Responses to Developmental Temperature Fluctuation in Life History Traits of Five Drosophila Species (Diptera: Drosophilidae) from Different Thermal Niches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Temperature Regimes
2.3. Phenotypic Traits
2.4. Statistical Analyses
3. Results
3.1. Constant Temperature Comparisons
3.2. Fluctuating Temperature Comparisons
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willmer, P. Biochemical adaptation—Mechanism and process in physiological evolution. Science 2002, 296, 473. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Parsons, P.A. Evolutionary Genetics and Environmental Stress; Oxford University Press: Oxford, UK, 1991; p. 284. [Google Scholar]
- Cossins, A.R.; Raynard, R.S. Adaptive responses of animal cell membranes to temperature. Symp. Soc. Exp. Biol. 1987, 41, 95–111. [Google Scholar] [PubMed]
- Bahrndorff, S.; Holmstrup, M.; Petersen, H.; Loeschcke, V. Geographic variation for climatic stress resistance traits in the springtail Orchesella cincta. J. Insect. Physiol. 2006, 52, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Chown, S.L.; Hoffmann, A.A.; Kristensen, T.N.; Angilletta, M.J.; Stenseth, N.C.; Pertoldi, C. Adapting to climate change: A perspective from evolutionary physiology. Clim. Res. 2010, 43, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Rezende, E.L.; Balanya, J.; Rodriguez-Trelles, F.; Rego, C.; Fragata, I.; Matos, M.; Serra, L.; Santos, M. Climate change and chromosomal inversions in Drosophila subobscura. Clim. Res. 2010, 43, 103–114. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. S 2006, 37, 637–669. [Google Scholar] [CrossRef] [Green Version]
- Devictor, V.; van Swaay, C.; Brereton, T.; Brotons, L.; Chamberlain, D.; Heliola, J.; Herrando, S.; Julliard, R.; Kuussaari, M.; Lindstrom, A.; et al. Differences in the climatic debts of birds and butterflies at a continental scale. Nat. Clim. Chang. 2012, 2, 121–124. [Google Scholar] [CrossRef]
- Petavy, G.; David, J.R.; Gibert, P.; Moreteau, B. Viability and rate of development at different temperatures in Drosophila: A comparison of constant and alternating thermal regimes. J. Therm. Biol. 2001, 26, 29–39. [Google Scholar] [CrossRef]
- Kristensen, T.N.; Barker, J.S.F.; Pedersen, K.S.; Loeschcke, V. Extreme temperatures increase the deleterious consequences of inbreeding under laboratory and semi-natural conditions. Proc. R. Soc. B Biol. Sci. 2008, 275, 2055–2061. [Google Scholar] [CrossRef] [Green Version]
- Bozinovic, F.; Calosi, P.; Spicer, J.I. Physiological correlates of geographic range in animals. Ann. Rev. Ecol. Evol. Syst. 2011, 42, 155–179. [Google Scholar] [CrossRef] [Green Version]
- Fischer, K.; Kolzow, N.; Holtje, H.; Karl, I. Assay conditions in laboratory experiments: Is the use of constant rather than fluctuating temperatures justified when investigating temperature-induced plasticity? Oecologia 2011, 166, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ketola, T.; Saarinen, K. Experimental evolution in fluctuating environments: Tolerance measurements at constant temperatures incorrectly predict the ability to tolerate fluctuating temperatures. J. Evol. Biol. 2015, 28, 800–806. [Google Scholar] [CrossRef] [Green Version]
- Manenti, T.; Loeschcke, V.; Moghadam, N.N.; Sørensen, J.G. Phenotypic plasticity is not affected by experimental evolution in constant, predictable or unpredictable fluctuating thermal environments. J. Evol. Biol. 2015, 28, 2078. [Google Scholar] [CrossRef] [PubMed]
- Tobler, R.; Hermisson, J.; Schlotterer, C. Parallel trait adaptation across opposing thermal environments in experimental Drosophila melanogaster populations. Evolution 2015, 69, 1745–1759. [Google Scholar] [CrossRef] [Green Version]
- Khelifa, R.; Blanckenhorn, W.U.; Roy, J.; Rohner, P.T.; Mandjoub, H. Usefulness and limitations of thermal performance curves in predicting ectotherm development under climatic variability. J. Anim. Ecol. 2019, 88, 1901–1912. [Google Scholar] [CrossRef]
- Milosavljevic, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effects of constant and fluctuating temperatures on development rates and longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef]
- Sharpe, P.J.H.; Demichele, D.W. Reaction kinetics of poikilotherm development. J. Theor. Biol. 1977, 64, 649–670. [Google Scholar] [CrossRef]
- Schoolfield, R.M.; Sharpe, P.J.H.; Magnuson, C.E. Non-linear regression of biological temperature-dependent rate models based on absolute reaction-rate theory. J. Theor. Biol. 1981, 88, 719–731. [Google Scholar] [CrossRef]
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009; p. 289. [Google Scholar]
