, L.; Vásquez, H. Phenology of the edaphic microarthropods in a chilean coastal desert site and their response to water and nutrient amendments to the soil. Pedobiologia 1996, 40, 352–363. [Google Scholar]
- MMA. Clasificación Según Estado de Conservación. Available online: https://clasificacionespecies.mma.gob.cl/ (accessed on 14 September 2021).
- Gjerde, I.; Saetersdal, M.; Rolstad, J.; Blom, H.H.; Storaunet, K.O. Fine-scale diversity and rarity hotspots in Northern forests. Conserv. Biol. 2004, 18, 1032–1042. [Google Scholar] [CrossRef]
- Grant, P.B.C.; Samways, M.J. Micro-hotspot determination and buffer zone value for Odonata in a globally significant biosphere reserve. Biol. Conserv. 2011, 144, 772–781. [Google Scholar] [CrossRef]
- Sander, J.; Wardell-Johnson, G. Fine-scale patterns of species and phylogenetic turnover in a global biodiversity hotspot: Implications for climate change vulnerability. J. Veg. Sci. 2011, 22, 766–780. [Google Scholar] [CrossRef]
- Maceda-Veiga, A.; Baselga, A.; Sousa, R.; Vila, M.; Doadrio, I.; de Sostoa, A. Fine-scale determinants of conservation value of river reaches in a hotspot of native and non-native species diversity. Sci. Total Environ. 2017, 574, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, J.; Talebi, A.; Doostmohammadi, M.; Rumpf, S.B.; Linder, H.P.; Schneeweiss, G.M. Hotspots within a global biodiversity hotspot-areas of endemism are associated with high mountain ranges. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Castuera-Oliveira, L.; Teixeira de Oliveira-Filho, A.; Eisenlohr, P. V Emerging hotspots of tree richness in Brazil. Acta Bot. Brasilica 2020, 34, 117–134. [Google Scholar] [CrossRef]
- Birkhofer, K.; Henschel, J.; Lubin, Y. Effects of extreme climatic events on small-scale spatial patterns: A 20-year study of the distribution of a desert spider. Oecologia 2012, 170, 651–657. [Google Scholar] [CrossRef]
- Hernández-Teixidor, D.; Santos, I.; Suárez, D.; Oromí, P. The importance of threatened host plants for arthropod diversity: The fauna associated with dendroid Euphorbia plants endemic to the Canary and Madeira archipelagos. J. Insect Conserv. 2020, 24, 867–876. [Google Scholar] [CrossRef]
- Ramírez-Fischer, F.J.; Benyamini, D.; Vargas, H.A. An endangered hemiparasitic shrub is the only host plant of the little-known Neotropical hairstreak Strymon flavaria (Lepidoptera: Lycaenidae) in the arid Andes. J. Insect Conserv. 2016, 20, 923–928. [Google Scholar] [CrossRef]
