Implications of Sublethal Insecticide Exposure and the Development of Resistance on Mosquito Physiology, Behavior, and Pathogen Transmission
Abstract
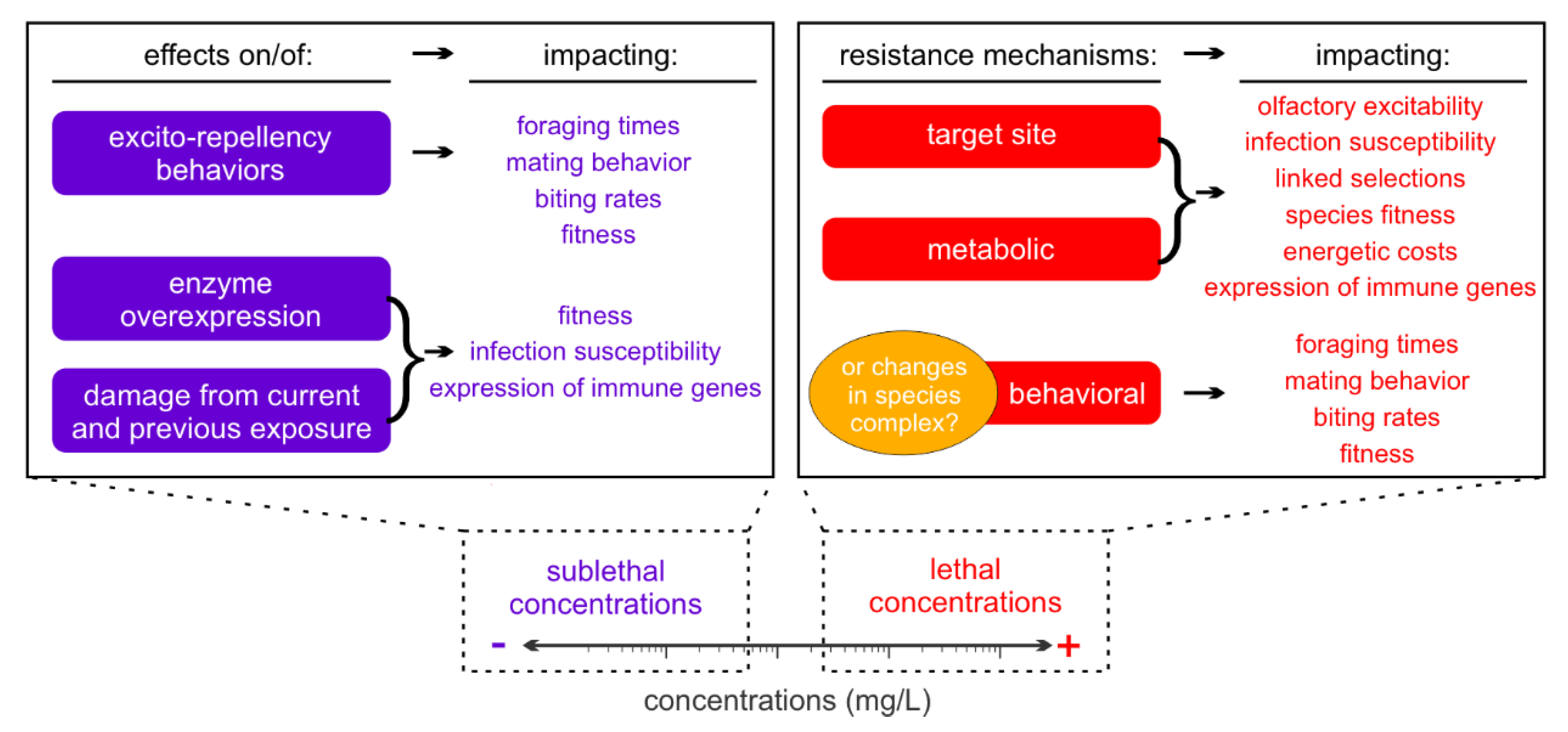
Simple Summary
Abstract
1. Background
2. Changes in Pathogen Dissemination by Mosquitoes That Survived an Insecticide Exposure
2.1. Sublethal Exposure of Adults to Insecticides
2.2. Sublethal Exposure of Larvae to Insecticides
3. Changes in Pathogen Dissemination in Insecticide-Resistant Mosquitoes
3.1. Target Site Insecticide Resistance
3.2. Metabolic Insecticide Resistance
3.3. Behavioral Insecticide Resistance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Fact-Sheets: Dengue and Severe Dengue 19 May 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- WHO. World Malaria Report 2020: 20 Years of Global Progress and Challenges; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Global Strategy for Dengue Prevention and Control 2012–2020; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Turell, M.J.; Beaman, J.R.; Tammariello, R.F. Susceptibility of selected strains of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to chikungunya virus. J. Med. Entomol. 1992, 29, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R.; Failloux, A.-B. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef] [PubMed]
- Gatton, M.L.; Chitnis, N.; Churcher, T.; Donnelly, M.J.; Ghani, A.C.; Godfray, H.C.J.; Gould, F.; Hastings, I.; Marshall, J.; Ranson, H. The importance of mosquito behavioural adaptations to malaria control in Africa. Evol. Int. J. Org. Evol. 2013, 67, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Chareonviriyaphap, T.; Bangs, M.J.; Suwonkerd, W.; Kongmee, M.; Corbel, V.; Ngoen-Klan, R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit. Vectors 2013, 6, 280. [Google Scholar] [CrossRef]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef]
- Elliott, M. Synthetic Pyrethroids; Elliott, M., Ed.; American Chemical Society: Washington, DC, USA, 1977; pp. 1–28. ISBN 1947-5918. [Google Scholar]
- Casida, J.E.; Quistad, G.B. Pyrethrum Flowers: Production, Chemistry, Toxicology, and Uses; Oxford University Press: New York, NY, USA, 1995; ISBN 9780195082104. [Google Scholar]
- Narahashi, T. Neuroreceptors and ion channels as the basis for drug action: Past, present, and future. J. Pharmacol. Exp. Ther. 2000, 294, 1–26. [Google Scholar]
- Soderlund, D.M.; Clark, J.M.; Sheets, L.P.; Mullin, L.S.; Piccirillo, V.J.; Sargent, D.; Stevens, J.T.; Weiner, M.L. Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology 2002, 171, 3–59. [Google Scholar] [CrossRef]
- Mengle, D.C.; Casida, J.E. Inhibition and recovery of brain cholinesterase activity in house flies poisoned with organophosphate and carbamate compounds. J. Econ. Entomol. 1958, 51, 750–757. [Google Scholar] [CrossRef]
- Estrada, J.G.; Mulla, M.S. Evaluation of two new insect growth regulators against mosquitoes in the laboratory. J. Am. Mosq. Control Assoc. 1986, 2, 57–60. [Google Scholar]
- Silva-Filha, M.H.N.L.; Romão, T.P.; Rezende, T.M.T.; da Silva Carvalho, K.; de Menezes, H.S.G.; do Nascimento, N.A.; Soberón, M.; Bravo, A. Bacterial toxins active against mosquitoes: Mode of action and resistance. Toxins 2021, 13, 523. [Google Scholar] [CrossRef]
- Ranson, H.; Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef] [PubMed]
- WHO. Fact-Sheets: Malaria 1 April 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Xu, Q.; Zhang, L.; Li, T.; Zhang, L.; He, L.; Dong, K.; Liu, N. Evolutionary adaptation of the amino acid and codon usage of the mosquito sodium channel following insecticide selection in the field mosquitoes. PLoS ONE 2012, 7, e47609. [Google Scholar] [CrossRef] [PubMed]
- Haddi, K.; Tomé, H.V.V.; Du, Y.; Valbon, W.R.; Nomura, Y.; Martins, G.F.; Dong, K.; Oliveira, E.E. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: A potential challenge for mosquito control. Sci. Rep. 2017, 7, 46549. [Google Scholar] [CrossRef] [PubMed]
- Lien, N.T.K.; Ngoc, N.T.H.; Hien, N.T.; Hoang, N.H.; Binh, N.T.H. Two novel mutations in the voltage-gated sodium channel associated with knockdown resistance (kdr) in the dengue vector Aedes aegypti in Vietnam. J. Vector Ecol. 2018, 43, 184–189. [Google Scholar] [CrossRef]
- Singh, O.P.; Dykes, C.L.; Das, M.K.; Pradhan, S.; Bhatt, R.M.; Agrawal, O.P.; Adak, T. Presence of two alternative kdr-like mutations, L1014F and L1014S, and a novel mutation, V1010L, in the voltage gated Na+ channel of Anopheles culicifacies from Orissa, India. Malar. J. 2010, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Tmimi, F.Z.; Faraj, C.; Bkhache, M.; Mounaji, K.; Failloux, A.B.; Sarih, M. Insecticide resistance and target site mutations (G119S ace-1 and L1014F kdr) of Culex pipiens in Morocco. Parasit Vectors 2018, 11, 51. [Google Scholar] [CrossRef]
