Unprecedented Density and Persistence of Feral Honey Bees in Urban Environments of a Large SE-European City (Belgrade, Serbia) †
Abstract
Simple Summary
Abstract
1. Introduction
- present evidence of the extraordinary frequent incidence of unmanaged honey bee colonies throughout the wider Belgrade area (based on the occurrence data compiled for 2011–2017), and a similarly frequent incidence of swarms (probably many of them from unmanaged colonies);
- assess the basic parameters of the recorded occurrences (the features of documented nesting/swarming sites: type of substrate/microspace, height, neighbourhood or habitat type) and the parameters of the recording and reporting process (features of the compiled data-set, its limitations, peculiarities, and utility);
- analyze the patterns of distribution of free-living honey bee colonies and observed swarms across the different local urban and sub-urban/peri-urban settings of a large city, relative to the distribution of managed hives;
- evaluate the status of unmanaged honey bee occurrences in Belgrade, hypothesizing that they indicate the existence of a large, long-lasting and self-sustaining feral population;
- present the experiences (advantages, problems and perspectives) of the citizen science approach for detecting, assessing the status and monitoring of feral honey bees in urban environments.
2. Materials and Methods
2.1. Case Study Area
2.2. Data Source
2.3. Data Processing
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaplin-Kramer, R.; Tuxen-Bettman, K.; Kremen, C. Value of Wildland Habitat for Supplying Pollination Services to Californian Agriculture. Rangelands 2011, 33, 33–41. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Mayer, D.F. Crop Pollination by Bees; CABI Publishing: New York, NY, USA, 2000. [Google Scholar]
- De la Rúa, P.; Jaffé, R.; Dall’Olio, R.; Muñoz, I.; Serrano, J. Biodiversity, Conservation and Current Threats to European Honeybees. Apidologie 2009, 40, 263–284. [Google Scholar] [CrossRef]
- Gaines-Day, H.R.; Gratton, C. Crop Yield is Correlated with Honey Bee Hive Density but not in High-Woodland Landscapes. Agric. Ecosyst. Environ. 2016, 218, 53–57. [Google Scholar] [CrossRef]
- Belsky, J.; Joshi, N.K. Impact of Biotic and Abiotic Stressors on Managed and Feral Bees. Insects 2019, 10, 223. [Google Scholar] [CrossRef]
- Aebi, A.; Neumann, P. Endosymbionts and Honey Bee Colony Losses? Trends Ecol. Evol. 2011, 26, 494. [Google Scholar] [CrossRef][Green Version]
- Ellis, J.D.; Evans, J.D.; Pettis, J. Colony Losses, Managed Colony Population Decline, and Colony Collapse Disorder in the United States. J. Apic. Res. 2010, 49, 134–136. [Google Scholar] [CrossRef]
- Higes, M.; Meana, A.; Bartolome, C.; Botías, C.; Martin-Hernandez, R. Nosema ceranae (Microsporidia), A Controversial 21st Century Honey Bee Pathogen. Environ. Microbiol. Rep. 2013, 5, 17–29. [Google Scholar] [CrossRef]
- Maini, S.; Medrzycki, P.; Porrini, C. The Puzzle of Honey Bee Losses: A Brief Review. Bull. Insectology 2010, 63, 153–160. [Google Scholar]
- Meixner, M.D.; Kryger, P.; Costa, C. Effects of Genotype, Environment, and Their Interactions on Honey Bee Health in Europe. Curr. Opin. Insect. 2015, 10, 177–184. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; de Miranda, J.; Fries, I.; Le Conte, Y.; Neumann, P.; Paxton, R.J. Research Strategies to Improve Honeybee Health in Europe. Apidologie 2010, 41, 227–242. [Google Scholar] [CrossRef]
- Naug, D. Nutritional Stress due to Habitat Loss may Explain Recent Honeybee Colony Collapses. Biol. Conserv. 2009, 142, 2369–2372. [Google Scholar] [CrossRef]
- Paudel, Y.P.; Mackereth, R.; Hanley, R.; Qin, W. Honey Bees (Apis mellifera L.) and Pollination Issues: Current Status, Impacts and Potential Drivers of Decline. J. Agric. Sci. 2015, 7, 93–109. [Google Scholar] [CrossRef]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Aleksić, N.; Jovanović, N.; Vejnović, B.; Stevanović, J. Looking for the Causes of and Solutions to the Issue of Honey Bee Colony Losses. Acta Vet. 2019, 69, 1–31. [Google Scholar] [CrossRef]
- van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of its Drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Fries, I.; Camazine, S.; Sneyd, J. Population Dynamics of Varroa jacobsoni: A Model and a Review. Bee World 1994, 75, 5–28. [Google Scholar] [CrossRef]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa Mites and Honey Bee Health: Can Varroa Explain Part of the Colony Losses? Apidologie 2010, 41, 353–363. [Google Scholar] [CrossRef]
- Sammataro, D.; Gerson, U.; Needham, G. Parasitic Mites of Honey Bees: Life History, Implications, and Impact. Annu. Rev. Entomol. 2000, 45, 519–548. [Google Scholar] [CrossRef]