- De Araujo Lira, A.; Badillo-Montaño, R.; Lira-Noriega, A.; Ribeiro de Albuquerque, C. Potential distribution patterns of scorpions in north-eastern Brazil under scenarios of future climate change. Austral Ecol. 2020, 45, 215–228. [Google Scholar] [CrossRef]
- Bellard, C.; Leclerc, C.; Leroy, B.; Bakkenes, M.; Veloz, S.; Thuiller, W.; Courchamp, F. Vulnerability of biodiversity hotspots to global change. Glob. Ecol. Biogeogr. 2014, 23, 1–11. [Google Scholar] [CrossRef]
- Khan, A.S.; Cundill, G. Hotspots 2.0: Toward an integrated understanding of stressors and response options. Ambio 2019, 48, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Baur, B.; Coray, A.; Lenzin, H.; Schmera, D. Factors contributing to the decline of an endangered flightless longhorn beetle: A 20-year study. Insect Conserv. Divers. 2020, 13, 175–186. [Google Scholar] [CrossRef]
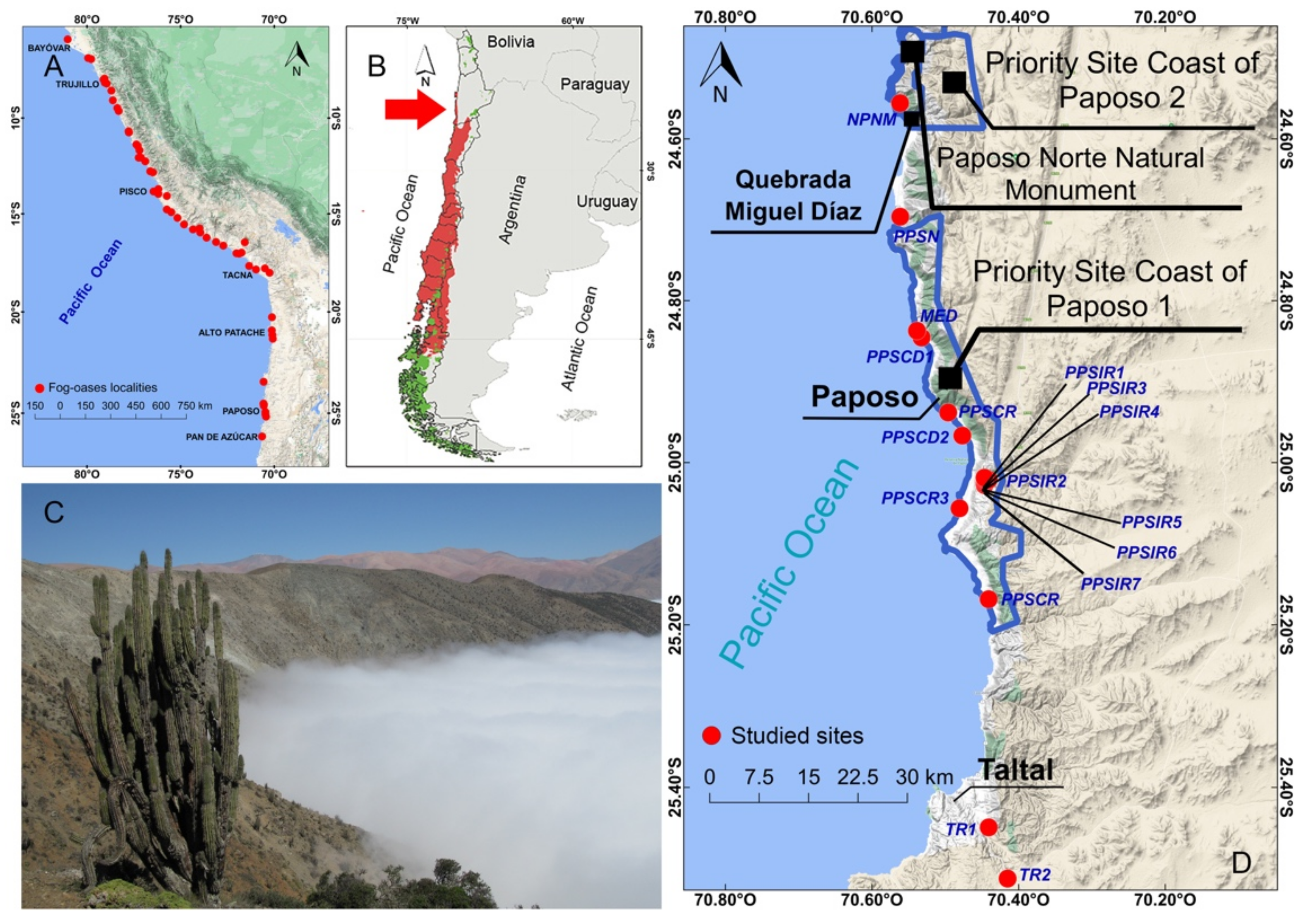


| N° Site | Site Code | ||||
|---|---|---|---|---|---|
| 1 | NPNM | Latitude | Longitude | Year | Habitat Description |
| 2 | PPSN | −24.578962° | −70.552316° | 2019 | Sandy beach with open scrub of Tetragonia sp. (Aizoceae). |
| 3 | PPSM | −24.694903° | −70.561567° | 2017 | Coastal plain (40 m asl) with scarce vegetation. |
| 4 | PPSCD1 | −24.840082° | −70.536391° | 2019 | Cliffs (70 m asl) with sandy-rocky substrate with scarce vegetation. |
| 5 | PPSCR | −24.851444° | −70.526972° | 2015 | Coastal plain, (50 m asl), sandy substrate, open scrub of Skytanthus acutus (Apocynaceae). |
| 6 | PPSCD2 | −24.939056° | −70.490389° | 2015 | Coastal plain, alluvial cone (140 m asl), open scrub of Euphorbia lactiflua (Euphorbiaceae). |
| 7 | PPSIR1 | −24.968222° | −70.476667° | 2015 | Coastal plain with reefs (escollos) (15 m asl), low scrub of Cristaria integerrima, Tetragonia sp., and Nolana sp. (Solanaceae). |
| 8 | PPSIR2 | −25.002861° | −70.446944° | 2015 | Ravine to leeward (676 m asl), low scrub of Frankenia chilensis (Frankeniaceae) and Mesembryanthemum crystallinum (Aizoceae). |
| 9 | PPSIR3 | −25.006307° | −70.426788° | 2017 | Interior ravine (1007 m asl), open scrub of Copiapoa sp. (Cactaceae) and Heliotropium sp. (Heliotropiaceae). |
| 10 | PPSIR4 | −25.005639° | −70.447556° | 2015 | Ravine to leeward (664 m asl), open scrub of Proustia cuneifolia ssp. tipia (Asteraceae) and cacti (Eulychnia iquiquensis). |
| 11 | PPSIR5 | −25.006528° | −70.446583° | 2015 | Ravine to leeward (645 m asl), interior scrub of Ophryosporus triangularis (Asteraceae) with cacti (Eulychnia iquiquensis) and Cistanthe sp. (Montiaceae). |