- Marcombe, S.; Farajollahi, A.; Healy, S.P.; Clark, G.G.; Fonseca, D.M. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS ONE 2014, 9, e101992. [Google Scholar] [CrossRef]
- Paris, M.; Tetreau, G.; Laurent, F.; Lelu, M.; Despres, L.; David, J. Persistence of Bacillus thuringiensis israelensis (Bti) in the environment induces resistance to multiple Bti toxins in mosquitoes. Pest Manag. Sci. 2011, 67, 122–128. [Google Scholar] [CrossRef]
- Tokponnon, F.T.; Ogouyémi, A.H.; Sissinto, Y.; Sovi, A.; Gnanguenon, V.; Cornélie, S.; Adéothy, A.A.; Ossè, R.; Wakpo, A.; Gbénou, D.; et al. Impact of long-lasting, insecticidal nets on anaemia and prevalence of Plasmodium falciparum among children under five years in areas with highly resistant malaria vectors. Malar. J. 2014, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.B.; Read, A.F. The threat (or not) of insecticide resistance for malaria control. Proc. Natl. Acad. Sci. USA 2016, 113, 8900–8902. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.L.; Houk, E.J.; Kramer, L.D.; Reeves, W.C. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu. Rev. Entomol. 1983, 28, 229–262. [Google Scholar] [CrossRef]
- Welling, W. Dynamic aspects of insect-insecticide interactions. Annu. Rev. Entomol. 1977, 22, 53–78. [Google Scholar] [CrossRef]
- Viana, M.; Hughes, A.; Matthiopoulos, J.; Ranson, H.; Ferguson, H.M. Delayed mortality effects cut the malaria transmission potential of insecticide-resistant mosquitoes. Proc. Natl. Acad. Sci. USA 2016, 113, 8975–8980. [Google Scholar] [CrossRef]
- Valbon, W.R.; Cruz, F.M.; Ramos, G.S.; Tome, H.V.V.; Oliveira, E.E. Sublethal exposure to deltamethrin reduces the abilities of giant water bugs to prey upon Aedes aegypti larvae. Chemosphere 2018, 191, 350–356. [Google Scholar] [CrossRef]
- Jones, C.M.; Sanou, A.; Guelbeogo, W.M.; Sagnon, N.; Johnson, P.C.D.; Ranson, H. Aging partially restores the efficacy of malaria vector control in insecticide-resistant populations of Anopheles gambiae sl. from Burkina Faso. Malar. J. 2012, 11, 24. [Google Scholar]
- Georghiou, G.P. The evolution of resistance to pesticides. Annu. Rev. Ecol. Syst. 1972, 3, 133–168. [Google Scholar] [CrossRef]
- Ritthison, W.; Titgratog, R.; Tainchum, K.; Bangs, M.J.; Manguin, S.; Chareonviriyaphap, T. Pyrethroid susceptibility and behavioral avoidance in Anopheles epiroticus, a malaria vector in Thailand. J. Vector Ecol. 2014, 39, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Tainchum, K.; Nararak, J.; Boonyuan, W.; Bangs, M.J.; Chareonviriyaphap, T. Behavioral responses of Anopheles species (Culicidae: Diptera) with varying surface exposure to pyrethroid-treated netting in an excito-repellency test system. J. Vector Ecol. 2016, 41, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Glunt, K.D. Understanding the Consequences of Sub-Lethal Insecticide Concentrations for Insecticide Resistance Management and Malaria Control; The Pennsylvania State University: State College, PA, USA, 2013. [Google Scholar]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef] [PubMed]
- Tomé, H.V.V.; Pascini, T.V.; Dângelo, R.A.C.; Guedes, R.N.C.; Martins, G.F. Survival and swimming behavior of insecticide-exposed larvae and pupae of the yellow fever mosquito Aedes aegypti. Parasit. Vectors 2014, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Stehle, S.; Schulz, R. Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. USA 2015, 112, 5750–5755. [Google Scholar] [CrossRef] [PubMed]
- Müller, C. Impacts of sublethal insecticide exposure on insects—Facts and knowledge gaps. Basic Appl. Ecol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Silva, S.M.; Haddi, K.; Viteri Jumbo, L.O.; Oliveira, E.E. Progeny of the maize weevil, Sitophilus zeamais, is affected by parental exposure to clove and cinnamon essential oils. Entomol. Exp. Appl. 2017, 163, 220–228. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Bass, C. Does resistance really carry a fitness cost? Curr. Opin. Insect Sci. 2017, 21, 39–46. [Google Scholar] [CrossRef]
- Siegert, P.Y.; Walker, E.; Miller, J.R. Differential behavioral responses of Anopheles gambiae (Diptera: Culicidae) modulate mortality caused by pyrethroid-treated bednets. J. Econ. Entomol. 2009, 102, 2061–2071. [Google Scholar] [CrossRef]
- Manda, H.; Arce, L.M.; Foggie, T.; Shah, P.; Grieco, J.P.; Achee, N.L. Effects of irritant chemicals on Aedes aegypti resting behavior: Is there a simple shift to untreated “safe sites”? PLoS Negl. Trop. Dis. 2011, 5, e1243. [Google Scholar] [CrossRef]
- Manda, H.; Shah, P.; Polsomboon, S.; Chareonviriyaphap, T.; Castro-Llanos, F.; Morrison, A.; Burrus, R.G.; Grieco, J.P.; Achee, N.L. Contact irritant responses of Aedes aegypti using sublethal concentration and focal application of pyrethroid chemicals. PLoS Negl. Trop. Dis. 2013, 7, e2074. [Google Scholar] [CrossRef]