- Jensen, J.L.W.V. On the convex functions and inequalities between mean values. Acta Math. 1906, 30, 175–193. [Google Scholar] [CrossRef]
- Ruel, J.J.; Ayres, M.P. Jensen′s inequality predicts effects of environmental variation. Trends Ecol. Evol. 1999, 14, 361–366. [Google Scholar] [CrossRef]
- Cooper, B.S.; Hammad, L.A.; Fisher, N.P.; Karty, J.A.; Montooth, K.L. In a variable thermal environment selection favors greater plasticity of cell membranes in Drosophila melanogaster. Evolution 2012, 66, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.S.; Hammad, L.A.; Montooth, K.L. Thermal adaptation of cellular membranes in natural populations of Drosophila melanogaster. Funct. Ecol. 2014, 28, 886–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeWitt, T.J.; Sih, A.; Wilson, D.S. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 1998, 13, 77–81. [Google Scholar] [CrossRef]
- Lynch, M.; Gabriel, W. Environmental tolerance. Am. Nat. 1987, 129, 283–303. [Google Scholar] [CrossRef] [Green Version]
- Huey, R.B.; Kingsolver, J.G. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol. Evol. 1989, 4, 131–135. [Google Scholar] [CrossRef]
- Gilchrist, G.W. Specialists and generalists in changing environments. Fitness landscapes of thermal sensitivity. Am. Nat. 1995, 146, 252–270. [Google Scholar] [CrossRef]
- Rank, N.E.; Dahlhoff, E.P. Allele frequency shifts in response to climate change and physiological consequences of allozyme variation in a montane insect. Evolution 2002, 56, 2278–2289. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Loeschcke, V. Effects of relative emergence time on heat stress resistance traits, longevity and hsp70 expression level in Drosophila melanogaster. J. Therm. Biol. 2004, 29, 195–203. [Google Scholar] [CrossRef]
- Kallioniemi, E.; Hanski, I. Interactive effects of Pgi genotype and temperature on larval growth and survival in the Glanville fritillary butterfly. Funct. Ecol. 2011, 25, 1032–1039. [Google Scholar] [CrossRef]
- Milosavljevic, I.; McCalla, K.A.; Morgan, D.J.W.; Hoddle, M.S. The effects of constant and fluctuating temperatures on development of Diaphorina citri (Hemiptera: Liviidae), the Asian citrus psyllid. J. Econ. Entomol. 2020, 113, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Manenti, T.; Sørensen, J.G.; Moghadam, N.N.; Loeschcke, V. Predictability rather than amplitude of temperature fluctuations determines stress resistance in a natural population of Drosophila simulans. J. Evol. Biol. 2014, 27, 2113–2122. [Google Scholar] [CrossRef]
- Manenti, T.; Sorensen, J.G.; Loeschcke, V. Environmental heterogeneity does not affect levels of phenotypic plasticity in natural populations of three Drosophila species. Ecol. Evol. 2017, 7, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, A.S.; Partridge, L. A comparison of the genetic basis of wing size divergence in three parallel body size clines of Drosophila melanogaster. Genetics 1999, 153, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Levins, R. Evolution in Changing Environments; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Condon, C.; Cooper, B.S.; Yeaman, S.; Angilletta, M.J. Temporal variation favors the evolution of generalists in experimental populations of Drosophila melanogaster. Evolution 2014, 68, 720–728. [Google Scholar] [CrossRef]
- Parson, P.A.; Stanley, S.M. Domesticated and widespread species. In The Genetics and Biology of Drosophila; Academic Press: London, UK, 1981. [Google Scholar]
- Gibbs, A.G.; Perkins, M.C.; Markow, T.A. No place to hide: Microclimates of sonoran desert Drosophila. J. Therm. Biol. 2003, 28, 353–362. [Google Scholar] [CrossRef]
- Parkash, R.; Singh, D.; Lambhod, C. Divergent strategies for adaptations to stress resistance in two tropical Drosophila species: Effects of developmental acclimation in D. bipectinata and the invasive species D. malerkotliana. J. Exp. Biol. 2014, 217, 924–934. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, J.; Ryan, A. Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. J. Fish Biol. 2006, 69, 722–734. [Google Scholar] [CrossRef]
- Hallsson, L.R.; Bjorklund, M. Selection in a fluctuating environment leads to decreased genetic variation and facilitates the evolution of phenotypic plasticity. J. Evol. Biol. 2012, 25, 1275–1290. [Google Scholar] [CrossRef]
- Kellermann, V.; Overgaard, J.; Hoffmann, A.A.; Flojgaard, C.; Svenning, J.C.; Loeschcke, V. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl. Acad. Sci. USA 2012, 109, 16228–16233. [Google Scholar] [CrossRef] [Green Version]
- Rohlf, F.K. TpsDig; Version 2.16; SUNY: Stony Brook, NY, USA, 2010; Available online: http://life.bio.sunysb.edu/morph/ (accessed on 10 October 2021).