| 12 | PPSIR6 | −25.010556° | −70.447583° | 2015 | Ravine to leeward (592 m asl), interior scrub of Euphorbia lactiflua with cacti (Eulychnia iquiquensis). |
| 13 | PPSIR7 | −25.010556° | −70.447194° | 2015 | Ravine to leeward (588 m asl), interior scrub of Ophryosporus triangularis with cacti (Eulychnia iquiquensis). |
| 14 | PPSCD3 | −25.010417° | −70.446111° | 2015 | Ravine to leeward (595 m asl), interior scrub of Euphorbia lactiflua with cacti (Eulychnia iquiquensis). |
| 15 | PPSCR | −25.055935° | −70.481731° | 2019 | Coastal terrace (8 m asl) with low open scrub of Nolana crassulifolia and Bakerolimon plumosum (Plumbaginaceae). |
| 16 | TR1 | −25.171344° | −70.436829° | 2019 | Coastal plain (88 m asl) with open scrub of Heliotropium sp., Eulychnia sp. and Copiapoa sp. |
| 17 | TR2 | −25.448125° | −70.435046° | 2017 | Interior ravine (620 m asl) with open scrub of Euphorbia lactiflua, Polyachyrus sp. (Asteraceae), Nolana crassulifolia, and Eulychnia iquiquensis. |
| Taxa | |||||
|---|---|---|---|---|---|
| Class-Order | Family | Genus | Species | Genus/Family | Species/Family |
| Arachnida | 14 | 21 | 27 | 1.50 | 1.92 |
| Acari | 2 | 3 | 7 | 1.50 | 3.50 |
| Araneae | 7 | 11 | 11 | 1.83 | 1.83 |
| Pseudoscorpiones | 1 | 1 | 1 | 1.00 | 1.00 |
| Scorpiones | 2 | 4 | 6 | 2.00 | 3.00 |
| Solifugae | 2 | 2 | 2 | 1.00 | 1.00 |
| Insecta | 43 | 97 | 146 | 2.25 | 3.39 |
| Coleoptera | 26 | 62 | 96 | 2.38 | 3.69 |
| Collembola | 5 | 8 | 8 | 1.60 | 1.60 |
| Hymenoptera | 3 | 11 | 21 | 3.67 | 7.00 |
| Orthoptera | 6 | 10 | 13 | 1.67 | 2.17 |
| Psocoptera | 2 | 4 | 6 | 2.00 | 3.00 |
| Thysanura | 1 | 2 | 2 | 2.00 | 2.00 |
| Order | Family | Species | Relative Abundance | Percentage |
|---|---|---|---|---|
| Acari | Caeculidae | 1 | 2 | 0.02 |
| Indeterminate | 6 | 14 | 0.15 | |
| Araneae | Araneidae | 3 | 6 | 0.07 |
| Thomisidae | 1 | 1 | 0.01 | |
| Gnaphosidae | 2 | 5 | 0.05 | |
| Sicariidae | 2 | 3 | 0.03 | |
| Theridiidae | 1 | 3 | 0.03 | |
| Anyphaenidae | 1 | 1 | 0.01 | |
| Indeterminate | 1 | 1 | 0.01 | |
| Pseudoscorpiones | Cheiridiidae | 1 | 1 | 0.01 |
| Scorpiones | Bothriuridae | 5 | 23 | 0.24 |
| Caraboctonidae | 1 | 5 | 0.05 | |
| Solifugae | Daesiidae | 1 | 1 | 0.01 |
| Mummuciidae | 1 | 4 | 0.04 | |
| Coleoptera | Anthicidae | 1 | 35 | 0.38 |
| Buprestidae | 2 | 53 | 0.58 | |
| Carabidae | 4 | 283 | 3.09 | |
| Cerambycidae | 1 | 1 | 0.01 | |
| Chrysomelidae | 3 | 11 | 0.12 | |
| Cleridae | 1 | 7 | 0.08 | |
| Corylophidae | 1 | 3 | 0.03 | |