- Solignac, M.; Cornuet, J.-M.; Vautrin, D.; Le Conte, Y.; Anderson, D.; Evans, J.; Cros-Arteil, S.; Navajas, M. The Invasive Korea and Japan Types of Varroa destructor, Ectoparasitic Mites of the Western Honeybee (Apis mellifera), are Two Partly Isolated Clones. Proc. Royal Soc. B 2005, 272, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.; Jones, R.; Neumann, P.; Settele, J. Declines of Managed Honey Bees and Beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and Control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, 96–119. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Eccles, L.; Calvete, Y.; McGowan, J.; Kelly, P.G.; Correa-Benítez, A. Varroa destructor is the Main Culprit for the Death and Reduced Populations of Overwintered Honey Bee (Apis mellifera) Colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef]
- van Dooremalen, C.; Gerritsen, L.; Cornelissen, B.; van der Steen, J.J.M.; van Langevelde, F.; Blacquière, T. Winter Survival of Individual Honey Bees and Honey Bee Colonies Depends On Level of Varroa destructor infestation. PLoS ONE 2012, 7, e36285. [Google Scholar] [CrossRef]
- Büchler, R.; Berg, S.; Le Conte, Y. Breeding for Resistance to Varroa destructor in Europe. Apidologie 2010, 41, 393–408. [Google Scholar] [CrossRef]
- Oleksa, A.; Gawroński, R.; Tofilski, A. Rural Avenues as a Refuge for Feral Honey Bee Population. J. Insect Conserv. 2013, 17, 465–472. [Google Scholar] [CrossRef]
- Le Conte, Y.; de Vaublanc, G.; Crauser, D.; Jeanne, F.; Rousselle, J.-C.; Bécard, J.-M. Honey Bee Colonies that Have Survived Varroa destructor. Apidologie 2007, 38, 566–572. [Google Scholar] [CrossRef]
- Seeley, T.D. Honey Bees of the Arnot Forest: A Population of Feral Colonies Persisting with Varroa destructor in the Northeastern United States. Apidologie 2007, 38, 19–29. [Google Scholar] [CrossRef]
- Seeley, T.D. Life-History Traits of Wild Honey Bee Colonies Living in Forests Around Ithaca, NY, USA. Apidologie 2017, 48, 743–754. [Google Scholar] [CrossRef]
- Seeley, T.D.; Tarpy, D.R.; Griffin, S.R.; Carcione, A.; Delaney, D.A. A Survivor Population of Wild Colonies of European Honeybees in the Northeastern United States: Investigating Its Genetic Structure. Apidologie 2015, 46, 654–666. [Google Scholar] [CrossRef]
- Rinderer, T.E.; De Guzman, L.I.; Delatte, G.T.; Stelzer, J.A.; Lancaster, V.A.; Kuznetsov, V.; Beaman, L.; Watts, R.; Harris, J.W. Resistance to the Parasitic Mite Varroa destructor in Honey Bees from Far-Eastern Russia. Apidologie 2001, 32, 381–394. [Google Scholar] [CrossRef]
- Fries, I.; Imdorf, A.; Rosenkranz, P. Survival of Mite Infested (Varroa destructor) Honey Bee (Apis mellifera) Colonies in a Nordic Climate. Apidologie 2006, 37, 564–570. [Google Scholar] [CrossRef]
- De Jong, D.; Soares, A.E.E. An Isolated Population of Italian Bees that Has Survived Varroa jacobsoni Infestation without Treatment for over 12 Years. Am. Bee J. 1997, 137, 742–745. [Google Scholar]
- Locke, B.; Fries, I. Characteristics of Honey Bee Colonies (Apis mellifera) in Sweden Surviving Varroa destructor Infestation. Apidologie 2011, 42, 533–542. [Google Scholar] [CrossRef]
- Kohl, P.L.; Rutschmann, B. The Neglected Bee Trees: European Beech Forests as a Home for Feral Honey Bee Colonies. PeerJ 2018, 6, e4602. [Google Scholar] [CrossRef]
- Locke, B.; Le Conte, Y.; Crauser, D.; Fries, I. Host Adaptations Reduce the Reproductive Success of Varroa destructor in Two Distinct European Honey Bee Populations. Ecol. Evol. 2012, 2, 1144–1150. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; Kraus, F.B.; Kryger, P.; Crewe, R.M. The Size of Wild Honeybee Populations (Apis mellifera) and Its Implications for the Conservation of Honeybees. J. Insect Conserv. 2007, 11, 391–397. [Google Scholar] [CrossRef]
- Alaux, C.; Le Conte, Y.; Decourtye, A. Pitting Wild Bees against Managed Honey Bees in Their Native Range, a Losing Strategy for the Conservation of Honey Bee Biodiversity. Front. Ecol. Evol. 2019, 7, 60. [Google Scholar] [CrossRef]
- Requier, F.; Garnery, L.; Kohl, P.L.; Njovu, H.K.; Pirk, C.W.W.; Crewe, R.M.; Steffan-Dewenter, I. The Conservation of Native Honey Bees is Crucial. Trends Ecol. Evol. 2019, 34, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.; Blacquière, T.; Panziera, D.; Dietemann, V.; Neumann, P. Host-Parasite Co-Evolution in Real-Time: Changes in Honey Bee Resistance Mechanisms and Mite Reproductive Strategies. Insects 2021, 12, 120. [Google Scholar] [CrossRef]
- Kurze, C.; Routtu, J.; Moritz, R.F. Parasite Resistance and Tolerance in Honeybees at the Individual and Social Level. Zoology 2016, 119, 290–297. [Google Scholar] [CrossRef]
- Blacquière, T.; Panziera, D. A Plea for Use of Honey Bees’ Natural Resilience in Beekeeping. Bee World 2018, 95, 34–38. [Google Scholar] [CrossRef]
- Moro, A.; Beaurepaire, A.; Dall’Olio, R.; Rogenstein, S.; Blacquière, T.; Dahle, B.; de Miranda, J.R.; Dietemann, V.; Locke, B.; Licón Luna, R.M.; et al. Using Citizen Science to scout Honey Bee Colonies that Naturally Survive Varroa destructor Infestations. Insects 2021, 12, 536. [Google Scholar] [CrossRef]
- Panziera, D.; van Langevelde, F.; Blacquière, T. Varroa Sensitive Hygiene Contributes to Naturally Selected Varroa Resistance in Honey Bees. J. Apic. Res. 2017, 56, 635–642. [Google Scholar] [CrossRef]
- Powell, J. Learning from Wild Bees and Tree Beekeeping [The Beekeepers Quarterly, Issue 123]. Available online: https://www.naturalbeekeepingtrust.org/learning-from-wild-bees-trees (accessed on 30 October 2021).