- Schielzeth, H. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol 2010, 1, 103–113. [Google Scholar] [CrossRef]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Stearns, S.C.; Kaiser, M.; Kawecki, T.J. The differential genetic and environmental canalization of fitness components in Drosophila melanogaster. J. Evol. Biol. 1995, 8, 539–557. [Google Scholar] [CrossRef]
- Ketola, T.; Mikonranta, L.; Zhang, J.; Saarinen, K.; Ormala, A.M.; Friman, V.P.; Mappes, J.; Laakso, J. Fluctuating temperature leads to evolution of thermal generalism and preadaptation to novel environments. Evolution 2013, 67, 2936–2944. [Google Scholar] [CrossRef] [PubMed]
- Cavieres, G.; Bogdanovich, J.M.; Toledo, P.; Bozinovic, F. Fluctuating thermal environments and time-dependent effects on fruit fly egg-hatching performance. Ecol. Evol. 2018, 8, 7014–7021. [Google Scholar] [CrossRef] [PubMed]
- Krebs, R.A.; Loeschcke, V. Costs and benefits of activation of the Heat shock response in Drosophila melanogaster. Funct. Ecol. 1994, 8, 730–737. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003, 6, 1025–1037. [Google Scholar] [CrossRef]
- Chen, P.P.; Zhang, J.Z. Antagonistic pleiotropy conceals molecular adaptations in changing environments. Nat. Ecol. Evol. 2020, 4, 461. [Google Scholar] [CrossRef]
- Slice, D.E. Introduction to Landmark Methods; Plenum Press: New York, NY, USA, 1996; Volume 284. [Google Scholar]
- Abrams, P.A.; Leimar, O.; Nylin, S.; Wiklund, C. The effect of flexible growth rates on optimal sizes and development times in a seasonal environment. Am. Nat. 1996, 147, 381–395. [Google Scholar] [CrossRef]