- Potikasikorn, J.; Chareonviriyaphap, T.; Bangs, M.J.; Prabaripai, A. Behavioral responses to DDT and pyrethroids between Anopheles minimus species A and C, malaria vectors in Thailand. Am. J. Trop. Med. Hyg. 2005, 73, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.; Shyong, J.; Lutz, E.K.; Yang, T.; Li, M.; Truong, K.; Arvidson, R.; Buchman, A.; Riffell, J.A.; Akbari, O.S. Live calcium imaging of Aedes aegypti neuronal tissues reveals differential importance of chemosensory systems for life-history-specific foraging strategies. BMC Neurosci. 2019, 20, 1–17. [Google Scholar] [CrossRef]
- Sanford, M.R.; Olson, J.K.; Lewis, W.J.; Tomberlin, J.K. The effect of sucrose concentration on olfactory-based associative learning in Culex quinquefasciatus Say (Diptera: Culicidae). J. Insect Behav. 2013, 26, 494–513. [Google Scholar] [CrossRef]
- Seenivasagan, T.; Guha, L.; Parashar, B.D.; Agrawal, O.P.; Sukumaran, D. Olfaction in Asian tiger mosquito Aedes albopictus: Flight orientation response to certain saturated carboxylic acids in human skin emanations. Parasitol. Res. 2014, 113, 1927–1932. [Google Scholar] [CrossRef]
- van Breugel, F.; Riffell, J.; Fairhall, A.; Dickinson, M.H. Mosquitoes use vision to associate odor plumes with thermal targets. Curr. Biol. 2015, 25, 2123–2129. [Google Scholar] [CrossRef]
- Vinauger, C.; Lahondere, C.; Cohuet, A.; Lazzari, C.R.; Riffell, J.A. Learning and memory in disease vector insects. Trends Parasitol. 2016, 32, 761–771. [Google Scholar] [CrossRef]
- Cohnstaedt, L.W.; Allan, S.A. Effects of sublethal pyrethroid exposure on the host-seeking behavior of female mosquitoes. J. Vector Ecol. 2011, 36, 395–403. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Wei, H.; Du, J. Sublethal effects of larval treatment with deltamethrin on moth sex pheromone communication system of the Asian corn borer, Ostrinia furnacalis. Pestic. Biochem. Physiol. 2004, 80, 12–20. [Google Scholar] [CrossRef]
- Kadala, A.; Charreton, M.; Jakob, I.; Le Conte, Y.; Collet, C. A use-dependent sodium current modification induced by type I pyrethroid insecticides in honeybee antennal olfactory receptor neurons. Neurotoxicology 2011, 32, 320–330. [Google Scholar] [CrossRef]
- Wang, G.; Huang, X.; Wei, H.; Fadamiro, H.Y. Sublethal effects of larval exposure to indoxacarb on reproductive activities of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Pestic. Biochem. Physiol. 2011, 101, 227–231. [Google Scholar] [CrossRef]
- Guedes, N.M.P.; Tolledo, J.; Corrêa, A.S.; Guedes, R.N.C. Insecticide-induced hormesis in an insecticide-resistant strain of the maize weevil, Sitophilus zeamais. J. Appl. Entomol. 2010, 134, 142–148. [Google Scholar] [CrossRef]
- Cordeiro, E.M.G.; De Moura, I.L.T.; Fadini, M.A.M.; Guedes, R.N.C. Beyond selectivity: Are behavioral avoidance and hormesis likely causes of pyrethroid-induced outbreaks of the southern red mite Oligonychus ilicis? Chemosphere 2013, 93, 1111–1116. [Google Scholar] [CrossRef]
- Zanuncio, J.C.; Jusselino-Filho, P.; Ribeiro, R.C.; Zanuncio, T.V.; Ramalho Fde, S.; Serrão, J.E. Hormetic responses of a stinkbug predator to sublethal doses of pyrethroid. Bull Env. Contam. Toxicol. 2011, 87, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.; Santos, R.L.; Tomé, H.V.V.; Barbosa, W.F.; Martins, G.F.; Guedes, R.N.C.; Oliveira, E.E. Imidacloprid-mediated effects on survival and fertility of the Neotropical brown stink bug Euschistus heros. J. Pest Sci. 2016, 89, 231–240. [Google Scholar] [CrossRef]
- Tuelher, E.S.; da Silva, É.H.; Freitas, H.L.; Namorato, F.A.; Serrão, J.E.; Guedes, R.N.C.; Oliveira, E.E. Chlorantraniliprole-mediated toxicity and changes in sexual fitness of the Neotropical brown stink bug Euschistus heros. J. Pest Sci. 2017, 90, 397–405. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Toxicology rethinks its central belief. Nature 2003, 421, 691. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Cutler, G.C. Insecticide-induced hormesis and arthropod pest management. Pest Manag. Sci. 2014, 70, 690–697. [Google Scholar] [CrossRef]
- Sunday, O.O.; Kayode, A.; Ashamo, M.O. Laboratory review of sublethal effects of cypermethrin on oviposition, life span and egg development in Culex quinquefasciatus, Say (Diptera: Culicidae). Int. J. Mosq. Res. 2016, 3, 20–26. [Google Scholar]
- Brogdon, W.G.; McAllister, J.C.; Corwin, A.M.; Cordon-Rosales, C. Independent selection of multiple mechanisms for pyrethroid resistance in Guatemalan Anopheles albimanus (Diptera: Culicidae). J. Econ. Entomol. 1999, 92, 298–302. [Google Scholar] [CrossRef]
- Vulule, J.M.; Beach, R.F.; Atieli, F.K.; McAllister, J.C.; Brogdon, W.G.; Roberts, J.M.; Mwangi, R.W.; Hawley, W.A. Elevated oxidase and esterase levels associated with permethrin tolerance in Anopheles gambiae from Kenyan villages using permethrin-impregnated nets. Med. Vet. Entomol. 1999, 13, 239–244. [Google Scholar] [CrossRef]