- Loftus, J.C.; Smith, M.L.; Seeley, T.D. How Honey Bee Colonies Survive in the Wild: Testing the Importance of Small Nests and Frequent Swarming. PLoS ONE 2016, 11, e0150362. [Google Scholar] [CrossRef]
- Thompson, C.E. The Health and Status of the Feral Honeybee (Apis mellifera sp) and Apis mellifera mellifera Population of the UK. Ph.D. Thesis, University of Leeds, Leeds, UK, November 2012. [Google Scholar]
- Baum, K.; Tchakerian, M.D.; Thoenes, S.C.; Coulson, R.N. Africanized Honey Bees in Urban Environments: A Spatio-Temporal Analysis. Landsc. Urban Plan. 2008, 85, 123–132. [Google Scholar] [CrossRef]
- Baum, K.A.; Tchakerian, M.D.; Birt, A.G.; Coulson, R.N. Honey Bee Ecology from an Urban Landscape Perspective. The Spatial Ecology of Feral Honey Bees. In Silico Bees; Devillers, J., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2014; Chapter 6; pp. 109–134. [Google Scholar]
- Wojcik, V.A.; Frankie, G.W.; Thorp, R.W.; Hernandez, J.L. Seasonality in Bees and Their Floral Resource Plants at a Constructed Urban Bee Habitat in Berkeley, California. J. Kans. Entomol. 2008, 81, 15–28. [Google Scholar] [CrossRef]
- Wenzel, A.; Grass, I.; Belavadi, V.V.; Tscharntke, T. How Urbanization is Driving Pollinator Diversity and Pollination—A Systematic Review. Biol. Conserv. 2020, 241, 108321. [Google Scholar] [CrossRef]
- Geslin, B.; Le Féon, V.; Folschweiller, M.; Flacher, F.; Carmignac, D.; Motard, E.; Perret, S.; Dajoz, I. The Proportion of Impervious Surfaces at the Landscape Scale Structures Wild Bee Assemblages in a Densely Populated Region. Ecol. Entomol. 2016, 6, 6599–6615. [Google Scholar] [CrossRef]
- Lawrence, T.J.; Culbert, E.M.; Felsot, A.S.; Hebert, V.R.; Sheppard, W.S. Survey and Risk Assessment of Apis mellifera (Hymenoptera: Apidae) Exposure to Neonicotinoid Pesticides in Urban, Rural, and Agricultural Settings. J. Econ. Entomol. 2016, 109, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Prudic, K.L.; Oliver, J.C.; Brown, B.V.; Long, E.C. Comparisons of Citizen Science Data-Gathering Approaches to Evaluate Urban Butterfly Diversity. Insects 2018, 9, 186. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen Science as an Ecological Research Tool: Challenges and Benefits. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 149–172. [Google Scholar] [CrossRef]
- Li, E.; Parker, S.S.; Pauly, G.B.; Randall, J.M.; Brown, B.V.; Cohen, B.S. An Urban Biodiversity Assessment Framework that Combines an Urban Habitat Classification Scheme and Citizen Science Data. Front. Ecol. Evol. 2019, 7, 277. [Google Scholar] [CrossRef]
- Statistical Office of the Republic of Serbia. Available online: https://www.stat.gov.rs/en-US (accessed on 1 October 2021).
- QGIS Development Team, QGIS Geographic Information System. Open Source Geospatial Foundation. Available online: http://qgis.org (accessed on 1 October 2021).
- Baddeley, A.; Rubak, E.; Turner, R. Spatial Point Patterns. Methodology and Applications with R; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Baddeley, A.; Turner, R. Spatstat: An R Package for Analyzing Spatial Point Patterns. J. Stat. Softw. 2005, 12, 1–42. [Google Scholar] [CrossRef]
- R Core Team: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 October 2021).
- Project SERBHIWE-Honey Bees of Serbia, Wild vs. Managed Colonies through the Eyes of Population Geneticists. Available online: http://www.ibiss.bg.ac.rs/index.php/en/present/national/item/1623-serbhiwe-honey-bees-of-serbia-wild-vs-managed-colonies-through-the-eyes-of-population-geneticists (accessed on 1 September 2021).