- Reeve, M.W.; Fowler, K.; Partridge, L. Increased body size confers greater fitness at lower experimental temperature in male Drosophila melanogaster. J. Evol. Biol. 2000, 13, 836–844. [Google Scholar] [CrossRef]
- Petavy, G.; David, J.R.; Debat, V.; Gibert, P.; Moreteau, B. Specific effects-of cycling stressful temperatures upon phenotypic and genetic variability of size traits in Drosophila melanogaster. Evol. Ecol. Res. 2004, 6, 873–890. [Google Scholar]
- Kjaersgaard, A.; Le, N.; Demontis, D.; Novicic, Z.K.; Loeschcke, V.; Pertoldi, C. The effect of developmental temperature fluctuation on wing traits and stressed locomotor performance in Drosophila melanogaster, and its dependence on heterozygosity. Evol. Ecol. Res. 2012, 14, 803–819. [Google Scholar]
- Czarnoleski, M.; Cooper, B.S.; Kierat, J.; Angilletta, M.J. Flies developed small bodies and small cells in warm and in thermally fluctuating environments. J. Exp. Biol. 2013, 216, 2896–2901. [Google Scholar] [CrossRef] [Green Version]
- Pertoldi, C.; Kristensen, T.N.; Andersen, D.H.; Loeschcke, V. Developmental instability as an estimator of genetic stress. Heredity 2006, 96, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Ann. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjaersgaard, A.; Pertoldi, C.; Loeschcke, V.; Blanckenhorn, W.U. The effect of fluctuating temperatures during development on fitness-related traits of Scatophaga stercoraria (Diptera: Scathophagidae). Environ. Entomol. 2013, 42, 1069–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, R.B.R.; James, A.C.; McCabe, J.; Partridge, L. Latitudinal variation of wing: Thorax size ratio and wing-aspect ratio in Drosophila melanogaster. Evolution 1998, 52, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Woods, R.E.; Collins, E.; Wallin, K.; White, A.; McKenzie, J.A. Wing shape versus asymmetry as an indicator of changing environmental conditions in insects. Aust. J. Entomol. 2005, 44, 233–243. [Google Scholar] [CrossRef]
