Nutrient Imbalance of the Host Plant for Larvae of the Pale Grass Blue Butterfly May Mediate the Field Effect of Low-Dose Radiation Exposure in Fukushima: Dose-Dependent Changes in the Sodium Content
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Butterfly
2.2. Sampling and Temperature Data
2.3. Sampling Procedure
2.4. Measurements of Ground Radiation Dose
2.5. Nutrient Analyses
2.6. Measurements of Radioactivity Concentrations
2.7. Statistical Analyses
3. Results
3.1. Comparisons of Nutrient Factors among the Tohoku, Niigata, and Kyushu Groups
3.2. Radioactive Factors and Nutrients: Sodium and Lipid Contents
3.3. Correlations of the Sodium Content with Other Nutrient Contents
3.4. Coefficient of Variation among the Nutrient Factors
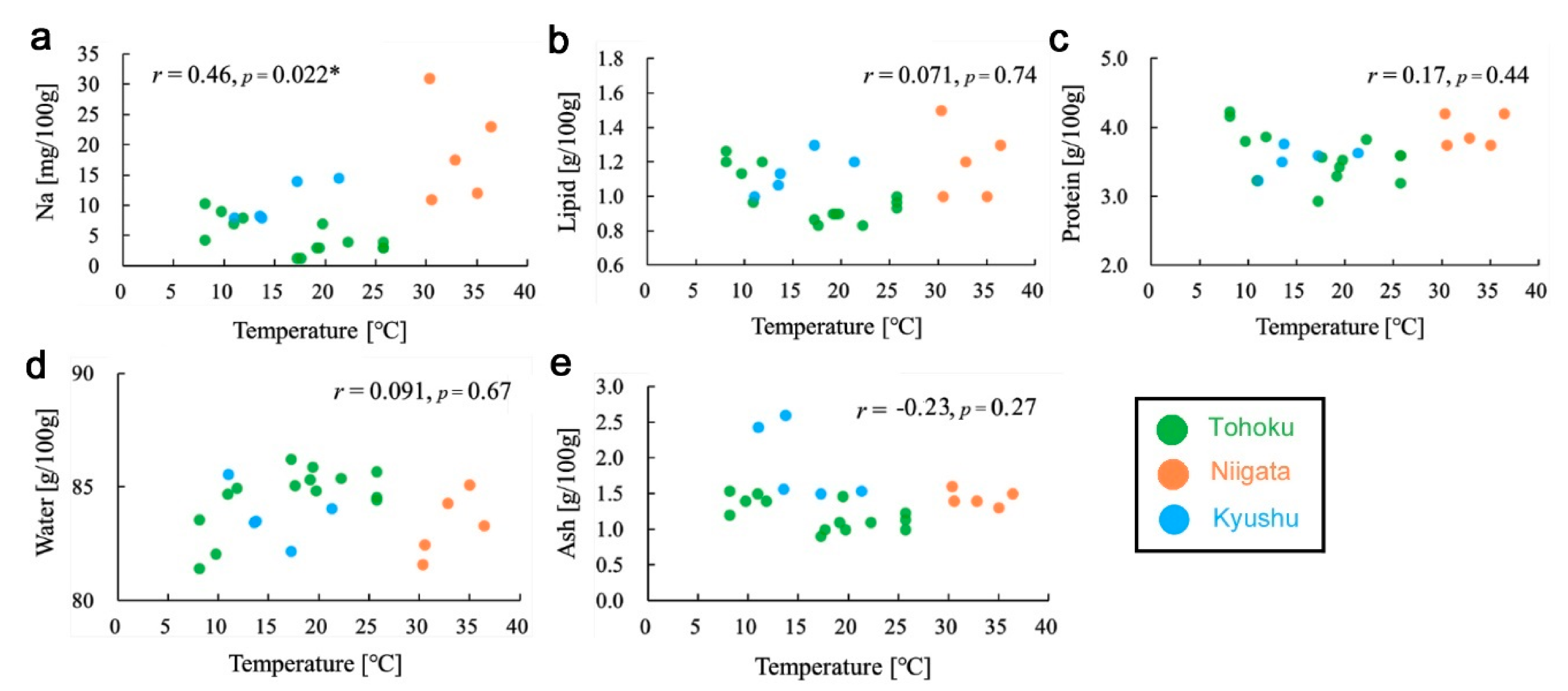
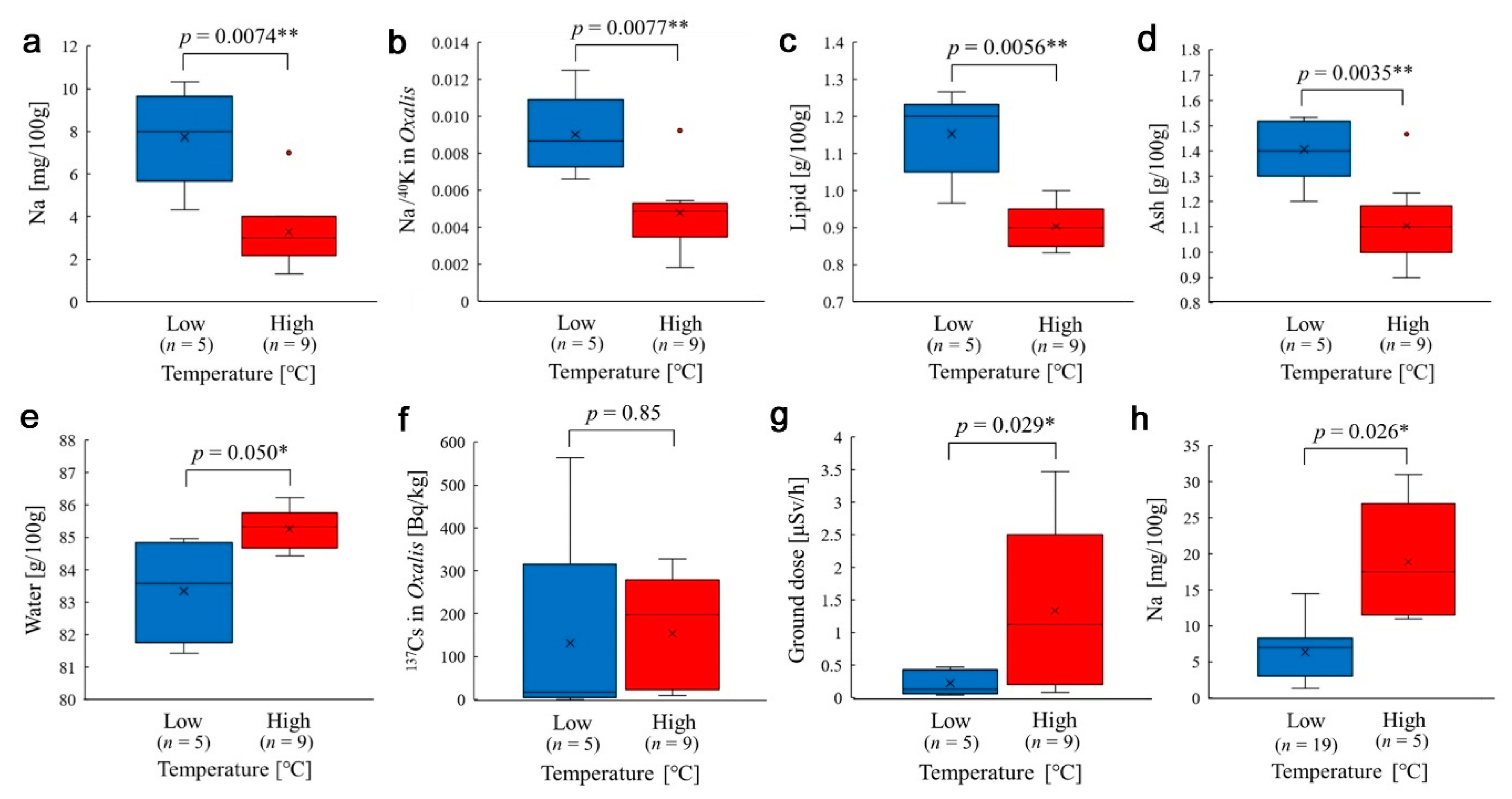
3.5. Temperature Effects on Nutrient Contents
3.6. Additional Analyses: Input Variables and Seasonal Comparisons
4. Discussion
4.1. Physiological Changes in Nutrient Contents in the Host Plant
4.2. Status of Kyushu and Tomioka-1
4.3. Temperature Effect in the Tohoku Group
4.4. Possible Function of Sodium and Radiation-Induced Stress Response in the Plant
4.5. Herbivorous Insects and Their Sodium Requirement
4.6. Transgenerational Effects of Ingesting Radioactively Polluted Diets
4.7. Essential Nutrients and Secondary Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chino, M.; Nakayama, H.; Nagai, H.; Terada, H.; Katata, G.; Yamazawa, H. Preliminary estimation of released amount of 131I and 137Cs accidentally discharged from the Fukushima Daiichi nuclear power plant into the atmosphere. J. Nucl. Sci. Technol. 2011, 48, 1129–1134. [Google Scholar] [CrossRef]
- Hirose, K. 2011 Fukushima Dai-ichi nuclear power plant accident: Summary of regional radioactive deposition monitoring results. J. Environ. Radioact. 2012, 111, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, N.; Sucki, K.; Sasa, K.; Kitagawa, J.; Ikarashi, S.; Nishimura, T.; Wong, Y.S.; Satou, Y.; Handa, K.; Takahashi, T.; et al. Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan. Proc. Natl. Acad. Sci. USA 2011, 108, 19526–19529. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, Y.; Ohara, T.; Nishizawa, M. Atmospheric behaviour, deposition, and budget of radioactive materials from the Fukushima Daiichi nuclear power plant in March 2011. Geophys. Res. Lett. 2011, 38, L00G11. [Google Scholar] [CrossRef] [Green Version]