- Casimiro, S.; Coleman, M.; Mohloai, P.; Hemingway, J.; Sharp, B. Insecticide resistance in Anopheles funestus (Diptera: Culicidae) from Mozambique. J. Med. Entomol. 2006, 43, 267–275. [Google Scholar] [CrossRef]
- Marcombe, S.; Mathieu, R.B.; Pocquet, N.; Riaz, M.-A.; Poupardin, R.; Sélior, S.; Darriet, F.; Reynaud, S.; Yébakima, A.; Corbel, V. Insecticide resistance in the dengue vector Aedes aegypti from Martinique: Distribution, mechanisms and relations with environmental factors. PLoS ONE 2012, 7, e30989. [Google Scholar] [CrossRef]
- Main, B.J.; Everitt, A.; Cornel, A.J.; Hormozdiari, F.; Lanzaro, G.C. Genetic variation associated with increased insecticide resistance in the malaria mosquito, Anopheles coluzzii. Parasit. Vectors 2018, 11, 225. [Google Scholar] [CrossRef]
- Meunier, L.; Prefontaine, G.; Van Munster, M.; Brousseau, R.; Masson, L. Transcriptional response of Choristoneura fumiferana to sublethal exposure of Cry1Ab protoxin from Bacillus thuringiensis. Insect Mol. Biol. 2006, 15, 475–483. [Google Scholar] [CrossRef]
- Xiao, C.; Luan, S.; Xu, Z.; Lang, J.; Rao, W.; Huang, Q. Tolerance potential of Chilo suppressalis larvae to fipronil exposure via the modulation of detoxification and GABA responses. J. Asia Pac. Entomol. 2017, 20, 1287–1293. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, H.; Wang, Z.; Long, G.; Jin, D. Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci. Rep. 2018, 8, 8773. [Google Scholar] [CrossRef]
- Hardstone, M.C.; Huang, X.; Harrington, L.C.; Scott, J.G. Differences in development, glycogen, and lipid content associated with cytochrome P450-mediated permethrin resistance in Culex pipiens quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 2010, 47, 188–198. [Google Scholar] [CrossRef]
- Rivero, A.; Magaud, A.; Nicot, A.; Vézilier, J. Energetic cost of insecticide resistance in Culex pipiens mosquitoes. J. Med. Entomol. 2011, 48, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Burgio, G.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 2017, 7, 1201. [Google Scholar] [CrossRef]
- Alkassab, A.T.; Kirchner, W.H. Assessment of acute sublethal effects of clothianidin on motor function of honeybee workers using video-tracking analysis. Ecotoxicol. Environ. Saf. 2018, 147, 200–205. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Corbett, S.; Rodriguez, M.; Goto, J.J.; Walse, S.S. Pesticide-mediated disruption of spotted wing Drosophila flight response to raspberries. J. Appl. Entomol. 2018, 142, 457–464. [Google Scholar] [CrossRef]
- Knecht, H.; Richards, S.L.; Balanay, J.A.G.; White, A.V. Impact of mosquito age and insecticide exposure on vector competence of Aedes albopictus (Diptera: Culicidae) for Zika virus. Pathogens 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; White, A.V.; Balanay, J.A.G. Potential for sublethal insecticide exposure to impact vector competence of Aedes albopictus (Diptera: Culicidae) for dengue and Zika viruses. Res. Rep. Trop. Med. 2017, 8, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, M.; Masetti, A.; Albieri, A.; Maccagnani, B.; Bellini, R. Larvicidal activity and influence of Bacillus thuringiensis var. israelensis on Aedes albopictus oviposition in ovitraps during a two-week check interval protocol. J. Am. Mosq. Control Assoc. 2009, 25, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Pechenik, J.A. Larval experience and latent effects—Metamorphosis is not a new beginning. Integr. Comp. Biol. 2006, 46, 323–333. [Google Scholar] [CrossRef]
- Fernandes, K.M.; Tomé, H.V.V.; Miranda, F.R.; Gonçalves, W.G.; Pascini, T.V.; Serrão, J.E.; Martins, G.F. Aedes aegypti larvae treated with spinosad produce adults with damaged midgut and reduced fecundity. Chemosphere 2019, 221, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.L.; Olson, J.K. Effects of sublethal dosages of insecticides on Culex quinquefasciatus. J. Am. Mosq. Control Assoc. 1989, 5, 239–246. [Google Scholar] [PubMed]
- Antonio, G.E.; Sanchez, D.; Williams, T.; Marina, C.F. Paradoxical effects of sublethal exposure to the naturally derived insecticide spinosad in the dengue vector mosquito, Aedes aegypti. Pest Manag. Sci. 2009, 65, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.J.; Montgomery, A.; Muturi, E.J. Sublethal effects of atrazine and glyphosate on life history traits of Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2014, 113, 2879–2886. [Google Scholar] [CrossRef]