- Williams, B.R.; van Heerwaarden, B.; Dowling, D.K.; Sgro, C.M. A multivariate test of evolutionary constraints for thermal tolerance in Drosophila melanogaster. J. Evol. Biol. 2012, 25, 1415–1426. [Google Scholar] [CrossRef]
- Terblanche, J.S.; Nyamukondiwa, C.; Kleynhans, E. Thermal variability alters climatic stress resistance and plastic responses in a globally invasive pest, the mediterranean fruit fly (Ceratitis capitata). Entomol. Exp. Appl. 2010, 137, 304–315. [Google Scholar] [CrossRef]
- Van Dooremalen, C.; Suring, W.; Ellers, J. Fatty acid composition and extreme temperature tolerance following exposure to fluctuating temperatures in a soil arthropod. J. Insect Physiol. 2011, 57, 1267–1273. [Google Scholar] [CrossRef]
- Folguera, G.; Bastias, D.A.; Caers, J.; Rojas, J.M.; Piulachs, M.D.; Belles, X.; Bozinovic, F. An experimental test of the role of environmental temperature variability on ectotherm molecular, physiological and life-history traits: Implications for global warming. Comp. Biochem. Phys. A 2011, 159, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M. Seeking the evolutionary regression coefficient—An analysis of what comparative methods measure. J. Theor. Biol. 1993, 164, 191–205. [Google Scholar] [CrossRef]
- Kellermann, V.; Loeschcke, V.; Hoffmann, A.A.; Kristensen, T.N.; Flojgaard, C.; David, J.R.; Svenning, J.C.; Overgaard, J. Phylogenetic constraints in key functional traits behind species′ climate niches: Patterns of desiccation and cold resistance across 95 Drosophila species. Evolution 2012, 66, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.N.; Loeschcke, V.; Bilde, T.; Hoffmann, A.A.; Sgro, C.; Noreikiene, K.; Ondresik, M.; Bechsgaard, J.S. No inbreeding depression for low temperature developmental acclimation across multiple Drosophila species. Evolution 2011, 65, 3195–3201. [Google Scholar] [CrossRef] [PubMed]
- Fasolo, A.G.; Krebs, R.A. A comparison of behavioural change in Drosophila during exposure to thermal stress. Biol. J. Linn. Soc. 2004, 83, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Schar, C.; Vidale, P.L.; Luthi, D.; Frei, C.; Haberli, C.; Liniger, M.A.; Appenzeller, C. The role of increasing temperature variability in European summer heatwaves. Nature 2004, 427, 332–336. [Google Scholar] [CrossRef]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [Green Version]
- Jentsch, A.; Kreyling, J.; Beierkuhnlein, C. A new generation of climate-change experiments: Events, not trends. Front. Ecol. Environ. 2007, 5, 365–374. [Google Scholar] [CrossRef]
- Pertoldi, C.; Bach, L.A.; Barker, J.S.F.; Lundberg, P.; Loeschcke, V. The consequences of the variance-mean rescaling effect on effective population size. Oikos 2007, 116, 769–774. [Google Scholar] [CrossRef]
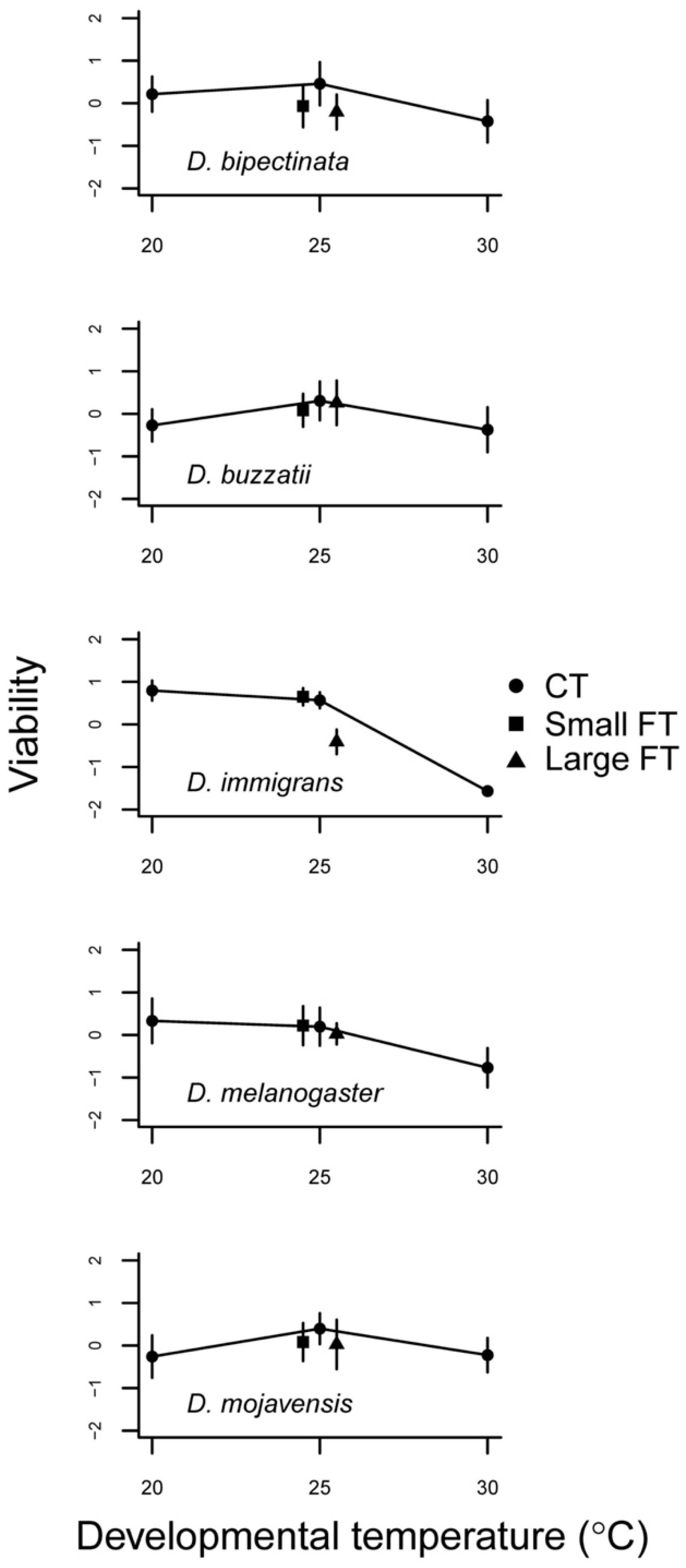

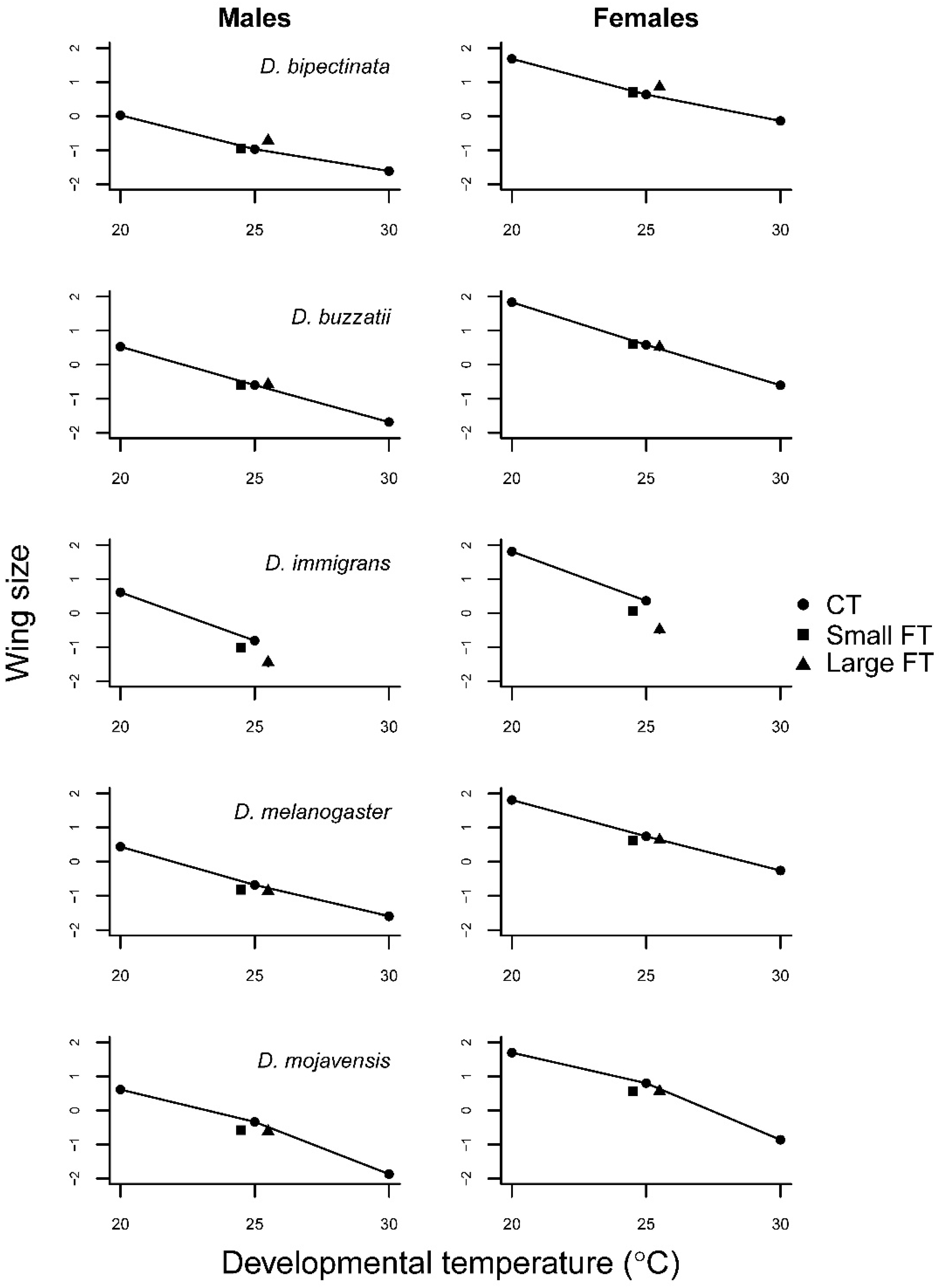
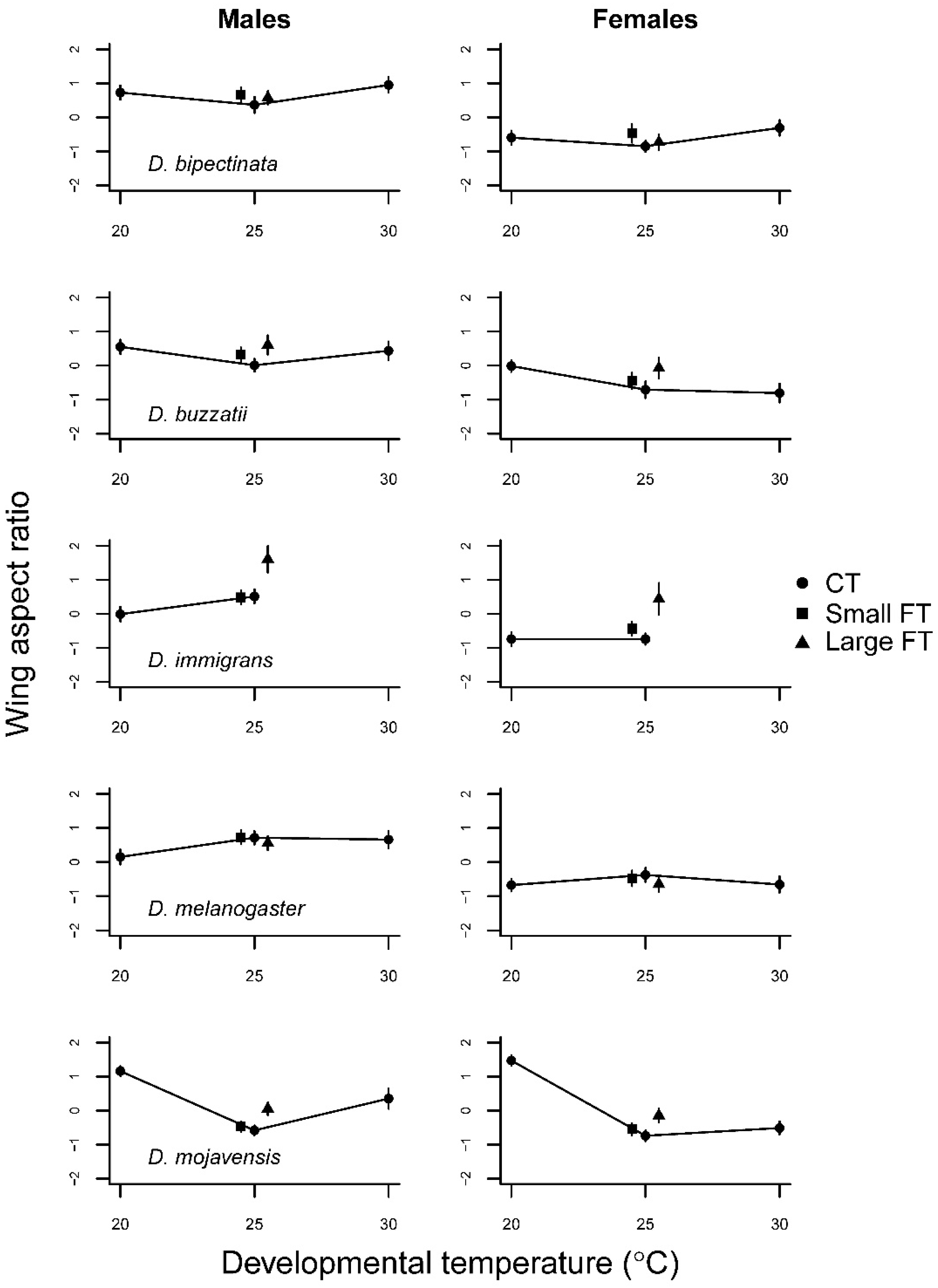
| Populations | Constant Temperature Viability | Fluctuating Temperature Viability | ||||
|---|---|---|---|---|---|---|
| Species | DF | MS | F | DF | MS | F |
| D. bipectinata | 2 (54) | 3.82 | 4.0 * | 2 (55) | 2.37 | 2.4 |
| D. buzzatii | 2 (57) | 2.67 | 2.8 | 2 (57) | 0.27 | 0.3 |
| D. immigrans | 2 (57) | 67.95 | 243.8 *** | 2 (56) | 6.87 | 28.5 *** |
| D. melanogaster | 2 (57) | 7.21 | 6.9 ** | 2 (57) | 0.22 | 0.3 |