- Endo, S.; Kimura, S.; Takatsuji, T.; Nanasawa, K.; Imanaka, T.; Shizuma, K. Measurement of soil contamination by radionuclides due to the Fukushima Dai-ichi Nuclear Power Plant accident and associated estimated cumulative external dose estimation. J. Environ. Radioact. 2012, 111, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Torii, T.; Sugita, T.; Okada, C.E.; Reed, M.S.; Blumenthal, D.J. Enhanced analysis methods to derive the spatial distribution of 131I deposition on the ground by airborne surveys at an early stage after the Fukushima Daiichi nuclear power plant accident. Health Phys. 2013, 105, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Le Petit, G.; Douysset, G.; Ducros, G.; Gross, P.; Achim, P.; Monfort, M.; Raymond, P.; Pontillon, Y.; Jutier, C.; Blanchard, X. Analysis of radionuclide releases from the Fukushima Dai-ichi nuclear power plant accident Part I. Pure Appl. Geophys. 2014, 171, 629–644. [Google Scholar] [CrossRef]
- Achim, P.; Monfort, M.; Le Pettit, G.; Gross, P.; Douysset, G.; Taffary, T.; Blanchard, X.; Moulin, C. Analysis of radionuclide releases from the Fukushima Dai-ichi nuclear power plant accident Part II. Pure Appl. Geophys. 2014, 171, 645–667. [Google Scholar] [CrossRef]
- Adachi, K.; Kajino, M.; Zaizen, Y.; Igarashi, Y. Emission of spherical cesium-bearing particles from an early stage of the Fukushima nuclear accident. Sci. Rep. 2013, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Doi, T.; Masumoto, K.; Toyoda, A.; Tanaka, A.; Shibata, Y.; Hirose, K. Anthropogenic radionuclides in the atmosphere observed at Tsukuba: Characteristics of the radionuclides derived from Fukushima. J. Environ. Radioact. 2013, 122, 55–62. [Google Scholar] [CrossRef]
- Scherb, H.H.; Mori, K.; Hayashi, K. Increases in perinatal mortality in prefectures contaminated by the Fukushima nuclear power plant accident in Japan: A spatially stratified longitudinal study. Medicine 2016, 95, e4958. [Google Scholar] [CrossRef] [PubMed]
- Murase, K.; Murase, J.; Moachidori, K.; Mizuno, K.; Hayashi, Y.; Kohri, K. Nationwide increase in cryptorchidism after the Fukushima nuclear accident. Urology 2018, 118, 65–70. [Google Scholar] [CrossRef]
- Murase, K.; Murase, J.; Mishima, A. nationwide increase in complex congenital heart diseases after the Fukushima nuclear accident. J. Am. Heart Assoc. 2019, 8, e009486. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Hayashi, K.; Scherb, H. Association between the detection rate of thyroid cancer and the external radiation dose-rate after the nuclear power plant accidents in Fukushima, Japan. Medicine 2019, 98, e17165. [Google Scholar] [CrossRef] [Green Version]
- Korblein, A.; Küchenhoff, H. Perinatal mortality after the Fukushima accident: A spatiotemporal analysis. J. Radiol. Prot. 2019, 39, 1021. [Google Scholar] [CrossRef]