- Patenković, A.; Tanasković, M.; Erić, P.; Erić, K.; Mihailović, M.; Tanasić, V.; Stanisavljević, L.; Davidović, S. Urban Ecosystem Drives Genetic Diversity in Feral Honey Bees’ (Apis mellifera) Colonies. manuscript in preparation.
- Spivak, M.; Reuter, G.S. Varroa destructor Infestation in Untreated Honey Bee (Hymenoptera: Apidae) Colonies Selected for Hygienic Behavior. J. Econ. Entomol. 2001, 94, 326–331. [Google Scholar] [CrossRef]
- Burley, L.M.; Fell, R.D.; Saacke, R.G. Survival of Honey Bee (Hymenoptera: Apidae) Spermatozoa Incubated at Room Temperature from Drones Exposed to Miticides. J. Econ. Entomol. 2008, 101, 1081–1087. [Google Scholar] [CrossRef]
- Fries, I.; Hansen, H.; Imdorf, A.; Rosenkranz, P. Swarming in Honey Bees (Apis mellifera) and Varroa destructor Population Development in Sweden. Apidologie 2003, 34, 389–397. [Google Scholar] [CrossRef]
- Fries, I.; Camazine, S. Implications of Horizontal and Vertical Pathogen Transmission for Honey Bee Epidemiology. Apidologie 2001, 32, 199–214. [Google Scholar] [CrossRef]
- van Alphen, J.J.M.; Fernhout, B.J. Natural Selection, Selective Breeding, and the Evolution of Resistance of Honeybees (Apis mellifera) against Varroa. Zool. Lett. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Blacquière, T.; Boot, W.; Calis, J.; Moro, A.; Neumann, P.; Panziera, D. Darwinian Black Box Selection for Resistance to Settled Invasive Varroa destructor Parasites in Honey Bees. Biol. Invasions 2019, 21, 2519–2528. [Google Scholar] [CrossRef]
- Rosenthal, J.S. A Review of the Role of Protected Areas in Conserving Global Domestic Animal Diversity. Anim. Genet. Resour. Inf. 2010, 47, 101–113. [Google Scholar] [CrossRef]
