Suitable Areas for Apiculture Expansion Determined by Antioxidant Power, Chemical Profiles, and Pesticide Residues in Caldcluvia paniculata Honey and Beeswax Samples
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey and Beeswax Samples
2.2. Mellisopalynological Analysis for Determining the Botanical Origin of Honey Samples
2.3. Preparation of Honey Solutions
2.4. Colorimetric Assays for Determining Total Phenolic Compounds
2.5. Ferric Reducing/Antioxidant Power (FRAP) Assays for Determining Antioxidant Power
2.6. Determinations of Antiradical Activity
2.7. Determination of Total Carbohydrate Content in Honey Samples
2.8. Determinations of Total Ash Content in Honey Samples
2.9. Direct Sample Analysis-Time of Flight-Mass Spectrometry (DSA-TOF-MS) for Determining Chromatography Profiles
2.10. Extraction Methodologies
2.10.1. Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS)
2.10.2. Solid-Phase Extraction
2.11. Chromatography
2.11.1. LC-MS/MS Analysis
2.11.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
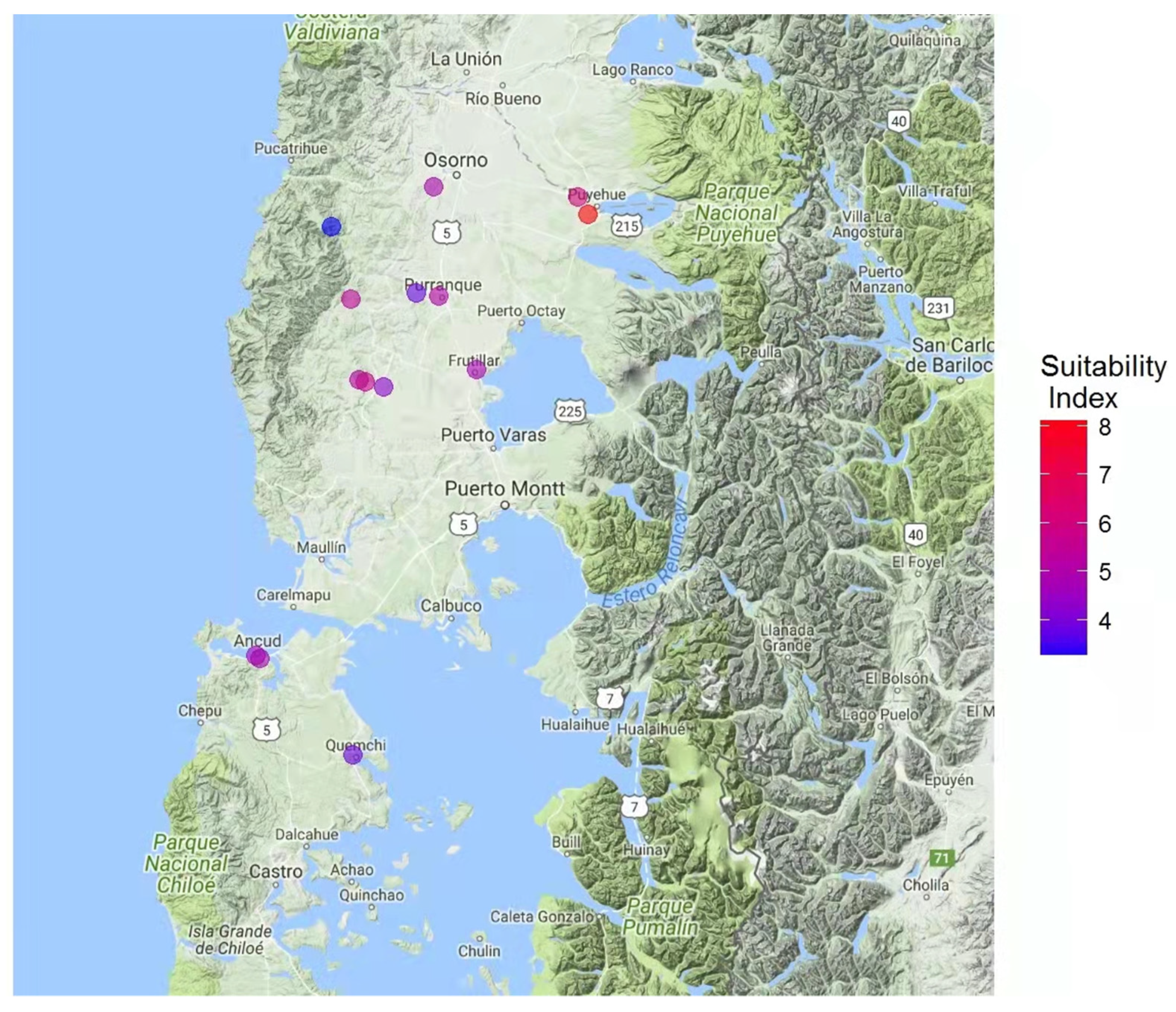
2.12. Suitability Index
2.13. Statistical Analysis
3. Results
3.1. Botanical Origin and Chemical Analyses of Honey Samples
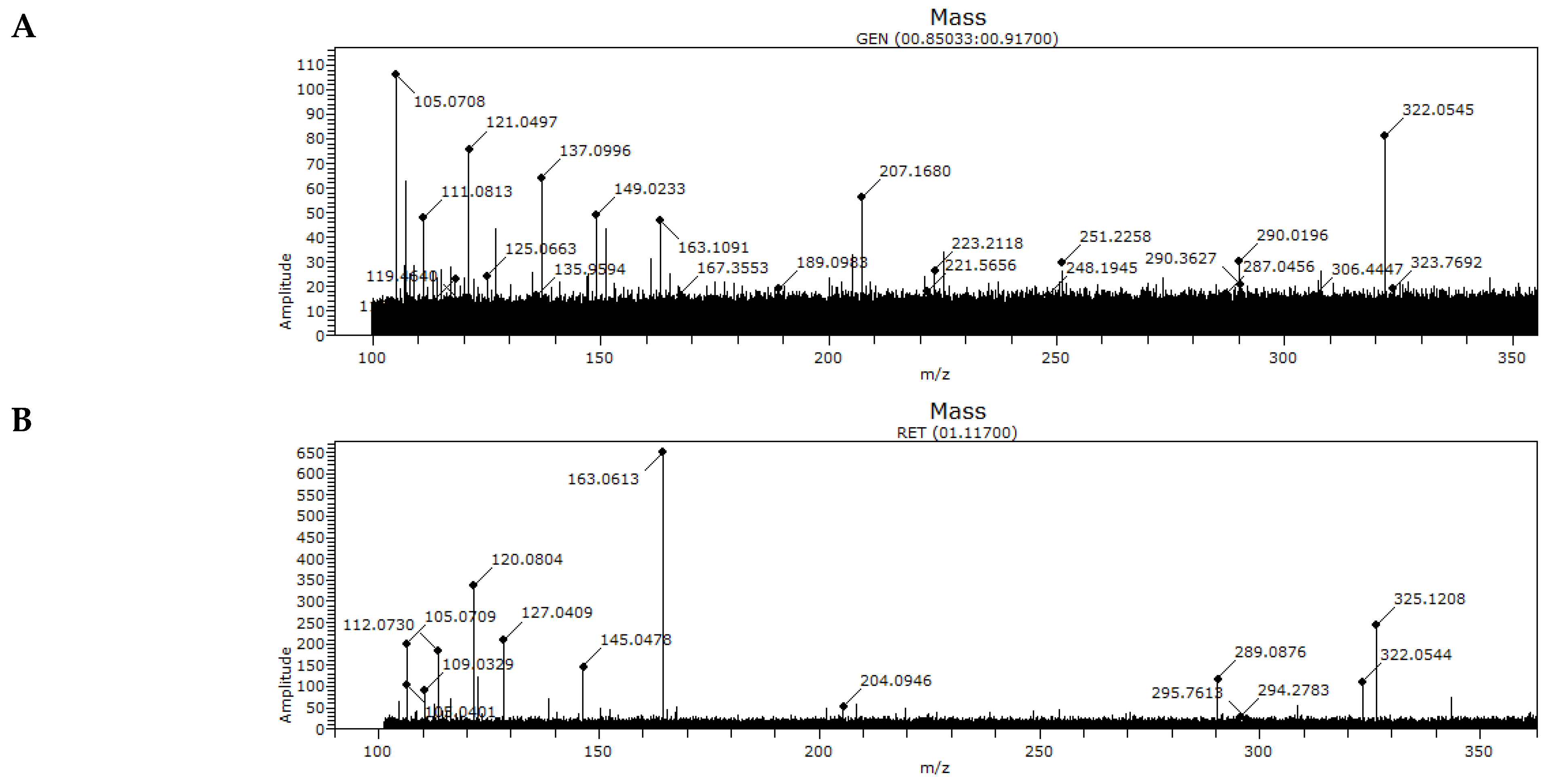