- Otaki, J.M. Fukushima nuclear accident: Potential health effects inferred from butterfly and human cases. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2019; pp. 497–514. [Google Scholar]
- Hayama, S.; Tsuchiya, M.; Ochiaki, K.; Nakiri, S.; Nakanishi, S.; Ishii, N.; Kato, T.; Tanaka, A.; Konno, F.; Kawamoto, Y.; et al. Small head size and delayed body weight growth in wild Japanese monkey fetuses after the Fukushima Daiichi nuclear disaster. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Ochiai, K.; Hayama, S.; Nakiri, S.; Nakanishi, S.; Ishii, N.; Uno, T.; Kato, T.; Konno, F.; Kawamoto, Y.; Tsuchida, S.; et al. Low blood cell counts in wild Japanese monkeys after the Fukushima Daiichi nuclear disaster. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urushihara, Y.; Suzuki, T.; Shimizu, Y.; Ohtaki, M.; Kuwahara, Y.; Suzuki, M.; Uno, T.; Fujita, S.; Saito, A.; Yamashiro, H.; et al. Haematological analysis of Japanese macaques (Macaca fuscata) in the area affected by the Fukushima Daiichi Nuclear Power Plant accident. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Yamashiro, H.; Abe, Y.; Fukuda, T.; Kino, Y.; Kawaguchi, I.; Kuwahara, Y.; Fukumoto, M.; Takahashi, S.; Suzuki, M.; Kobayashi, J.; et al. Effects of radioactive caesium on bull testes after the Fukushima nuclear accident. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, Y.; Tsuji, H.; Kawagoshi, T.; Shiomi, N.; Takahashi, H.; Watanabe, Y.; Fuma, S.; Doi, K.; Kawaguchi, I.; Aoki, M.; et al. Chromosomal aberrations in wild mice captured in areas differentially contaminated by the Fukushima Dai-ichi Nuclear power plant accident. Environ. Sci. Technol. 2015, 49, 10074–10083. [Google Scholar] [CrossRef]
- Yamashiro, H.; Abe, Y.; Hayashi, G.; Urushihara, Y.; Kuwahara, Y.; Suzuki, M.; Kobayashi, J.; Kino, Y.; Fukuda, T.; Tong, B.; et al. Electron probe X-ray microanalysis of boar and inobuta testes after the Fukushima accident. J. Radiat. Res. 2015, 56, i42–i47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, H.; Yamaguchi, Y.; Yoshimura, T.; Fukumoto, M.; Todo, T. Fukushima simulation experiment: Assessing the effects of chronic low-dose-rate internal 137Cs radiation exposure on litter size, sex ratio, and biokinetics in mice. J. Radiat. Res. 2015, 56, i29–i35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okano, T.; Ishiniwa, H.; Onuma, M.; Shindo, J.; Yokohata, Y.; Tamaoki, M. Effects of environmental radiation on testes and spermatogenesis in wild large Japanese field mice (Apodemus speciosus) from Fukushima. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Urushihara, Y.; Kawasumi, K.; Endo, S.; Tanaka, K.; Hirakawa, Y.; Hayashi, G.; Sekine, T.; Kino, Y.; Kuwahara, Y.; Suzuki, M.; et al. Analysis of plasma protein concentrations and enzyme activities in cattle within the ex-evacuation zone of the Fukushima Daiichi nuclear plant accident. PLoS ONE 2016, 11, e0155069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, M.; Kato, A.; Kobayashi, J.; Okuda, K.; Kuwahara, Y.; Kino, Y.; Abe, Y.; Sekine, T.; Fukuda, T.; Isogai, E.; et al. Gene expression analyses of the small intestine of pigs in the ex-evacuation zone of the Fukushima Daiichi Nuclear Power Plant. BMC Vet. Res. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Nakamura, A.J.; Suzuki, M.; Redon, C.E.; Kuwahara, Y.; Yamashiro, H.; Abe, Y.; Takahashi, S.; Fukuda, T.; Isogai, E.; Bonner, W.M.; et al. The causal relationship between DNA damage induction in bovine lymphocytes and the Fukushima Nuclear Power Plant Accident. Radiat. Res. 2017, 187, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Takino, S.; Yamashiro, H.; Sugano, Y.; Fujishima, Y.; Nakata, A.; Kasai, K.; Hayashi, G.; Urushihara, Y.; Suzuki, M.; Shinoda, H.; et al. Analysis of the effect of chronic and low-dose radiation exposure on spermatogenic cells of male large Japanese field mice (Apodemus speciosus) after the Fukushima Daiichi Nuclear Power Plant Accident. Radiat. Res. 2017, 187, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kawagoshi, T.; Shiomi, N.; Takahashi, H.; Watanabe, Y.; Fuma, S.; Doi, K.; Kawaguchi, I.; Aoki, M.; Kubota, M.; Furuhata, Y.; et al. Chromosomal aberrations in large Japanese field mice (Apodemus speciosus) captured near Fukushima Dai-ichi nuclear power plant. Environ. Sci. Technol. 2017, 51, 4632–4641. [Google Scholar] [CrossRef]
- Sato, I.; Sasaki, J.; Satoh, H.; Deguchi, Y.; Chida, H.; Natsuhori, M.; Otani, K.; Okada, K. Decreased blood cell counts were not observed in cattle living in the “difficult-to-return zone” of the Fukushima nuclear accident. Anim. Sci. J. 2019, 90, 128–134. [Google Scholar] [CrossRef]
- Sasaki, J.; Uehara, M.; Sato, I.; Satoh, H.; Deguchi, Y.; Chida, H.; Natsuhori, M.; Murata, T.; Ochiai, K.; Otani, K.; et al. Pathological characteristics of thyroid glands from Japanese Black Cattle living in the restricted area of the Fukushima Daiichi Nuclear Power Plant accident. Anim. Sci. J. 2019, 90, 1333–1339. [Google Scholar] [CrossRef]