- López-Uribe, M.M.; Soro, A.; Jha, S. Conservation Genetics of Bees: Advances in the Application of Molecular Tools to Guide Bee Pollinator Conservation. Conserv. Genet. 2017, 18, 501–506. [Google Scholar] [CrossRef]
- Broeckx, B.J.G.; De Smet, L.; Blacquiere, T.; Maebe, K.; Khalenkow, M.; Van Poucke, M.; Dahle, B.; Neumann, P.; Bach Nguyen, K.; Smagghe, G.; et al. Honey Bee Predisposition of Resistance to Ubiquitous Mite Infestations. Sci. Rep. 2019, 9, 7794. [Google Scholar] [CrossRef]
- Uzunov, A.; Brascamp, E.W.; Büchler, R. The Basic Concept of Honey Bee Breeding Programs. Bee World 2017, 94, 84–87. [Google Scholar] [CrossRef]
- Costa, C.; Büchler, R.; Berg, S.; Bienkowska, M.; Bouga, M.; Bubalo, D.; Charistos, L.; Le Conte, Y.; Drazic, M.; Dyrba, W.; et al. A Europe-Wide Experiment for Assessing the Impact of Genotype-Environment Interactions on the Vitality and Performance of Honey Bee Colonies: Experimental Design and Trait Evaluation. J. Apic. Sci. 2012, 56, 147–158. [Google Scholar] [CrossRef]
- Requier, F.; Paillet, Y.; Laroche, F.; Rutschmann, B.; Zhang, J.; Lombardi, F.; Svoboda, M.; Steffan-Dewenter, I. Contribution of European Forests to Safeguard Wild Honeybee Populations. Conserv. Lett. 2019, 13, e12693. [Google Scholar] [CrossRef]
- Mitchell, D. Ratios of Colony Mass to Thermal Conductance of Tree And Man-Made Nest Enclosures of Apis mellifera: Implications for Survival, Clustering, Humidity Regulation and Varroa destructor. Int. J. Biometeorol. 2016, 60, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.A.; Hassett, J.; Geary, M.; Moore, E.; Henriques, D.; Soland-Reckeweg, G.; Ferrari, R.; Mac Loughlin, E.; O’Brien, E.; O’Driscoll, S.; et al. Investigation of Free-Living Honey Bee Colonies in Ireland. J. Apic. Res. 2020, 60, 229–240. [Google Scholar] [CrossRef]