| D. mojavensis | 2 (56) | 2.63 | 3.2 * | 2 (56) | 0.77 | 0.7 |
| Wing Size | |||||||
|---|---|---|---|---|---|---|---|
| Constant Temperature | Fluctuating Temperature | ||||||
| Species | Factor | DF | MS | F | DF | MS | F |
| D. bipectinata | Temperature | 2 | 9.27 | 1775.6 *** | 2 | 0.21 | 41.7 *** |
| Sex | 1 | 24.09 | 4617.2 *** | 1 | 24.41 | 4862.8 *** | |
| Temp × sex | 2 | 0.03 | 5.1 ** | 2 | 0.01 | 1.18 | |
| Error DF | 237 | 243 | |||||
| D. buzzatii | Temperature | 2 | 17.13 | 2248.4 *** | 2 | 0.004 | 0.4 |
| Sex | 1 | 14.43 | 1893.8 *** | 1 | 12.24 | 1382.0 *** | |
| Temp × sex | 2 | 0.04 | 5.3 ** | 2 | 0.01 | 0.9 | |
| Error DF | 277 | 266 | |||||
| D. immigrans | Temperature | 1 | 37.14 | 2836.6 *** | 2 | 2.87 | 191.0 *** |
| Sex | 1 | 26.69 | 2037.8 *** | 1 | 28.85 | 1923.0 *** | |
| Temp × sex | 1 | 0.00 | 0.3 | 2 | 0.06 | 4.3 | |
| Error DF | 162 | 198 | |||||
| D. melanogaster | Temperature | 2 | 21.94 | 1776.3 *** | 2 | 0.13 | 13.8 *** |
| Sex | 1 | 28.76 | 2328.4 *** | 1 | 33.26 | 3416.1 *** | |
| Temp × sex | 2 | 0.01 | 0.9 | 2 | 0.01 | 0.6 | |
| Error DF | 277 | 280 | |||||
| D. mojavensis | Temperature | 2 | 19.86 | 3741.2 *** | 2 | 0.26 | 60.2 *** |
| Sex | 1 | 9.72 | 1831.1 *** | 1 | 12.33 | 2888.2 *** | |
| Temp × sex | 2 | 0.01 | 2.4 | 2 | 0.001 | 0.3 | |
| Error DF | 281 | 281 | |||||
| Wing aspect ratio | |||||||
| D. bipectinata | Temperature | 2 | 4.56 | 16.2 *** | 2 | 1.69 | 5.1 ** |
| Sex | 1 | 72.44 | 256.4 *** | 1 | 67.98 | 205.0 *** | |
| Temp × sex | 2 | 0.05 | 0.2 | 2 | 0.11 | 0.3 | |
| Error DF | 238 | 244 | |||||
| D. buzzatii | Temperature | 2 | 3.32 | 17.7 *** | 2 | 2.81 | 14.9 *** |
| Sex | 1 | 15.95 | 85.2 *** | 1 | 12.20 | 64.6 *** | |