- Møller, A.P.; Hagiwara, A.; Matsui, S.; Kasahara, S.; Kawatsu, K.; Nishiumi, I.; Suzuki, H.; Mousseau, T.A. Abundance of birds in Fukushima as judges from Chernobyl. Environ. Pollut. 2012, 164, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Bonisoli-Alquati, A.; Koyama, K.; Tedeschi, D.J.; Kitamura, W.; Sukuzi, H.; Ostermiller, S.; Arai, E.; Møller, A.P.; Mousseau, T.A. Abundance and genetic damage of barn swallows from Fukushima. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murase, K.; Murase, J.; Horie, R.; Endo, K. Effects of the Fukushima Daiichi nuclear accident on goshawk reproduction. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, T.; Yoshii, H.; Mizuno, S.; Shiraishi, H. Decline in intertidal biota after the 2011 Great East Japan Earthquake and Tsunami and the Fukushima nuclear disaster: Field observations. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Møller, A.P.; Nishiumi, I.; Suzuki, H.; Ueda, K.; Mousseau, T.A. Differences in effects of radiation on abundance of animals in Fukushima and Chernobyl. Ecol. Indic. 2013, 24, 75–81. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Møller, A.P. Genetic and ecological studies of animals in Chernobyl and Fukushima. J. Hered. 2014, 105, 704–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiyama, A.; Nohara, C.; Kinjo, S.; Taira, W.; Gima, S.; Tanahara, A.; Otaki, J.M. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci. Rep. 2012, 2, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akimoto, S. Morphological abnormalities in gall-forming aphids in a radiation-contaminated area near Fukushima Daiichi: Selective impact of fallout? Ecol. Evol. 2014, 4, 355–369. [Google Scholar] [CrossRef]
- Akimoto, S.I.; Li, Y.; Imanaka, T.; Sato, H.; Ishida, K. Effects of radiation from contaminated soil and moss in Fukushima on embryogenesis and egg hatching of the aphid Prociphilus oriens. J. Hered. 2018, 109, 199–205. [Google Scholar] [CrossRef]
- Yoshioka, A.; Mishima, Y.; Fukasawa, K. Pollinators and other flying insects inside and outside the Fukushima evacuation zone. PLoS ONE 2015, 10, e0140957. [Google Scholar] [CrossRef]
- Tanaka, S.; Hatakeyama, K.; Takahashi, S.; Adati, T. Radioactive contamination of arthropods from different trophic levels in hilly and mountainous areas after the Fukushima Daiichi nuclear power plant accident. J. Environ. Radioact. 2016, 164, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, Y.; Hayashi, S.; Takamura, N. Radiocesium transfer in forest insect communities after the Fukushima Dai-ichi nuclear power plant accident. PLoS ONE 2017, 12, e0171133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, M.; Kajihara, R.; Kato, Y.; Takano-Shimizu, T.; Inoue, Y. Frequencies of chromosomal inversions in Drosophila melanogaster in Fukushima after the nuclear power plant accident. PLoS ONE 2018, 13, e0192096. [Google Scholar] [CrossRef] [Green Version]