- Vantaux, A.; Ouattarra, I.; Lefèvre, T.; Dabiré, K.R. Effects of larvicidal and larval nutritional stresses on Anopheles gambiae development, survival and competence for Plasmodium falciparum. Parasit. Vectors 2016, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Pigeault, R.; Bataillard, D.; Glaizot, O.; Christe, P. Effect of neonicotinoid exposure on the life history traits and susceptibility to Plasmodium infection on the major avian malaria vector Culex pipiens (Diptera: Culicidae). Parasitologia 2021, 1, 3. [Google Scholar] [CrossRef]
- Bataillard, D.; Christe, P.; Pigeault, R. Impact of field-realistic doses of glyphosate and nutritional stress on mosquito life history traits and susceptibility to malaria parasite infection. Ecol. Evol. 2020, 10, 5079–5088. [Google Scholar] [CrossRef]
- Muturi, E.J.; Alto, B.W. Larval environmental temperature and insecticide exposure alter Aedes aegypti competence for arboviruses. Vector-Borne Zoonotic Dis. 2011, 11, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.M.; Gonzaga, W.G.; Pascini, T.V.; Miranda, F.R.; Tomé, H.V.V.; Serrão, J.E.; Martins, G.F. Imidacloprid impairs the post-embryonic development of the midgut in the yellow fever mosquito Stegomyia aegypti (= Aedes aegypti). Med. Vet. Entomol. 2015, 29, 245–254. [Google Scholar] [CrossRef]
- Lopes, M.P.; Fernandes, K.M.; Tomé, H.V.V.; Gonçalves, W.G.; Miranda, F.R.; Serrão, J.E.; Martins, G.F. Spinosad-mediated effects on the walking ability, midgut, and Malpighian tubules of Africanized honey bee workers. Pest Manag. Sci. 2018, 74, 1311–1318. [Google Scholar] [CrossRef]
- Santos, H.P.; Gutierrez, Y.; Oliveira, E.E.; Serrão, J.E. Sublethal dose of deltamethrin damage the midgut cells of the mayfly Callibaetis radiatus. Environ. Sci. Pollut. Res. 2018, 25, 1418–1427. [Google Scholar] [CrossRef]
- Michalski, M.L.; Erickson, S.M.; Bartholomay, L.C.; Christensen, B.M. Midgut barrier imparts selective resistance to filarial worm infection in Culex pipiens pipiens. PLoS Negl. Trop. Dis. 2010, 4, e875. [Google Scholar] [CrossRef]
- Moltini-Conclois, I.; Stalinski, R.; Tetreau, G.; Després, L.; Lambrechts, L. Larval exposure to the bacterial insecticide Bti enhances dengue virus susceptibility of adult Aedes aegypti mosquitoes. Insects 2018, 9, 193. [Google Scholar] [CrossRef]
- Hauser, G.; Thiévent, K.; Koella, J.C. Consequences of larval competition and exposure to permethrin for the development of the rodent malaria Plasmodium berghei in the mosquito Anopheles gambiae. Parasit. Vectors 2020, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.A.; Kline, D.L.; Walker, T. Environmental factors affecting efficacy of bifenthrin-treated vegetation for mosquito control. J. Am. Mosq. Control Assoc. 2009, 25, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, Y.; Liu, Q.; Liu, J. Photodegradation mechanism of deltamethrin and fenvalerate. J. Environ. Sci. 2010, 22, 1123–1128. [Google Scholar] [CrossRef]
- Dong, K.; Du, Y.; Rinkevich, F.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B.S. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, T.; Feng, Y.; Liu, N. The function of two P450s, CYP9M10 and CYP6AA7, in the permethrin resistance of Culex quinquefasciatus. Sci. Rep. 2017, 7, 587. [Google Scholar] [CrossRef]
- Romero, A.; Potter, M.F.; Haynes, K.F. Behavioral responses of the bed bug to insecticide residues. J. Med. Entomol. 2009, 46, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Haddi, K.; Mendonça, L.P.; Dos Santos, M.F.; Guedes, R.N.C.; Oliveira, E.E. Metabolic and behavioral mechanisms of indoxacarb resistance in Sitophilus zeamais (Coleoptera: Curculionidae). J. Econ. Entomol. 2015, 108, 362–369. [Google Scholar] [CrossRef]
- Silverman, J.; Ross, M.H. Behavioral resistance of field-collected German cockroaches (Blattodea: Blattellidae) to baits containing glucose. Environ. Entomol. 1994, 23, 425–430. [Google Scholar] [CrossRef]
- Freitas, R.C.P.; Faroni, L.R.D.; Haddi, K.; Jumbo, L.O.V.; Oliveira, E.E. Allyl isothiocyanate actions on populations of Sitophilus zeamais resistant to phosphine: Toxicity, emergence inhibition and repellency. J. Stored Prod. Res. 2016, 69, 257–264. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhang, Z.W.; Fu, Y.F.; Zhang, G.C.; Yuan, S. Carbon dioxide, odorants, heat and visible cues affect wild mosquito landing in open spaces. Front Behav. Neurosci. 2018, 12, 86. [Google Scholar] [CrossRef]
- Valbon, W.R.; Haddi, K.; Carvalho, G.A.; Souza, R.A.; Guedes, R.N.C.; Martins, G.F.; Oliveira, E.E. “Armed to the teeth”: The multiple ways to survive insecticidal and predatory challenges in Aedes aegypti larvae. Pestic. Biochem. Physiol. 2019, 156, 87–95. [Google Scholar] [CrossRef]
- Silver, K.; Dong, K.S.; Zhorov, B. Molecular mechanism of action and selectivity of sodium channel blocker insecticides. Curr. Med. Chem. 2017, 24, 2912–2924. [Google Scholar] [CrossRef][Green Version]
- Silver, K.S.; Du, Y.; Nomura, Y.; Oliveira, E.E.; Salgado, V.L.; Zhorov, B.S.; Dong, K. Voltage-gated sodium channels as insecticide targets. Adv. Insect Phys. 2014, 46, 389–433. [Google Scholar]