- Baum, K.A.; Rubink, W.L.; Pinto, A.M.; Coulson, R.N. Spatial and Temporal Distribution and Nest Site Characteristics of Feral Honey Bee (Hymenoptera: Apidae) Colonies in a Coastal Prairie Landscape. Environ. Entomol. 2005, 34, 610–618. [Google Scholar] [CrossRef]
- Collins, A. Feral Friend or Foe? Nat. Bee Husb. 2017, 4, 4–7. [Google Scholar]
- Geoghegan, H.; Dyke, A.; Pateman, R.; West, S.; Everett, G. Understanding Motivations for Citizen Science. Final Report on Behalf of UKEOF; University of Reading, Stockholm Environment Institute (University of York) and University of the West of England: Wilthsire, UK, 2016. [Google Scholar]
- Saunders, M.E.; Goodwin, E.K.; Santos, K.C.B.S.; Sonter, C.A.; Rader, R. Cavity Occupancy by Wild Honey Bees: Need for Evidence of Ecological Impacts. Front. Ecol. Environ. 2021, 19, 349–354. [Google Scholar] [CrossRef]
- van Strien, A.J.; van Swaay, C.A.M.; Termaat, T.; Devictor, V. Opportunistic Citizen Science Data of Animal Species Produce Reliable Estimates of Distribution Trends If Analysed with Occupancy Models. J. Appl. Ecol. 2013, 50, 1450–1458. [Google Scholar] [CrossRef]
- Jackson, M.M.; Gergel, S.E.; Martin, K. Citizen Science and Field Survey Observations Provide Comparable Results for Mapping Vancouver Island White-Tailed Ptarmigan (Lagopus leucura saxatilis) Distributions. Biol. Conserv. 2015, 181, 162–172. [Google Scholar] [CrossRef]