- Taira, W.; Toki, M.; Kakinohana, K.; Sakauchi, K.; Otaki, J.M. Developmental and hemocytological effects of ingesting Fukushima’s radiocesium on the cabbage white butterfly Pieris rapae. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshihara, T.; Matsumura, H.; Hashida, S.N.; Nagaoka, T. Radiocesium contaminations of 20 wood species and the corresponding gamma-ray dose rates around the canopies at 5 months after the Fukushima nuclear power plant accident. J. Environ. Radioact. 2013, 115, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Ohmori, Y.; Kajikawa, M.; Nishida, S.; Tanaka, N.; Kobayashi, N.I.; Tanoi, K.; Furukawa, J.; Fujiwara, T. The effect of fertilization on cesium concentration of rice grown in a paddy field in Fukushima Prefecture in 2011 and 2012. J. Plant Res. 2014, 127, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, G.; Shibato, J.; Imanaka, T.; Cho, K.; Kubo, A.; Kikuchi, S.; Satoh, K.; Kimura, S.; Ozawa, S.; Fukutani, S.; et al. Unraveling low-level gamma radiation-responsive changes in expression of early and late genes in leaves of rice seedlings at Iitate Village, Fukushima. J. Hered. 2014, 105, 723–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, Y.; Ichikawa, S.; Kubota, M.; Hoshino, J.; Kubota, Y.; Maruyama, K.; Fuma, S.; Kawaguchi, I.; Yoschenko, V.I.; Yoshida, S. Morphological defects in native Japanese fir trees around the Fukushima Daiichi Nuclear Power Plant. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Yoschenko, V.; Nanba, K.; Yoshida, S.; Watanabe, Y.; Takase, T.; Sato, N.; Keitoku, K. Morphological abnormalities in Japanese red pine (Pinus densiflora) at the territories contaminated as a result of the accident at Fukushima Dai-ichi Nuclear Power Plant. J. Environ. Radioact. 2016, 165, 60–67. [Google Scholar] [CrossRef]
- Diedidi, S.; Kojima, K.; Ohkama-Ohtsu, N.; Bellingrath-Kimura, S.D.; Yokoyama, T. Growth and 137Cs uptake and accumulation among 56 Japanese cultivars of Brassica rapa, Brassica juncea and Brassica napus grown in a contaminated field in Fukushima: Effect of inoculation with a Bacillus pumilus strain. J. Environ. Radioact. 2016, 157, 27–37. [Google Scholar] [CrossRef]
- Rakwal, R.; Hayashi, G.; Shibato, J.; Deepak, S.A.; Gundimeda, S.; Simha, U.; Padmanaban, A.; Gupta, R.; Han, S.I.; Kim, S.T.; et al. Progress toward rice seed OMICS in low-level gamma radiation environment in Iitate Village, Fukushima. J. Hered. 2017, 109, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Horemans, N.; Nauts, R.; Vives i Batlle, J.; van Hees, M.; Jacobs, G.; Voorspoels, S.; Gaschak, S.; Nanba, K.; Saenen, E. Genome-wide DNA methylation changes in two Brassicaceae species sampled alongside a radiation gradient in Chernobyl and Fukushima. J. Environ. Radioact. 2018, 192, 405–416. [Google Scholar] [CrossRef]