- Du, Y.; Song, W.; Groome, J.R.; Nomura, Y.; Luo, N.; Dong, K. A negative charge in transmembrane segment 1 of domain II of the cockroach sodium channel is critical for channel gating and action of pyrethroid insecticides. Toxicol. Appl. Pharmacol. 2010, 247, 53–59. [Google Scholar] [CrossRef]
- Oliveira, E.E.; Du, Y.; Nomura, Y.; Dong, K. A residue in the transmembrane segment 6 of domain I in insect and mammalian sodium channels regulate differential sensitivities to pyrethroid insecticides. Neurotoxicology 2013, 38, 42–50. [Google Scholar] [CrossRef]
- Vais, H.; Williamson, M.S.; Goodson, S.J.; Devonshire, A.L.; Warmke, J.W.; Usherwood, P.N.; Cohen, C.J. Activation of Drosophila sodium channels promotes modification by deltamethrin. Reductions in affinity caused by knock-down resistance mutations. J. Gen. Physiol. 2000, 115, 305–318. [Google Scholar] [CrossRef]
- Jung, J.W.; Baeck, S.-J.; Perumalsamy, H.; Hansson, B.S.; Ahn, Y.-J.; Kwon, H.W. A novel olfactory pathway is essential for fast and efficient blood-feeding in mosquitoes. Sci. Rep. 2015, 5, 13444. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.M.; Moiroux, N.; Chandre, F.; Martin-Herrou, H.; Milesi, P.; Boussari, O.; Porciani, A.; Duchon, S.; Labbé, P.; Pennetier, C. Behavioral cost & overdominance in Anopheles gambiae. PLoS ONE 2015, 10, e0121755. [Google Scholar]
- Porciani, A.; Diop, M.; Moiroux, N.; Kadoke-Lambi, T.; Cohuet, A.; Chandre, F.; Dormont, L.; Pennetier, C. Influence of pyrethroid-treated bed net on host seeking behavior of Anopheles gambiae s. s. carrying the kdr allele. PLoS ONE 2017, 12, e0164518. [Google Scholar]
- Alout, H.; Djègbè, I.; Chandre, F.; Djogbénou, L.S.; Dabiré, R.K.; Corbel, V.; Cohuet, A. Insecticide exposure impacts vector–parasite interactions in insecticide-resistant malaria vectors. Proc. R. Soc. 2014, 281, 20140389. [Google Scholar] [CrossRef]
- Ndiath, M.O.; Cailleau, A.; Diedhiou, S.M.; Gaye, A.; Boudin, C.; Richard, V.; Trape, J.-F. Effects of the kdr resistance mutation on the susceptibility of wild Anopheles gambiae populations to Plasmodium falciparum: A hindrance for vector control. Malar. J. 2014, 13, 340. [Google Scholar] [CrossRef][Green Version]
- Mitri, C.; Markianos, K.; Guelbeogo, W.M.; Bischoff, E.; Gneme, A.; Eiglmeier, K.; Holm, I.; Sagnon, N.; Vernick, K.D.; Riehle, M.M. The kdr-bearing haplotype and susceptibility to Plasmodium falciparum in Anopheles gambiae: Genetic correlation and functional testing. Malar. J. 2015, 14, 391. [Google Scholar] [CrossRef]
- Kristan, M.; Lines, J.; Nuwa, A.; Ntege, C.; Meek, S.R.; Abeku, T.A. Exposure to deltamethrin affects development of Plasmodium falciparum inside wild pyrethroid resistant Anopheles gambiae ss mosquitoes in Uganda. Parasit. Vectors 2016, 9, 100. [Google Scholar] [CrossRef]
- Ellegren, H.; Galtier, N. Determinants of genetic diversity. Nat. Rev. Genet. 2016, 17, 422. [Google Scholar] [CrossRef]
- Essandoh, J.; Yawson, A.E.; Weetman, D. Acetylcholinesterase (Ace-1) target site mutation 119S is strongly diagnostic of carbamate and organophosphate resistance in Anopheles gambiae s.s. and Anopheles coluzzii across southern Ghana. Malar. J. 2013, 12, 404. [Google Scholar] [CrossRef]
- Alout, H.; Djogbénou, L.; Berticat, C.; Chandre, F.; Weill, M. Comparison of Anopheles gambiae and Culex pipiens acetycholinesterase 1 biochemical properties. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 150, 271–277. [Google Scholar] [CrossRef]
- Weetman, D.; Mitchell, S.N.; Wilding, C.S.; Birks, D.P.; Yawson, A.E.; Essandoh, J.; Mawejje, H.D.; Djogbenou, L.S.; Steen, K.; Rippon, E.J. Contemporary evolution of resistance at the major insecticide target site gene Ace-1 by mutation and copy number variation in the malaria mosquito Anopheles gambiae. Mol. Ecol. 2015, 24, 2656–2672. [Google Scholar] [CrossRef]
- Berticat, C.; Boquien, G.; Raymond, M.; Chevillon, C. Insecticide resistance genes induce a mating competition cost in Culex pipiens mosquitoes. Genet. Res. 2002, 79, 41–47. [Google Scholar] [CrossRef]
- Alout, H.; Ndam, N.T.; Sandeu, M.M.; Djégbe, I.; Chandre, F.; Dabiré, R.K.; Djogbénou, L.S.; Corbel, V.; Cohuet, A. Insecticide resistance alleles affect vector competence of Anopheles gambiae s.s. for Plasmodium falciparum field isolates. PLoS ONE 2013, 8, e63849. [Google Scholar] [CrossRef]
- Beerntsen, B.T.; James, A.A.; Christensen, B.M. Genetics of mosquito vector competence. Microbiol. Mol. Biol. Rev. 2000, 64, 115–137. [Google Scholar] [CrossRef]
- Platt, N.; Kwiatkowska, R.M.; Irving, H.; Diabaté, A.; Dabire, R.; Wondji, C.S. Target-site resistance mutations (kdr and RDL), but not metabolic resistance, negatively impact male mating competiveness in the malaria vector Anopheles gambiae. Heredity 2015, 115, 243. [Google Scholar] [CrossRef]