| Cryptophagidae | 2 | 4 | 0.04 | |
| Curculionidae | 26 | 205 | 2.24 | |
| Elateridae | 1 | 1 | 0.01 | |
| Histeridae | 1 | 5 | 0.05 | |
| Latridiidae | 5 | 25 | 0.27 | |
| Leiodidae | 1 | 101 | 1.10 | |
| Mauroniscidae | 1 | 5 | 0.05 | |
| Meloidae | 3 | 16 | 0.17 | |
| Melyridae | 3 | 1.800 | 19.68 | |
| Mordellidae | 1 | 5 | 0.05 | |
| Nitidulidae | 2 | 10 | 0.11 | |
| Oedemeridae | 1 | 26 | 0.28 | |
| Phengodidae | 1 | 27 | 0.30 | |
| Ptiliidae | 4 | 6 | 0.07 | |
| Ptinidae | 3 | 4 | 0.04 | |
| Scarabaeidae | 2 | 2 | 0.02 | |
| Staphylinidae | 3 | 62 | 0.68 | |
| Tenebrionidae | 22 | 537 | 5.87 | |
| Zopheridae | 1 | 2 | 0.02 | |
| Collembola | Entomobryidae | 2 | 510 | 5.58 |
| Hypogastruridae | 1 | 9 | 0.10 | |
| Poduridae | 2 | 591 | 6.46 | |
| Sminthuridae | 2 | 205 | 2.24 | |
| Indeterminate | 1 | 78 | 0.85 | |
| Hymenoptera | Bradynobaenidae | 2 | 4 | 0.04 |
| Formicidae | 13 | 3.446 | 37.68 | |
| Mutillidae | 6 | 147 | 1.61 | |
| Orthoptera | Acrididae | 1 | 1 | 0.01 |
| Mogoplistidae | 2 | 188 | 2.06 | |
| Ommexechidae | 2 | 9 | 0.10 | |
| Proscopiidae | 1 | 1 | 0.01 | |
| Tettigoniidae | 5 | 35 | 0.38 | |
| Tristiridae | 2 | 20 | 0.22 | |
| Psocoptera | Liposcelidae | 2 | 5 | 0.05 |
| Indeterminate | 4 | 535 | 5.85 | |
| Thysanura | Lepismatidae | 2 | 56 | 0.61 |
| Totals | 57 | 173 | 9.146 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizarro-Araya, J.; Alfaro, F.M.; Ojanguren-Affilastro, A.A.; Moreira-Muñoz, A. A Fine-Scale Hotspot at the Edge: Epigean Arthropods from the Atacama Coast (Paposo-Taltal, Antofagasta Region, Chile). Insects 2021, 12, 916. https://doi.org/10.3390/insects12100916
Pizarro-Araya J, Alfaro FM, Ojanguren-Affilastro AA, Moreira-Muñoz A. A Fine-Scale Hotspot at the Edge: Epigean Arthropods from the Atacama Coast (Paposo-Taltal, Antofagasta Region, Chile). Insects. 2021; 12(10):916. https://doi.org/10.3390/insects12100916
Chicago/Turabian StylePizarro-Araya, Jaime, Fermín M. Alfaro, Andrés A. Ojanguren-Affilastro, and Andrés Moreira-Muñoz. 2021. "A Fine-Scale Hotspot at the Edge: Epigean Arthropods from the Atacama Coast (Paposo-Taltal, Antofagasta Region, Chile)" Insects 12, no. 10: 916. https://doi.org/10.3390/insects12100916
APA StylePizarro-Araya, J., Alfaro, F. M., Ojanguren-Affilastro, A. A., & Moreira-Muñoz, A. (2021). A Fine-Scale Hotspot at the Edge: Epigean Arthropods from the Atacama Coast (Paposo-Taltal, Antofagasta Region, Chile). Insects, 12(10), 916. https://doi.org/10.3390/insects12100916