- Tagami, K.; Uchida, S. Changes of effective half-lives of 137Cs in three herbaceous plants and bioavailable 137Cs fraction in soil after the Fukushima nuclear accident. Appl. Geochem. 2017, 85, 162–168. [Google Scholar] [CrossRef]
- Uchida, S.; Tagami, K. Comparison of radiocesium concentration changes in leguminous and non-leguminous herbaceous plants observed after the Fukushima Dai-ichi Nuclear Power Plant accident. J. Environ. Radioact. 2017, 186, 3–8. [Google Scholar] [CrossRef]
- Tagami, K.; Uchida, S. Effective half-lives of 137Cs in giant butterbur and field horsetail, and the distribution differences of potassium and 137Cs in aboveground tissue parts. J. Environ. Radioact. 2015, 141, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, T.A.; Møller, A.P. Plants in the light of ionizing radiation: What we have learned from Chernobyl, Fukushima, and other “hot” places? Front. Plant Sci. 2020, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Nohara, C.; Taira, W.; Kinjo, S.; Iwata, M.; Otaki, J.M. The Fukushima nuclear accident and the pale grass blue butterfly: Evaluating biological effects of long-term low-dose exposures. BMC Evol. Biol. 2013, 13, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Taira, W.; Nohara, C.; Hiyama, A.; Otaki, J.M. Fukushima’s biological impacts: The case of the pale grass blue butterfly. J. Hered. 2014, 105, 710–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nohara, C.; Hiyama, A.; Taira, W.; Tanahara, A.; Otaki, J.M. The biological impacts of ingested radioactive materials on the pale grass blue butterfly. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nohara, C.; Taira, W.; Hiyama, A.; Tanahara, A.; Takatsuji, T.; Otaki, J.M. Ingestion of radioactively contaminated diets for two generations in the pale grass blue butterfly. BMC Evol. Biol. 2014, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Taira, W.; Hiyama, A.; Nohara, C.; Sakauchi, K.; Otaki, J.M. Ingestional and transgenerational effects of the Fukushima nuclear accident on the pale grass blue butterfly. J. Radiat. Res. 2015, 56, i2–i18. [Google Scholar] [CrossRef]
- Nohara, C.; Hiyama, A.; Taira, W.; Otaki, J.M. Robustness and radiation resistance of the pale grass blue butterfly from radioactively contaminated areas: A possible case of adaptive evolution. J. Hered. 2018, 109, 188–198. [Google Scholar] [CrossRef]