- Fawaz, E.Y.; Allan, S.A.; Bernier, U.R.; Obenauer, P.J.; Diclaro, J.W. Swarming mechanisms in the yellow fever mosquito: Aggregation pheromones are involved in the mating behavior of Aedes aegypti. J. Vector Ecol. 2014, 39, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Weerashinghe, I.S.; Shono, T. P450 monooxygenases are an important mechanism of permethrin resistance in Culex quinquefasciatus Say larvae. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 1998, 37, 47–56. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Rascalou, G.; Pontier, D.; Menu, F.; Gourbière, S. Emergence and prevalence of human vector-borne diseases in sink vector populations. PLoS ONE 2012, 7, e36858. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M.J.; Rohani, P. Modeling Infectious Diseases in Humans and Animals; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Arnaud, L.; Brostaux, Y.; Assie, L.K.; Gaspar, C.; Haubruge, E. Increased fecundity of malathion-specific resistant beetles in absence of insecticide pressure. Heredity 2002, 89, 425. [Google Scholar] [CrossRef] [PubMed]
- Gazave, É.; Chevillon, C.; Lenormand, T.; Marquine, M.; Raymond, M. Dissecting the cost of insecticide resistance genes during the overwintering period of the mosquito Culex pipiens. Heredity 2001, 87, 441. [Google Scholar] [CrossRef] [PubMed]
- Vézilier, J.; Nicot, A.; Gandon, S.; Rivero, A. Insecticide resistance and malaria transmission: Infection rate and oocyst burden in Culex pipiens mosquitoes infected with Plasmodium relictum. Malar. J. 2010, 9, 379. [Google Scholar] [CrossRef]
- Vézilier, J.; Nicot, A.; Lorgeril, J.; Gandon, S.; Rivero, A. The impact of insecticide resistance on Culex pipiens immunity. Evol. Appl. 2013, 6, 497–509. [Google Scholar] [CrossRef]
- Lucas, K.J.; Zhao, B.; Liu, S.; Raikhel, A.S. Regulation of physiological processes by microRNAs in insects. Curr. Opin. Insect Sci. 2015, 11, 1–7. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, Y.; Zou, F.; Liu, B.; Tian, M.; Ye, W.; Guo, J.; Sun, X.; Zhou, D.; Sun, Y. The role of miR-2~ 13~ 71 cluster in resistance to deltamethrin in Culex pipiens pallens. Insect Biochem. Mol. Biol. 2017, 84, 15–22. [Google Scholar] [CrossRef]
- Sun, X.H.; Xu, N. A novel miRNA, miR-13664, targets CpCYP314A1 to regulate deltamethrin resistance in Culex pipiens pallens. Parasitology 2019, 146, 197–205. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Pottier, M.A.; Bozzolan, F.; Chertemps, T.; Jacquin-Joly, E.; Lalouette, L.; Siaussat, D.; Maibeche-Coisne, M. Cytochrome P450s and cytochrome P450 reductase in the olfactory organ of the cotton leafworm Spodoptera littoralis. Insect Mol. Biol. 2012, 21, 568–580. [Google Scholar] [CrossRef]
- Cooke, M.K.; Kahindi, S.C.; Oriango, R.M.; Owaga, C.; Ayoma, E.; Mabuka, D.; Nyangau, D.; Abel, L.; Atieno, E.; Awuor, S. ’A bite before bed’: Exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar. J. 2015, 14, 259. [Google Scholar] [CrossRef] [PubMed]
- Gould, F. Role of behavior in the evolution of insect adaptation to insecticides and resistant host plants. Bull. ESA 1984, 30, 34–41. [Google Scholar] [CrossRef]
- Lynch, P.A.; Boots, M. Using evolution to generate sustainable malaria control with spatial repellents. eLife 2016, 5, e15416. [Google Scholar] [CrossRef]
- Andreazza, F.; Valbon, W.R.; Wang, Q.; Liu, F.; Xu, P.; Bandason, E.; Chen, M.; Wu, S.; Smith, L.B.; Scott, J.G. Sodium channel activation underlies transfluthrin repellency in Aedes aegypti. PLoS Negl. Trop. Dis. 2021, 15, e0009546. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Q.; Xu, P.; Andreazza, F.; Valbon, W.R.; Bandason, E.; Chen, M.; Yan, R.; Feng, B.; Smith, L.; et al. A dual-target molecular mechanism of pyrethrum repellency against mosquitoes. Nat. Commun. 2021, 12, 2553. [Google Scholar] [CrossRef]
- Yang, L.; Norris, E.J.; Jiang, S.; Bernier, U.R.; Linthicum, K.J.; Bloomquist, J.R. Reduced effectiveness of repellents in a pyrethroid-resistant strain of Aedes aegypti (Diptera: Culicidae) and its correlation with olfactory sensitivity. Pest Manag. Sci. 2020, 76, 118–124. [Google Scholar] [CrossRef]
- Deletre, E.; Martin, T.; Duménil, C.; Chandre, F. Insecticide resistance modifies mosquito response to DEET and natural repellents. Parasit. Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Tirados, I.; Costantini, C.; Gibson, G.; Torr, S.J. Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: Implications for vector control. Med. Vet. Entomol. 2006, 20, 425–437. [Google Scholar] [CrossRef]
- Russell, T.L.; Govella, N.J.; Azizi, S.; Drakeley, C.J.; Kachur, S.P.; Killeen, G.F. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 2011, 10, 80. [Google Scholar] [CrossRef]