- Zapponi, L.; Cini, A.; Bardiani, M.; Hardersen, S.; Maura, M.; Maurizi, E.; Redolfi De Zan, L.; Audisio, P.; Bologna, M.A.; Carpaneto, G.M.; et al. Citizen Science Data as an Efficient Tool for Mapping Protected Saproxylic Beetles. Biol. Conserv. 2016, 208, 139–145. [Google Scholar] [CrossRef]
- Olkowski, H.; Olkowski, W. Entomophobia in the Urban Ecosystem, Some Observations and Suggestions. Ann. Entomol. Soc. 1976, 22, 313–318. [Google Scholar] [CrossRef]
- Schönfelder, M.L.; Bogner, F.X. Individual Perception Of Bees: Between Perceived Danger and Willingness to Protect. PLoS ONE 2017, 12, e0180168. [Google Scholar] [CrossRef]
- Sumner, S.; Law, G.; Cini, A. Why We Love Bees and Hate Wasps. Ecol. Entomol. 2018, 43, 836–845. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Thomas, C.D. Bias in Butterfly Distribution Maps: The Influence of Hot Spots and Recorder’s Home Range. J. Insect Conserv. 2000, 4, 73–77. [Google Scholar] [CrossRef]
- Thompson, C.E.; Budge, G.E.; Biesmeijer, J.C. Feral Bees in the UK: The Real Story. Bee Craft 2010, 92, 22–24. [Google Scholar]
- Wastian, L.; Unterweger, P.A.; Betz, O. Influence of the Reduction of Urban Lawn Mowing on Wild Bee Diversity (Hymenoptera, Apoidea). J. Hymenopt. Res. 2016, 49, 51–63. [Google Scholar] [CrossRef]



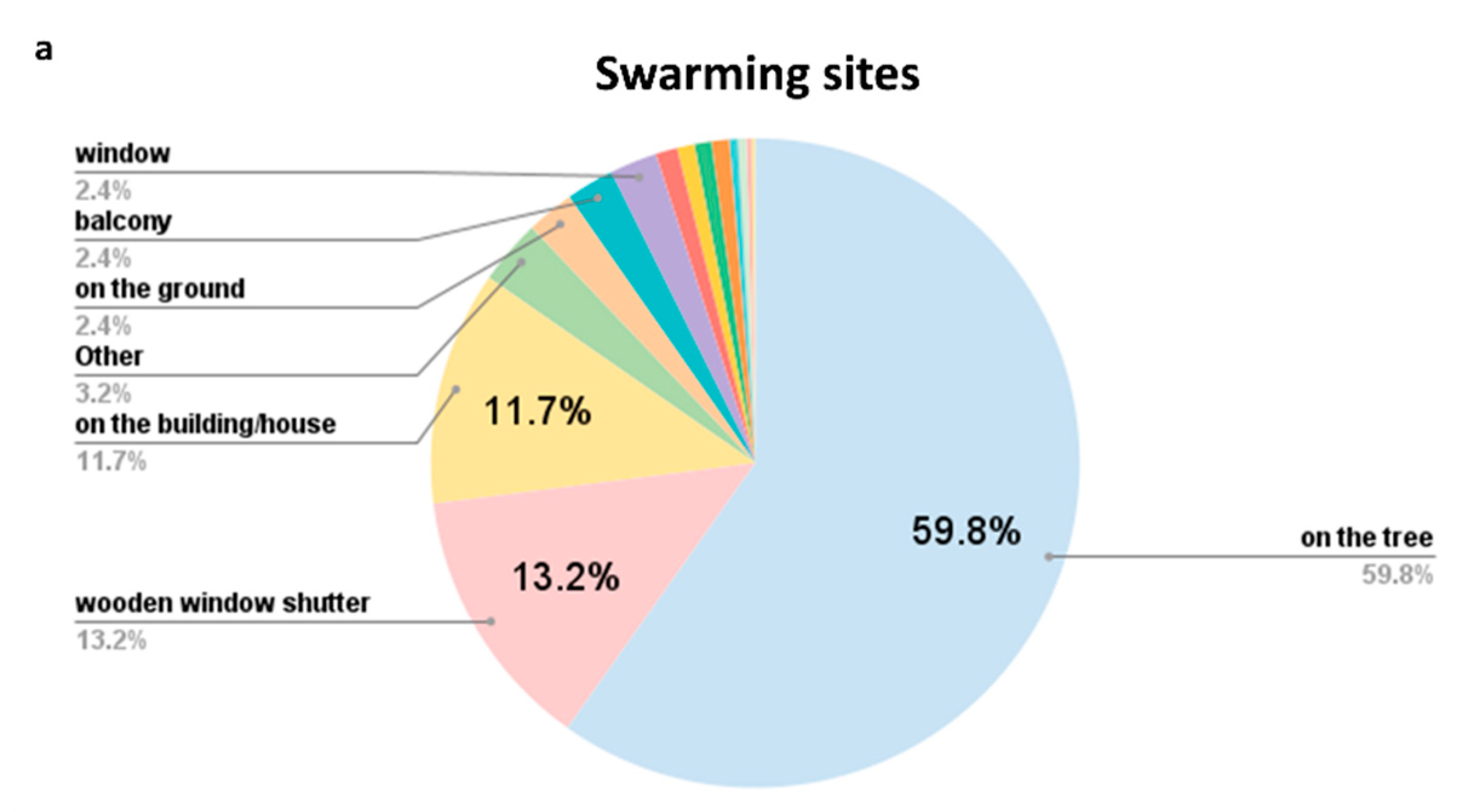
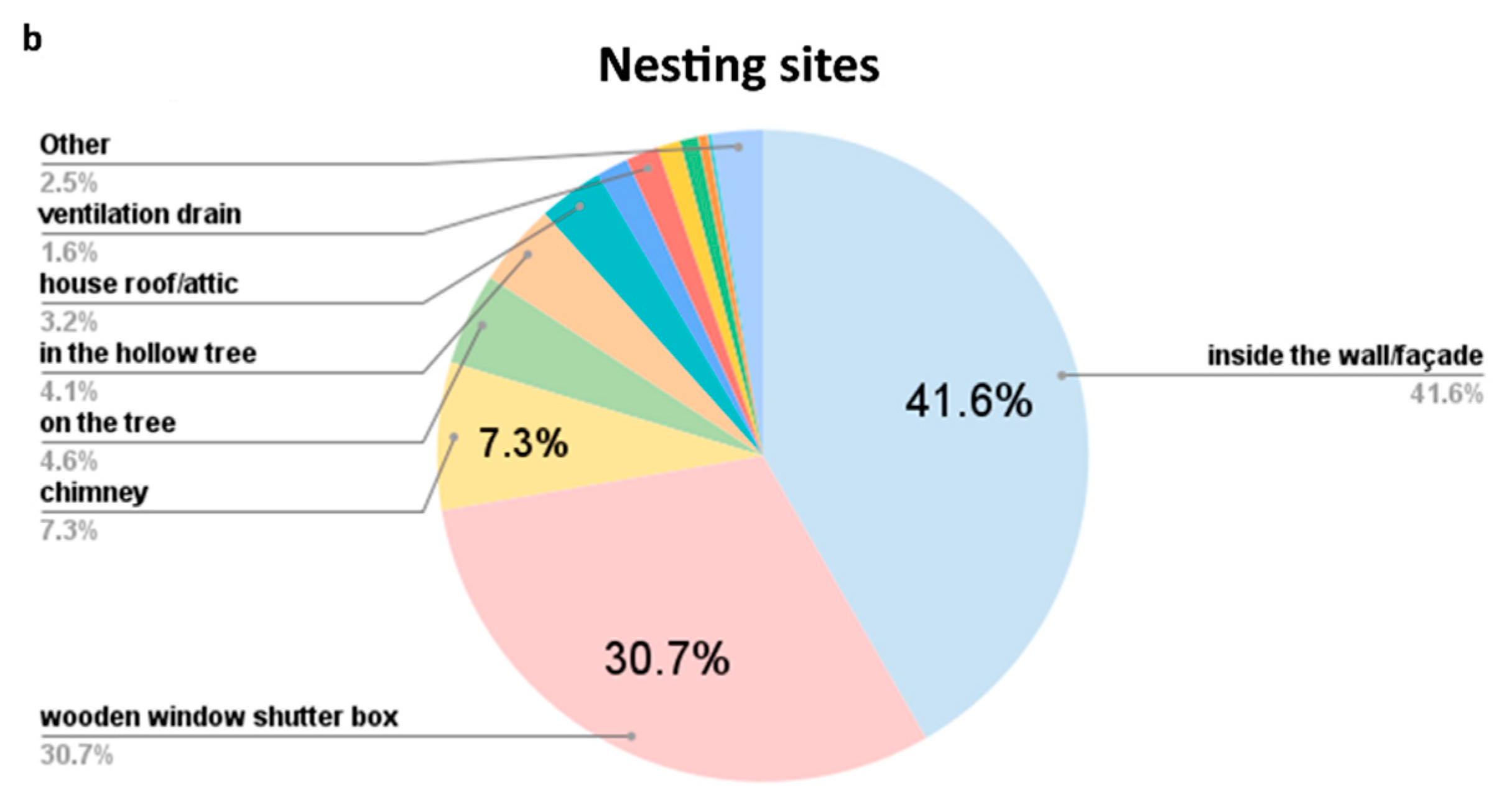


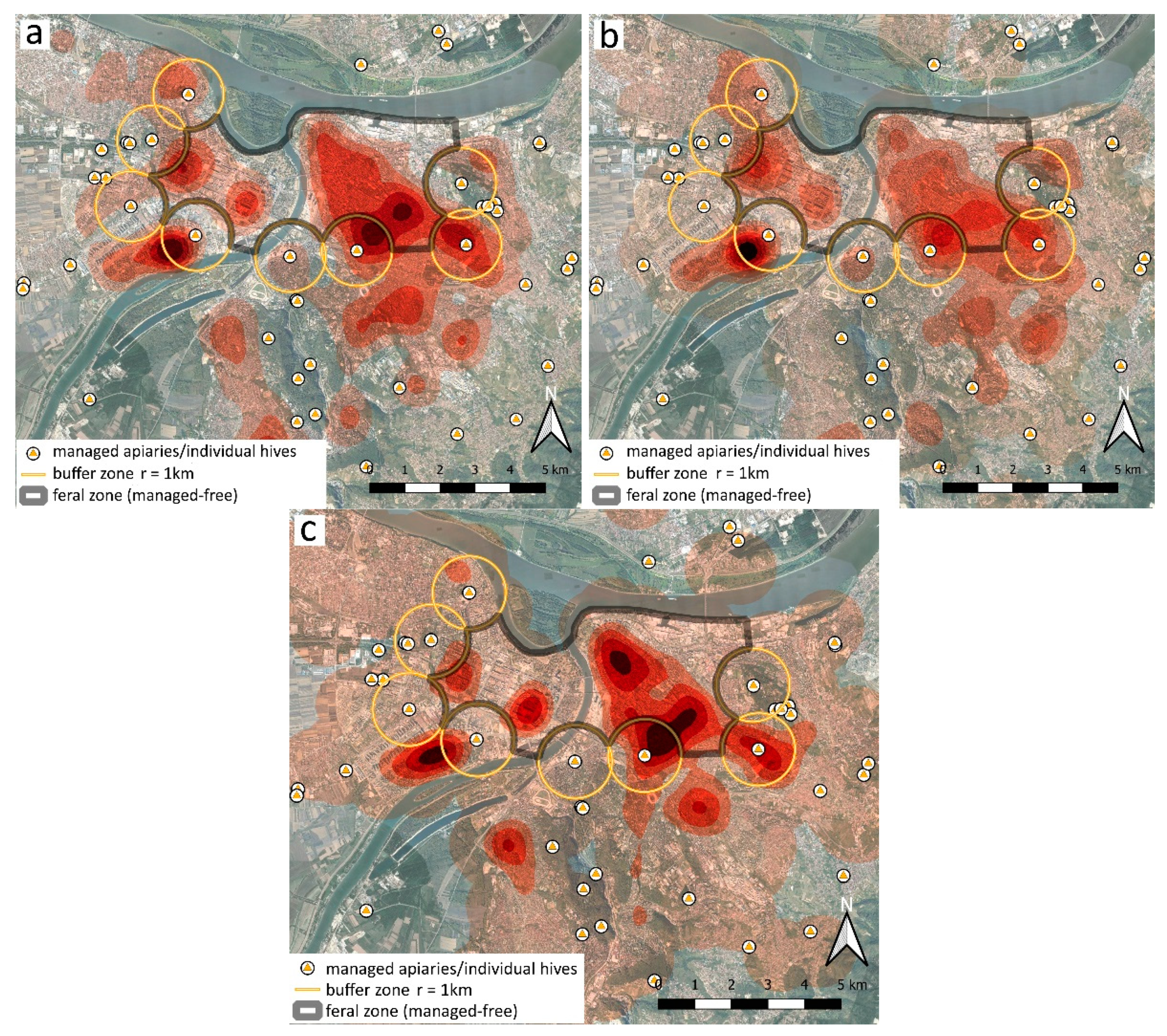
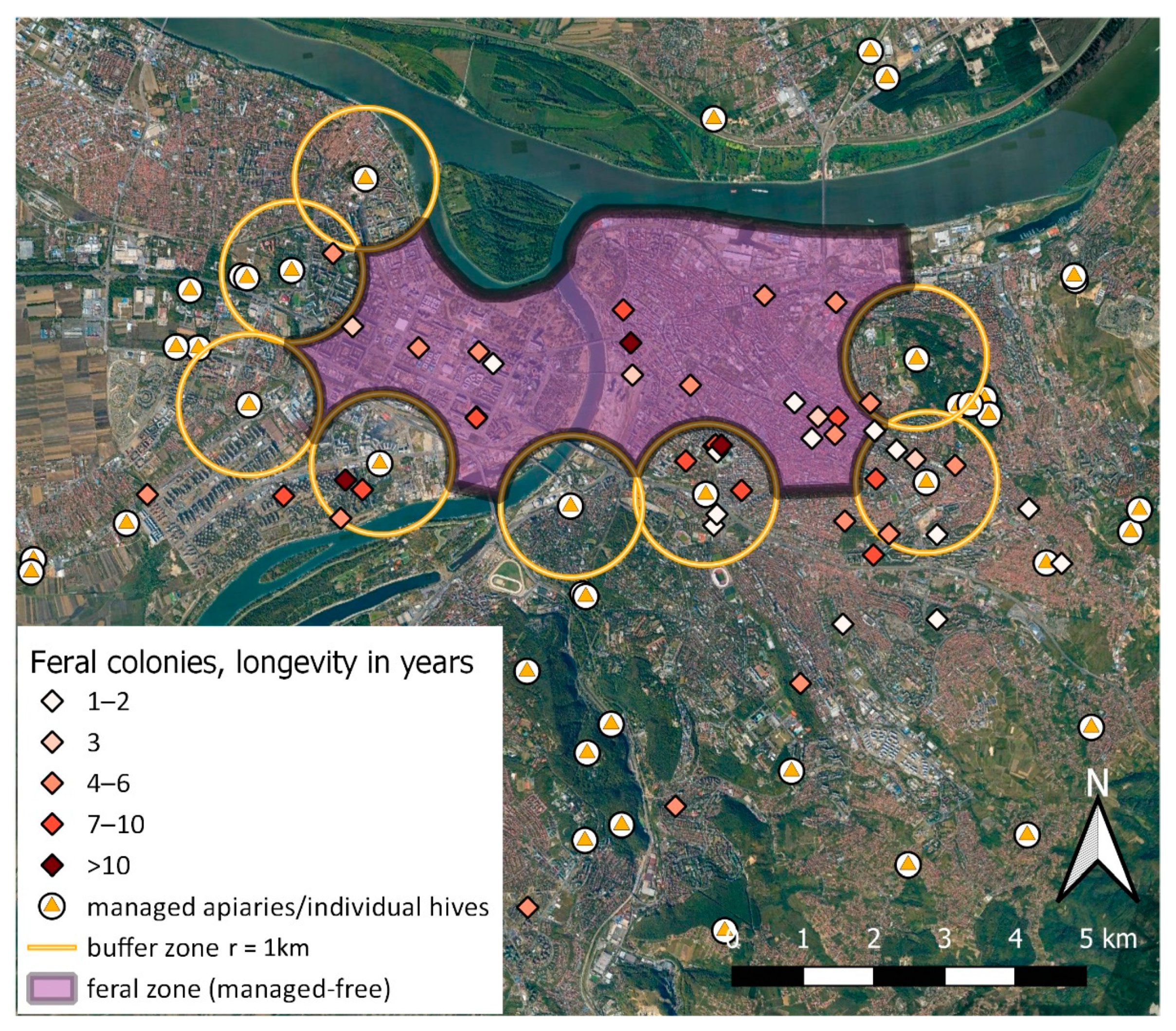
| Year | Colonies | Swarms | Ambiguous/Unspecified | Total Honey Bees |
|---|---|---|---|---|
| 2011 | 157 | 95 | - | 252 |
| 2012 | 116 | 78 | - | 194 |
| 2013 | 34 | 38 | 38 | 110 |
| 2014 | 54 | 115 | 109 | 278 |
| 2015 | 33 | 56 | 78 | 167 |
| 2016 | 44 | 95 | 110 | 249 |
| 2017 | 22 | 60 | 39 | 121 |
| ∑ | 460 | 537 | 374 | 1371 |