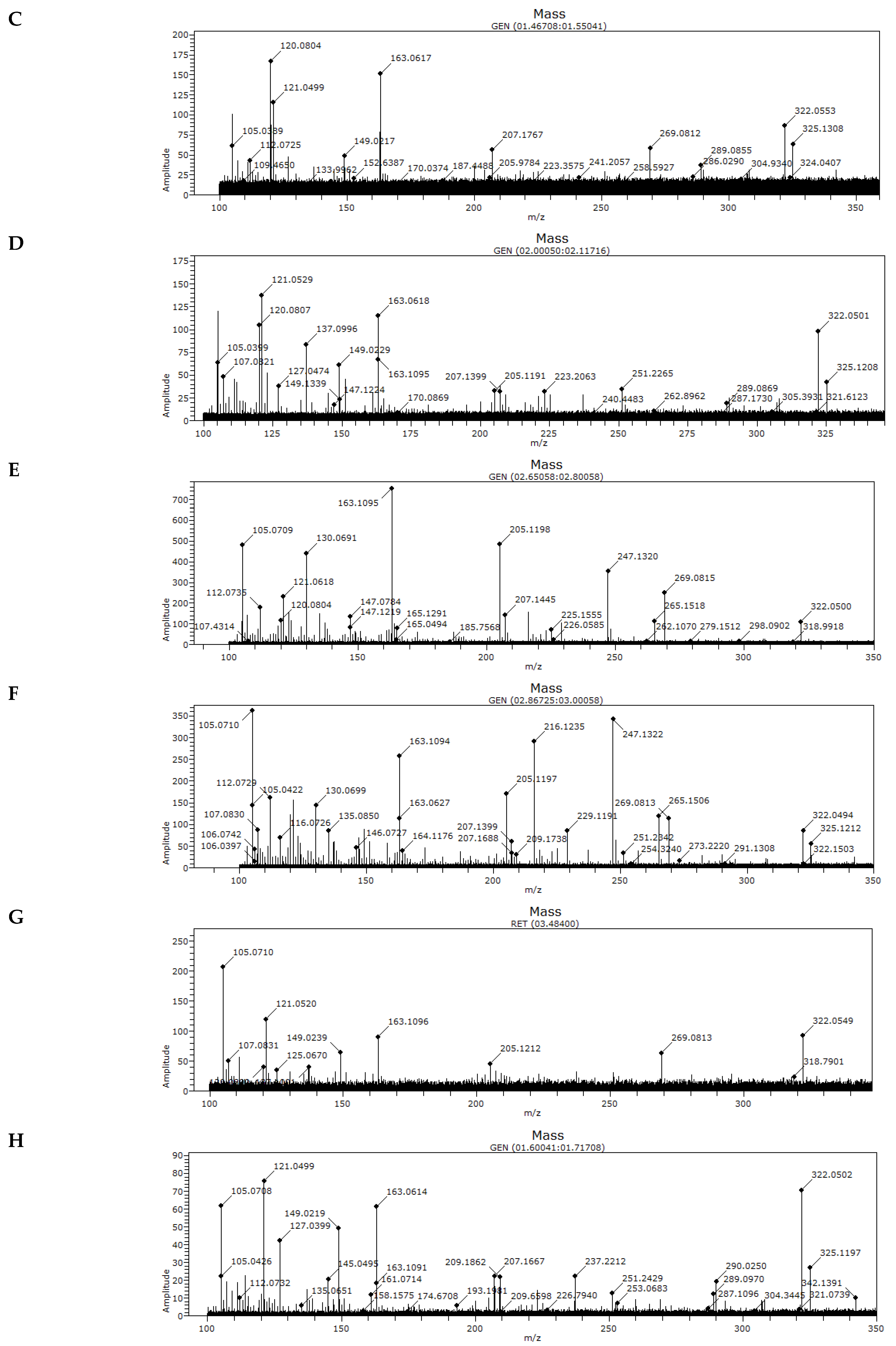
3.2. MS Analysis of Honey Samples
3.3. Presence of Pesticides in Honey/Beeswax Samples
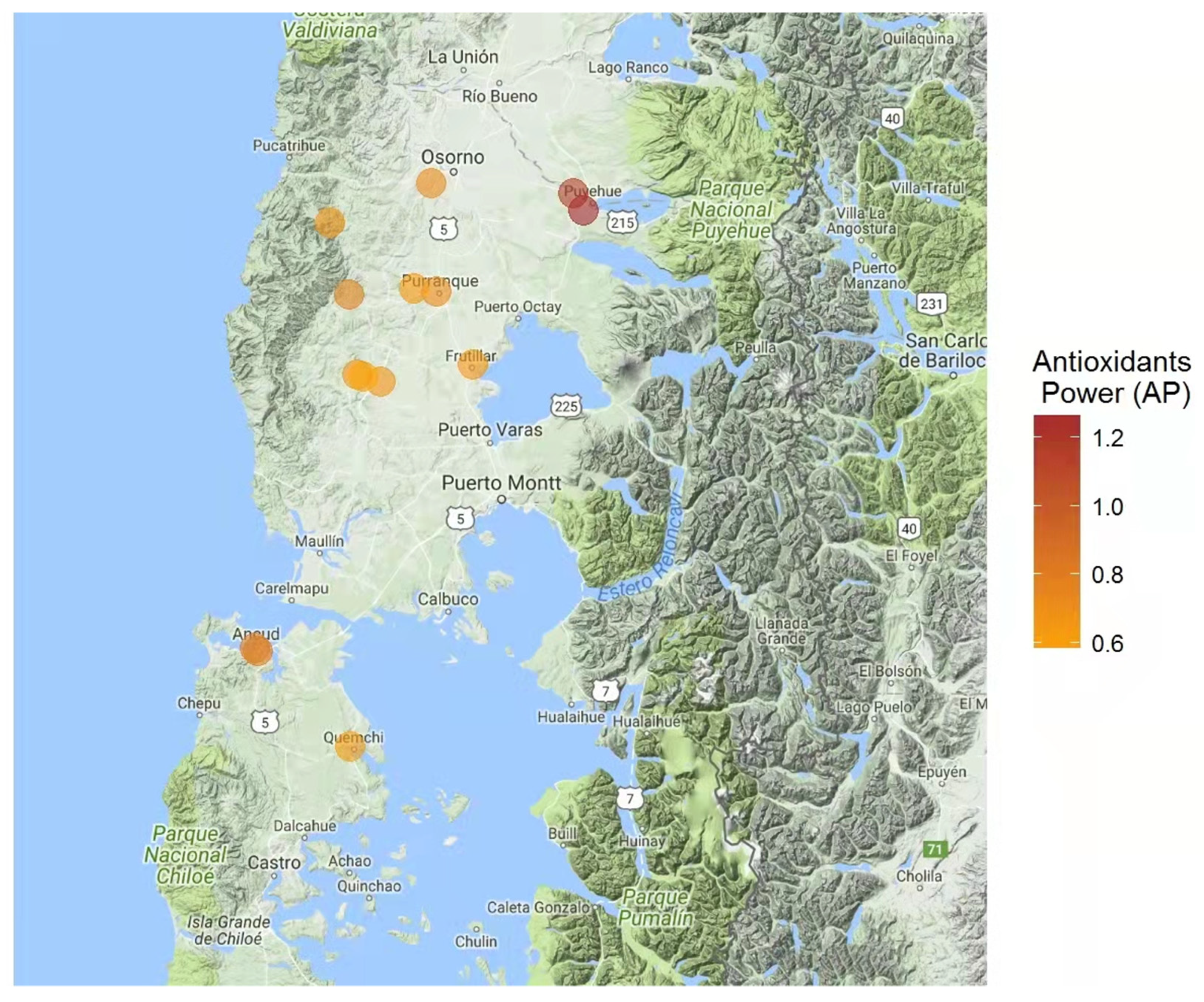
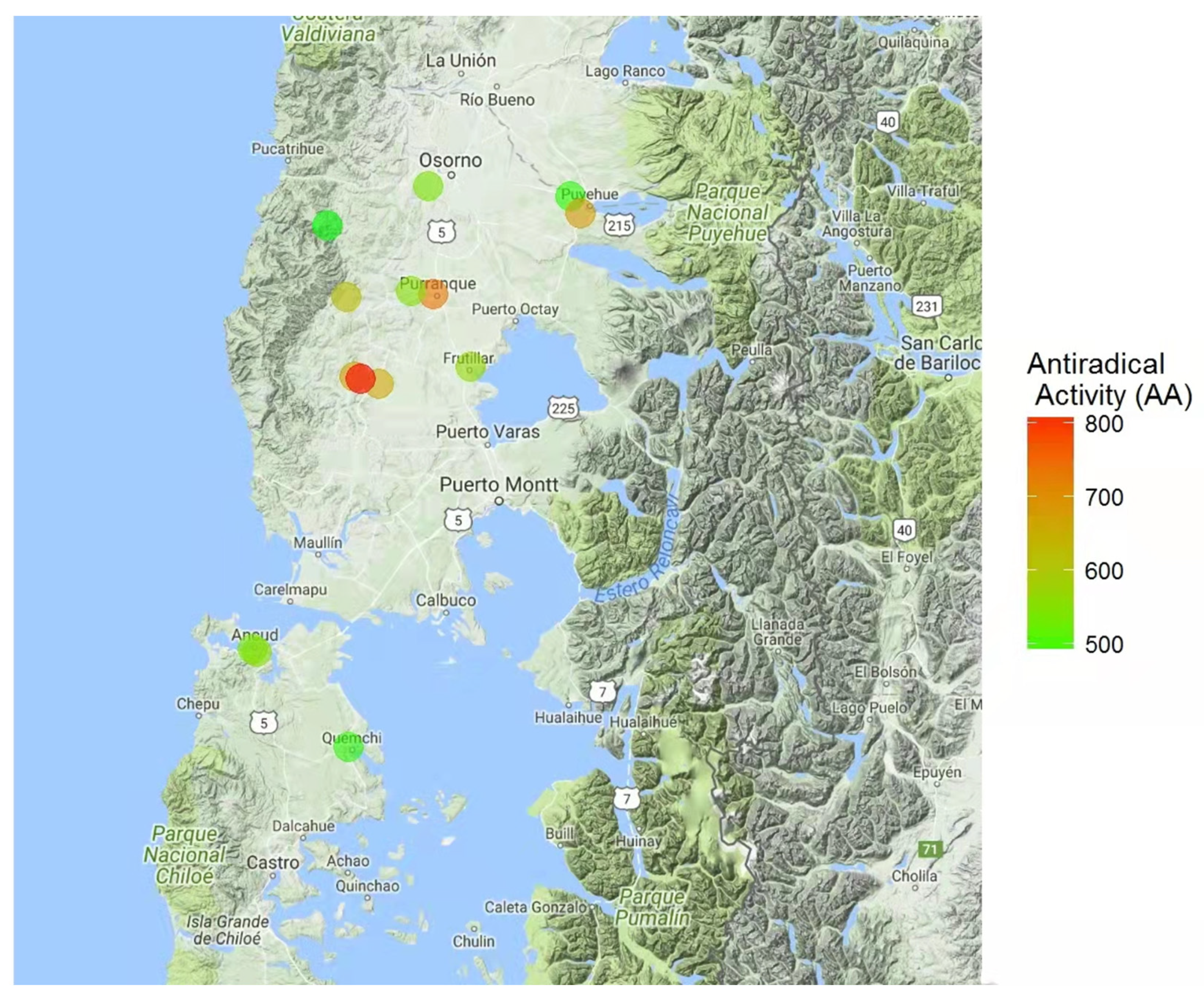
3.4. Suitable Areas for the Pesticide-Free Production of Honey and Beeswax
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Cooper, J.; Dobson, H. The benefits of pesticides to mankind and the environment. Crop. Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Garrido-Reyes, T.I.; Mendoza-Crisosto, J.E.; Varela-Echeverría, P.S.; Mejias-Barrios, E.G.; Alvarez-Salgado, X.A. Interaction between polychlorinated biphenyls and dissolved organic matter of different molecular weights from natural and anthropic sources. J. Environ. Manag. 2021, 299, 113645. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C. Environmental fate and exposure; neonico-tinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ. Health Perspect. 2015, 125, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Hernández, A.F.; Parrón, T.; Tsatsakis, A.M.; Requena, M.; Alarcón, R.; López-Guarnido, O. Toxic effects of pesticide mixtures at a molecular level: Their relevance to human health. Toxicology 2012, 307, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Fernández-de Córdova, M.L.; Ruiz-Medina, A. Trends in flow-based analytical methods applied to pesticide detection: A review. Anal. Chim. Acta 2011, 684, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Souza Tette, P.A.; Guidi, L.R.; De Abreu Glória, M.B.; Fernandes, C. Pesticides in honey: A review on chromatographic analytical methods. Talanta 2016, 149, 124–141. [Google Scholar] [CrossRef]
- Williamson, S.; Wright, A. Exposure to multiple cholinergic pesticides impairs olfatory learning and memory in honeybees. J. Exp. Biol. 2013, 216, 1799–1807. [Google Scholar]
- Tomizawa, M.; Casida, J.E. Neonicotinoid Insecticide Toxicology: Mechanisms of Selective Action. Annu. Rev. Pharm. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Barganska, Z.; Slebioda, M.; Namiesnik, J. Pesticide residues levels in honey from apiaries located of Northern Poland. Food Control 2013, 31, 196–201. [Google Scholar] [CrossRef]
- Codling, G.; Al Naggar, Y.; Giesy, J.P.; Robertson, A.J. Concentrations of neonicotinoid insecticides in honey, pollen and honey bees (Apis mellifera L.) in central Saskatchewan, Canada. Chemosphere 2016, 144, 2321–2328. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Anagnostopoulos, C.; Anastasiadou, P.; Machera, K. Pesticide residues in honeybees, honey and bee pollen by LC-MS/MS screening: Reported death incidents in honeybees. Sci. Total Environ. 2014, 485–486, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A worldwide survey of neonicotinoids in honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez López, D.; Ahumada, D.A.; Díaz, A.C.; Guerrero, J.A. Evaluation of pesticide residues in honey from different geographic regions of Colombia. Food Control 2014, 37, 33–40. [Google Scholar] [CrossRef]
- Boily, M.; Sarrasin, B.; DeBlois, C.; Aras, P.; Chagnon, M. Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonico-tinoids, atrazine and glyphosate: Laboratory and field experiments. Environ. Sci. Pollut. Res. 2013, 20, 5603–5614. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Demares, F.; Nicolson, S.; Medrzycki, P.; Pirk, C.; Human, H. Effects of a neonicotinoid pesticide on thermoregulation of African honey bees (Apis mellifera scutellata). J. Insect Physiol. 2016, 93–94, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Aliouane, Y.; El Hassani, A.K.; Gary, V.; Armengaud, C.; Lambin, M.; Gauthier, M. Subchronic Exposure of Honeybees to Sublethal Doses of Pesticides: Effects on Behavior. Environ. Toxicol. Chem. 2009, 28, 113. [Google Scholar] [CrossRef]
- El Hassani, A.K.; Dacher, M.; Gary, V.; Lambin, M.; Gauthier, M.; Armengaud, C. Effects of sublethal doses of acetamiprid and thiamethoxam on the behavior of the honeybee (Apis mellifera). Arch. Environ. Contam. Toxicol. 2008, 54, 653–661. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Javed Ansari, M. Antibiotic, Pesticide, and Microbial Contaminants of Honey: Human Health Hazards. Sci. World J. 2012, 11, 930849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanov, S. Contaminants of bee products. Apidologie 2006, 37, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, A.; Sharma, D.C. Pesticide Residues in Honey Samples from Himachal Pradesh (India). Bull. Environ. Contam. Toxicol. 2008, 80, 417–422. [Google Scholar] [CrossRef]
- Oficina de Estudios y Políticas Agrarias; Ministerio de Agricultura de Chile. Apicultura Chilena: Actualización de Mercado y Estadísticas Sectoriales. 2018. Available online: https://www.odepa.gob.cl/publicaciones/apicultura-chilena-actualizacion-de-mercado-y-estadisticas-sectoriales-octubre-de-2018 (accessed on 26 November 2021).