- Yohannes, M.; Boelee, E. Early biting rhythm in the Afro-tropical vector of malaria, Anopheles arabiensis, and challenges for its control in Ethiopia. Med. Vet. Entomol. 2012, 26, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, M.; Haile, M.; Ghebreyesus, T.A.; Witten, K.H.; Getachew, A.; Byass, P.; Lindsay, S.W. Can source reduction of mosquito larval habitat reduce malaria transmission in Tigray, Ethiopia? Trop. Med. Int. Heal. 2005, 10, 1274–1285. [Google Scholar] [CrossRef]
- Reddy, M.R.; Overgaard, H.J.; Abaga, S.; Reddy, V.P.; Caccone, A.; Kiszewski, A.E.; Slotman, M.A. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar. J. 2011, 10, 184. [Google Scholar] [CrossRef]
- Niitepõld, K.; Smith, A.D.; Osborne, J.L.; Reynolds, D.R.; Carreck, N.L.; Martin, A.P.; Marden, J.H.; Ovaskainen, O.; Hanski, I. Flight metabolic rate and Pgi genotype influence butterfly dispersal rate in the field. Ecology 2009, 90, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Z.; Li, T.; Zhang, R.; Xue, Y.; Zhong, Y.; Bai, W.; Zhou, D.; Zhao, Z. Regulation of Drosophila circadian rhythms by miRNA let-7 is mediated by a regulatory cycle. Nat. Commun. 2014, 5, 5549. [Google Scholar] [CrossRef] [PubMed]
- Bayoh, M.N.; Mathias, D.K.; Odiere, M.R.; Mutuku, F.M.; Kamau, L.; Gimnig, J.E.; Vulule, J.M.; Hawley, W.A.; Hamel, M.J.; Walker, E.D. Anopheles gambiae: Historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar. J. 2010, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Mutuku, F.M.; King, C.H.; Mungai, P.; Mbogo, C.; Mwangangi, J.; Muchiri, E.M.; Walker, E.D.; Kitron, U. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar. J. 2011, 10, 356. [Google Scholar] [CrossRef]
- Zhou, G.; Afrane, Y.A.; Vardo-Zalik, A.M.; Atieli, H.; Zhong, D.; Wamae, P.; Himeidan, Y.E.; Minakawa, N.; Githeko, A.K.; Yan, G. Changing patterns of malaria epidemiology between 2002 and 2010 in Western Kenya: The fall and rise of malaria. PLoS ONE 2011, 6, e20318. [Google Scholar] [CrossRef]
- Moiroux, N.; Gomez, M.B.; Pennetier, C.; Elanga, E.; Djènontin, A.; Chandre, F.; Djègbé, I.; Guis, H.; Corbel, V. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J. Infect. Dis. 2012, 206, 1622–1629. [Google Scholar] [CrossRef]
- Sougoufara, S.; Diédhiou, S.M.; Doucouré, S.; Diagne, N.; Sembène, P.M.; Harry, M.; Trape, J.-F.; Sokhna, C.; Ndiath, M.O. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: A new challenge to malaria elimination. Malar. J. 2014, 13, 1–7. [Google Scholar] [CrossRef]
- Dukeen, M.Y.H. Ecology of the malaria vector Anopheles arabiensis Patton (Diptera: Culicidae) by the Nile in northern Sudan. Bull. Entomol. Res. 1986, 76, 451–467. [Google Scholar] [CrossRef]
- White, M.T.; Griffin, J.T.; Churcher, T.S.; Ferguson, N.M.; Basanez, M.G.; Ghani, A.C. Modelling the impact of vector control interventions on Anopheles gambiae population dynamics. Parasit Vectors 2011, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Lyimo, E.O.; Koella, J.C. Relationship between body size of adult Anopheles gambiae sl and infection with the malaria parasite Plasmodium falciparum. Parasitology 1992, 104, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.T.; Hollingsworth, T.D.; Okell, L.C.; Churcher, T.S.; White, M.; Hinsley, W.; Bousema, T.; Drakeley, C.J.; Ferguson, N.M.; Basáñez, M.-G. Reducing Plasmodium falciparum malaria transmission in Africa: A model-based evaluation of intervention strategies. PLoS Med. 2010, 7, e1000324. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Maire, N.; Ross, A.; Penny, M.; Chitnis, N.; Schapira, A.; Studer, A.; Genton, B.; Lengeler, C.; Tediosi, F. Towards a comprehensive simulation model of malaria epidemiology and control. Parasitology 2008, 135, 1507–1516. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreazza, F.; Oliveira, E.E.; Martins, G.F. Implications of Sublethal Insecticide Exposure and the Development of Resistance on Mosquito Physiology, Behavior, and Pathogen Transmission. Insects 2021, 12, 917. https://doi.org/10.3390/insects12100917
Andreazza F, Oliveira EE, Martins GF. Implications of Sublethal Insecticide Exposure and the Development of Resistance on Mosquito Physiology, Behavior, and Pathogen Transmission. Insects. 2021; 12(10):917. https://doi.org/10.3390/insects12100917
Chicago/Turabian StyleAndreazza, Felipe, Eugênio E. Oliveira, and Gustavo Ferreira Martins. 2021. "Implications of Sublethal Insecticide Exposure and the Development of Resistance on Mosquito Physiology, Behavior, and Pathogen Transmission" Insects 12, no. 10: 917. https://doi.org/10.3390/insects12100917
APA StyleAndreazza, F., Oliveira, E. E., & Martins, G. F. (2021). Implications of Sublethal Insecticide Exposure and the Development of Resistance on Mosquito Physiology, Behavior, and Pathogen Transmission. Insects, 12(10), 917. https://doi.org/10.3390/insects12100917