| Variable | Coefficient (SE) | 95% CI | z Value | z Test |
|---|---|---|---|---|
| Intercept | 0.842421 (0.046938) | 0.7504–0.9344 | 17.947 | <0.05 |
| Population density | 0.000128 (0.000004) | 0.000121–0.000136 | 34.384 | <0.05 |
| Years | 1–2 | 3 | 4–6 | 7–10 | >10 | ∑ |
|---|---|---|---|---|---|---|
| Number of Colonies | 16 | 28 | 19 | 12 | 3 | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bila Dubaić, J.; Simonović, S.; Plećaš, M.; Stanisavljević, L.; Davidović, S.; Tanasković, M.; Ćetković, A. Unprecedented Density and Persistence of Feral Honey Bees in Urban Environments of a Large SE-European City (Belgrade, Serbia). Insects 2021, 12, 1127. https://doi.org/10.3390/insects12121127
Bila Dubaić J, Simonović S, Plećaš M, Stanisavljević L, Davidović S, Tanasković M, Ćetković A. Unprecedented Density and Persistence of Feral Honey Bees in Urban Environments of a Large SE-European City (Belgrade, Serbia). Insects. 2021; 12(12):1127. https://doi.org/10.3390/insects12121127
Chicago/Turabian StyleBila Dubaić, Jovana, Slađan Simonović, Milan Plećaš, Ljubiša Stanisavljević, Slobodan Davidović, Marija Tanasković, and Aleksandar Ćetković. 2021. "Unprecedented Density and Persistence of Feral Honey Bees in Urban Environments of a Large SE-European City (Belgrade, Serbia)" Insects 12, no. 12: 1127. https://doi.org/10.3390/insects12121127
APA StyleBila Dubaić, J., Simonović, S., Plećaš, M., Stanisavljević, L., Davidović, S., Tanasković, M., & Ćetković, A. (2021). Unprecedented Density and Persistence of Feral Honey Bees in Urban Environments of a Large SE-European City (Belgrade, Serbia). Insects, 12(12), 1127. https://doi.org/10.3390/insects12121127