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a reduce reliance on conventional pesticides in European agriculture. Plant Dis. 2015, 100, 10–24. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Conclusion on the peer review of the pesticide risk assessment for bees for the active substance imidacloprid. EFSA J. 2013, 11, 1–55. [Google Scholar]
- EFSA. Conclusion on the peer review of the pesticide risk assessment for bees for the active substance thiamethoxam. EFSA J. 2013, 11, 12–68. [Google Scholar]
- EFSA. Conclusion on the peer review of the pesticide risk assessment for bees for Clothianidin. EFSA J. 2013, 11, 1–58. [Google Scholar]
- Eissa, F.; El-Sawi, S.; Zidan, N.E.-H. Determining Pesticide Residues in Honey and their Potential Risk to Consumers. Pol. J. Environ. Stud. 2014, 23, 1573–1580. [Google Scholar]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple routes of pesticide exposure for honey bees living near ag-ricultural fields. PLoS ONE 2012, 7, e29268. [Google Scholar]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Montenegro, G.; Gómez, M.; Díaz-Forestier, J.; Pizarro, R. Aplicación de la Norma Chilena Oficial de denominación de origen botánico de la miel para la caracterización de la producción apícola. Cienc. Investig. Agrar. 2008, 35, 181–190. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Buratti, S.; Benedetti, S.; Cosio, M.S. Evaluation of the antioxidant power of honey, propolis and royal jelly by amperometric flow injection analysis. Talanta 2007, 71, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Mejías, E.; Montenegro, G. The Antioxidant Activity of Chilean Honey and Bee Pollen Produced in the Llaima Volcano’s Zones. J. Food Qual. 2012, 35, 315–322. [Google Scholar] [CrossRef]
- del Campo, G.; Zuriarrain, J.; Zuriarrain, A.; Berregi, I. Quantitative determination of carboxylic acids, amino acids, carbohydrates, ethanol and hydroxymethylfurfural in honey by 1H NMR. Food Chem. 2016, 196, 1031–1039. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC, 17th ed.; Association of Official Analytical Chemists: Gaithersburgs, MD, USA, 2000. [Google Scholar]
- Bohm, D.A.; Stachel, C.S.; Gowik, P. Confirmatory method for the determination of streptomycin and dihydrostreptomycin in honey by LC–MS/MS. Food Addit. Contam. Part A 2012, 29, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Borsdorf, A.; Marchant, C.; Rovira, A.; Sánchez, R. Chile cambiando. In Revisitando la Geografía Regional de Wolfgang Weischet; Serie GEOlibros N°36; Instituto de Geografía, Pontificia Universidad Católica de Chile/Instituto de Ciencias Ambientales y Evolutivas, Facultad de Ciencias, Universidad Austral de Chile: Santiago, Chile, 2020; pp. 123–144. [Google Scholar]
- Gayoso, J.; Iroume, A. Impacto del manejo de plantaciones sobre el ambiente físico. Bosque 1995, 16, 3–12. [Google Scholar] [CrossRef]
- Meneses, M.; Gayoso, J. Estudio de Impacto Ambiental Proyecto Forestal de los Predios Tepuhueico y El Canelo; Golden Spring Forestal: Santiago, Chile, 1995. [Google Scholar]
- Montenegro, G.; Díaz-Forestier, J.; Fredes, C.; Rodríguez, S. Phenolic profiles of nectar and honey of Quillaja saponaria Mol. (Quillajaceae) as potential chemical markers. Biol. Res. 2013, 46, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Viteri, R.; Zacconi, F.; Montenegro, G.; Giordano, A. Bioactive compounds in Apis mellifera monofloral honeys. J. Food Sci. 2021, 86, 1552–1582. [Google Scholar] [CrossRef]
- Cuevas-Glory, L.F.; Pino, J.A.; Santiago, L.S.; Sauri-Duch, E. A review of volatile analytical methods for determining the botanical origin of honey. Food Chem. 2007, 103, 1032–1043. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Tuberoso, C.; Floris, I.; Reniero, F.; Guillou, C. Homogentisic acid: A phenolic acid as a marker of straw-berry-tree (Arbutus unedo) honey. J. Agric. Food Chem. 1999, 47, 4064–4067. [Google Scholar] [CrossRef]
- Ferreres, F.; García-Viguera, C.; Tomás-Lorente, F.; Tomás-Barberán, F.A. Hesperetin: A marker of the floral origin of citrus honey. J. Sci. Food Agric. 1993, 61, 121–123. [Google Scholar] [CrossRef]