| Temp × sex | 2 | 0.99 | 5.29 ** | 2 | 0.02 | 0.1 | |
| Error DF | 277 | 266 | |||||
| D. immigrans | Temperature | 1 | 0.91 | 7.0 ** | 2 | 5.97 | 44.9 *** |
| Sex | 1 | 14.82 | 113.1 *** | 1 | 23.3 | 175.5 *** | |
| Temp × sex | 1 | 0.98 | 7.5 ** | 2 | 0.22 | 1.99 *** | |
| Error DF | 163 | 199 | |||||
| D. melanogaster | Temperature | 2 | 2.50 | 8.6 *** | 2 | 0.68 | 2.3 † |
| Sex | 1 | 47.49 | 162.3 *** | 1 | 54.82 | 188.3 *** | |
| Temp × sex | 2 | 0.81 | 2.8 † | 2 | 0.06 | 0.2 | |
| Error DF | 278 | 279 | |||||
| D. mojavensis | Temperature | 2 | 24.12 | 250.6 *** | 2 | 2.40 | 32.0 *** |
| Sex | 1 | 1.23 | 12.8 *** | 1 | 0.41 | 5.5 * | |
| Temp × sex | 2 | 2.02 | 21.0 *** | 2 | 0.02 | 0.3 | |
| Error DF | 282 | 281 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manenti, T.; Kjærsgaard, A.; Schou, T.M.; Pertoldi, C.; Moghadam, N.N.; Loeschcke, V. Responses to Developmental Temperature Fluctuation in Life History Traits of Five Drosophila Species (Diptera: Drosophilidae) from Different Thermal Niches. Insects 2021, 12, 925. https://doi.org/10.3390/insects12100925
Manenti T, Kjærsgaard A, Schou TM, Pertoldi C, Moghadam NN, Loeschcke V. Responses to Developmental Temperature Fluctuation in Life History Traits of Five Drosophila Species (Diptera: Drosophilidae) from Different Thermal Niches. Insects. 2021; 12(10):925. https://doi.org/10.3390/insects12100925
Chicago/Turabian StyleManenti, Tommaso, Anders Kjærsgaard, Toke Munk Schou, Cino Pertoldi, Neda N. Moghadam, and Volker Loeschcke. 2021. "Responses to Developmental Temperature Fluctuation in Life History Traits of Five Drosophila Species (Diptera: Drosophilidae) from Different Thermal Niches" Insects 12, no. 10: 925. https://doi.org/10.3390/insects12100925