- Kaškoniene, V.; Venskutonis, P.R. Floral Markers in Honey of Various Botanical and Geographic Origins: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Baltrušaityte, V.; Venskutonis, P.R.; Čeksteryte, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contribu-tions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Serem, J.C.; Bester, M.J. Physicochemical properties, antioxidant activity and cellular protective effects of honeys from southern Africa. Food Chem. 2012, 133, 1544–1550. [Google Scholar] [CrossRef]
- Perna, A.; Simonetti, A.; Intaglietta, I.; Sofo, A.; Gambacorta, E. Metal content of southern Italy honey of different botanical origins and its correlation with polyphenol content and antioxidant activity. Int. J. Food Sci. Technol. 2012, 47, 1909–1917. [Google Scholar] [CrossRef]
- Ulusoy, E.; Kolayli, S. Phenolic composition and antioxidant properties of anzer bee pollen. J. Food Biochem. 2014, 38, 73–82. [Google Scholar] [CrossRef]
- Kishore, R.K.; Halim, A.S.; Syazana, M.S.N.; Sirajudeen, K.N.S. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr. Res. 2011, 31, 322–325. [Google Scholar] [CrossRef]
- Grabke, A.; Fernández-Ortuño, D.; Schnabel, G. Fenhexamid Resistance in Botrytis cinerea from Strawberry Fields in the Carolinas Is Associated with Four Target Gene Mutations. Plant Dis. 2013, 97, 271–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoner, K.A.; Eitzer, B.D. Using a Hazard Quotient to Evaluate Pesticide Residues Detected in Pollen Trapped from Honey Bees (Apis mellifera) in Connecticut. PLoS ONE 2013, 8, e10. [Google Scholar] [CrossRef]
- Malhat, F.M.; El Sharkawi, H.M.; Loutfy, N.M.; Ahmed, M.T. Field dissipation and health hazard assessment of Fenhexamid on Egyptian grapes. Toxicol. Environ. Chem. 2014, 96, 722–729. [Google Scholar] [CrossRef]
- Ravoet, J.; Reybroeck, W.; De Graaf, D.C. Pesticides for apicultural and/or agricultural application found in Belgian honey bee wax combs. Bull. Environ. Contam. Toxicol. 2015, 94, 543–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacífico da Silva, I.; Oliveira, F.A.S.; Pedroza, H.P.; Gadelha, I.C.N.; Melo, M.M.; Soto-Blanco, B. Pesticide exposure of honeybees (Apis mellifera) pollinating melon crops. Apidologie 2015, 46, 703–715. [Google Scholar] [CrossRef] [Green Version]
- Heard, M.S.; Baas, J.; Dorne, J.L.; Lahive, E.; Robinson, A.G. Comparative toxicity of pesticides and environmental contaminants in bees: Are honey bees a useful proxy for wild bee species? Sci. Total Environ. 2017, 578, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Bastias, A. Biodiversidad y Plantas Nativas de Chile, 1st ed.; Universidad Autónoma de Chile: Santiago, Chile, 2020; pp. 61–90. [Google Scholar]
- Harriet, J.; Campá, J.P.; Grajales, M.; Lhéritier, C.; Gómez-Pajuelo, A. Agricultural pesticides and veterinary substances in Uruguayan beeswax. Chemosphere 2017, 177, 77.e83. [Google Scholar] [CrossRef]
- Mejias, E.; Gómez, C.; Garrido, T.; Godoy, P.; Gómez, M.; Montenegro, G. Natural attributes of Chilean honeys modified by the presence of neonicotinoids residues. Agrofor. Syst. 2019, 93, 2257–2266. [Google Scholar] [CrossRef]



| Apiary (*)-Total Pollen Grains | Caldcluvia paniculata | Luma/Myrceugenia | Weinmannia trichosperma | Other Species |
|---|---|---|---|---|
| A (1.851) | 35 ± 0.02 h | 0 | 0 | 65 |
| B (1.908) | 72 ± 0.02 c | 5 ± 0.01 b | 6 ± 0.01 b | 17 |
| C (2.232) | 52 ± 0.03 f | 2 ± 0.01 c | 0 | 46 |
| D (2.241) | 33 ± 0.02 h | 0 | 0 | 67 |
| E (1.836) | 85 ± 0.04 a | 6 ± 0.02 b | 6 ± 0.01 b | 3 |
| F (1.986) | 81 ± 0.04 a | 7 ± 0.01 a | 5 ± 0.01 b | 7 |
| G (1.968) | 28 ± 0.03 i | 0 | 0 | 72 |
| H (1.953) | 58 ± 0.02 e | 2 ± 0.02 c | 2 ± 0.01 c | 38 |
| I (2.049) | 75 ± 0.01 b | 5 ± 0.02 b | 5 ± 0.02 b | 15 |
| J (2.169) | 63 ± 0.02 d | 2 ± 0.01 c | 2 ± 0.01 c | 33 |
| K (1.965) | 70 ± 0.03 c | 6 ± 0.01 b | 7 ± 0.02 a | 17 |
| L (2.073) | 71 ± 0.02 c | 5 ± 0.02 b | 6 ± 0.01 b | 18 |
| M (2.001) | 48 ± 0.03 f | 0 | 0 | 52 |
| N (1.827) | 43 ± 0.03 g | 0 | 0 | 57 |
| Sample | m/z | |||||
|---|---|---|---|---|---|---|
| A | 105.0708 | ND | 121.0497 | 163.1091 | 207.1680 | 322.0545 |
| B | 105.0709 | 120.0804 | ND | 163.0613 | ND | 322.0544 |
| C | 105.0389 | 120.0804 | 121.0499 | 163.0617 | 207.1767 | 322.0553 |
| D | 105.0399 | 120.0807 | 121.0529 | 163.0618 | 207.1399 | 322.0501 |
| E | 105.0709 | 120.0804 | 121.0618 | 163.1095 | 207.1445 | 322.0500 |
| F | 105.0422 | ND | ND | 163.1094 | 207.1399 | 322.0494 |
| G | 105.0710 | ND | 121.0520 | 163.1096 | ND | 322.0549 |
| H | 105.0708 | ND | 121.0499 | 163.0614 | 207.1667 | 322.0502 |
| I | 105.0709 | ND | 121.0475 | 163.0704 | 207.1666 | 322.0501 |
| J | 105.0708 | 120.0807 | 121.1037 | 163.1093 | 207.1635 | 322.0506 |
| K | 105.0709 | ND | 121.0501 | 163.0615 | ND | 322.0506 |
| L | 105.0709 | ND | 121.0500 | 163.0615 | 207.1569 | 322.0509 |
| M | 105.0709 | ND | 121.0499 | 163.0616 | 207.1666 | 322.0508 |
| N | 105.0709 | ND | ND | 163.0616 | ND | 322.0511 |
| Z (CONTROL) | ND | ND | ND | 163.0465 | ND | ND |
| Sample | Phenols † ± SD | Antioxidant Power ‡ ± SD | Antiradical Activity ≡ ± SD | Total Carbohydrates Ʇ ± SD | Energy # | Total Ash * | Phenol Index | Antioxidant Index | Antiradical Index | Carbohydrate Index | Energy Index | Ash Index | Suitability Index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.222 ± 0.011 a | 0.65 ± 0.09 a | 485.5 ± 0.01 a | 82.1 ± 0.5 a | 329 ± 4 a | 0.08 ± 0.008 i | 1.1 | 1.3 | 1.0 | 2.1 | 2.1 | 1.0 | 6.4 |
| B | 0.320 ± 0.008 b | 1.15 ± 0.06 b | 490.1 ± 0.01 a | 83.4 ± 0.9 a | 332 ± 4 a | 0.19 ± 0.006 b | 2.4 | 2.7 | 1.0 | 2.9 | 2.5 | 2.8 | 11.8 |
| C | 0.303 ± 0.010 c | 0.64 ± 0.06 a | 558.5 ± 0.06 b | 80.4 ± 0.5 a | 321 ± 6 a | 0.21 ± 0.008 a | 2.2 | 1.2 | 1.4 | 1.0 | 1.0 | 3.0 | 8.8 |
| D | 0.272 ± 0.013 d | 0.61 ± 0.07 a | 492.4 ± 0.02 a | 81.5 ± 0.6 a | 326 ± 5 a | 0.12 ± 0.007 g | 1.7 | 1.2 | 1.0 | 1.7 | 1.7 | 1.7 | 7.3 |
| E | 0.366 ± 0.011 e | 1.27 ± 0.04 b | 681.8 ± 0.06 c | 83.6 ± 0.6 a | 334 ± 4 a | 0.15 ± 0.006 d | 3.0 | 3.0 | 2.2 | 3.0 | 2.7 | 2.2 | 13.3 |
| F | 0.227 ± 0.008 f | 0.71 ± 0.07 d | 737.5 ± 0.07 d | 80.7 ± 0.1 a | 323 ± 6 a | 0.22 ± 0.008 a | 1.1 | 1.4 | 2.5 | 1.2 | 1.3 | 3.0 | 9.3 |
| G | 0.239 ± 0.005 g | 0.62 ± 0.05 e | 546.0 ± 0.03 b | 82.5 ± 0.5 a | 331 ± 7 a | 0.09 ± 0.006 j | 1.3 | 1.2 | 1.4 | 2.3 | 2.3 | 1.2 | 7.3 |
| H | 0.282 ± 0.010 d | 0.79 ± 0.01 d | 539.2 ± 0.06 b | 83.6 ± 0.8 a | 330 ± 6 a | 0.17 ± 0.005 c | 1.9 | 1.6 | 1.3 | 3.0 | 2.2 | 2.5 | 10.3 |
| I | 0.263 ± 0.010 d | 0.67 ± 0.08 f | 678.5 ± 0.01 c | 82.2 ± 0.3 a | 327 ± 5 a | 0.12 ± 0.007 f | 1.6 | 1.3 | 2.2 | 2.1 | 1.8 | 1.7 | 8.9 |
| J | 0.308 ± 0.004 h | 0.70 ± 0.07 f | 536.8 ± 0.01 a | 81.8 ± 0.4 a | 327 ± 4 a | 0.14 ± 0.006 e | 2.2 | 1.4 | 1.3 | 1.9 | 1.8 | 2.0 | 8.8 |
| K | 0.284 ± 0.005 d | 0.77 ± 0.08 g | 624.1 ± 0.04 c | 81.6 ± 0.7 a | 326 ± 7 a | 0.16 ± 0.005 d | 1.9 | 1.6 | 1.8 | 1.8 | 1.7 | 2.3 | 9.4 |
| L | 0.273 ± 0.009 i | 0.95 ± 0.06 h | 817.5 ± 0.03 e | 81.2 ± 0.9 a | 323 ± 5 a | 0.09 ± 0.007 i | 1.8 | 2.0 | 3.0 | 1.5 | 1.3 | 1.2 | 8.4 |
| M | 0.265 ± 0.008 d | 0.79 ± 0.06 g | 560.1 ± 0.06 b | 83.5 ± 0.7 a | 336 ± 8 a | 0.18 ± 0.006 b | 1.6 | 1.7 | 1.4 | 2.9 | 3.0 | 2.7 | 10.3 |
| N | 0.217 ± 0.004 j | 0.66 ± 0.09 c | 654.7 ± 0.05 c | 81.6 ± 0.5 a | 326 ± 5 a | 0.12 ± 0.007 h | 1.0 | 1.3 | 2.0 | 1.8 | 1.7 | 1.7 | 7.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejías, E.; Gómez, C.; Garrido, T. Suitable Areas for Apiculture Expansion Determined by Antioxidant Power, Chemical Profiles, and Pesticide Residues in Caldcluvia paniculata Honey and Beeswax Samples. Insects 2022, 13, 31. https://doi.org/10.3390/insects13010031
Mejías E, Gómez C, Garrido T. Suitable Areas for Apiculture Expansion Determined by Antioxidant Power, Chemical Profiles, and Pesticide Residues in Caldcluvia paniculata Honey and Beeswax Samples. Insects. 2022; 13(1):31. https://doi.org/10.3390/insects13010031
Chicago/Turabian StyleMejías, Enrique, Carlos Gómez, and Tatiana Garrido. 2022. "Suitable Areas for Apiculture Expansion Determined by Antioxidant Power, Chemical Profiles, and Pesticide Residues in Caldcluvia paniculata Honey and Beeswax Samples" Insects 13, no. 1: 31. https://doi.org/10.3390/insects13010031
APA StyleMejías, E., Gómez, C., & Garrido, T. (2022). Suitable Areas for Apiculture Expansion Determined by Antioxidant Power, Chemical Profiles, and Pesticide Residues in Caldcluvia paniculata Honey and Beeswax Samples. Insects, 13(1), 31. https://doi.org/10.3390/insects13010031