- Hiyama, A.; Taira, W.; Nohara, C.; Iwasaki, M.; Kinjo, S.; Iwata, M.; Otaki, J.M. Spatiotemporal abnormality dynamics of the pale grass blue butterfly: Three years of monitoring (2011–2013) after the Fukushima nuclear accident. BMC Evol. Biol. 2015, 15, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taira, W.; Iwasaki, M.; Otaki, J.M. Body size distributions of the pale grass blue butterfly in Japan: Size rules and the status of the Fukushima population. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Otaki, J.M. Fukushima’s lessons from the blue butterfly: A risk assessment of the human living environment in the post-Fukushima era. Integr. Environ. Assess. Manag. 2016, 12, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M.; Taira, W. Current status of the blue butterfly in Fukushima research. J. Hered. 2018, 109, 178–187. [Google Scholar] [CrossRef]
- Hiyama, A.; Taira, W.; Iwasaki, M.; Sakauchi, K.; Gurung, R.; Otaki, J.M. Geographical distribution of morphological abnormalities and wing color pattern modifications of the pale grass blue butterfly in northeastern Japan. Entomol. Sci. 2017, 20, 100–110. [Google Scholar] [CrossRef]
- Hiyama, A.; Taira, W.; Iwasaki, M.; Sakauchi, K.; Iwata, M.; Otaki, J.M. Morphological abnormality rate of the pale grass blue butterfly Zizeeria maha (Lepidoptera: Lycaenidae) in southwestern Japan: A reference data set for environmental monitoring. J. Asia-Pac. Entomol. 2017, 20, 1333–1339. [Google Scholar] [CrossRef]
- Hiyama, A.; Taira, W.; Sakauchi, K.; Otaki, J.M. Sampling efficiency of the pale grass blue butterfly Zizeeria maha (Lepidoptera: Lycaenidae): A versatile indicator species for environmental risk assessment in Japan. J. Asia-Pac. Entomol. 2018, 21, 609–615. [Google Scholar] [CrossRef]
- Otaki, J.M. Understanding low-dose exposure and field effects to resolve the field-laboratory paradox: Multifaceted biological effects from the Fukushima nuclear accident. In New Trends in Nuclear Science; Awwad, N.S., AlFaify, S.A., Eds.; IntechOpen: London, UK, 2018; pp. 49–71. [Google Scholar] [CrossRef] [Green Version]
- Gurung, R.D.; Taira, W.; Sakauchi, K.; Iwata, M.; Hiyama, A.; Otaki, J.M. Tolerance of high oral doses of nonradioactive and radioactive caesium chloride in the pale grass blue butterfly Zizeeria maha. Insects 2019, 10, 290. [Google Scholar] [CrossRef] [Green Version]
- Hancock, S.; Vo, N.T.K.; Omar-Nazir, L.; Batlle, J.V.I.; Otaki, J.M.; Hiyama, A.; Byun, S.H.; Seymour, C.B.; Mothersill, C. Transgenerational effects of historic radiation dose in pale grass blue butterflies around Fukushima following the Fukushima Dai-ichi Nuclear Power Plant meltdown accident. Environ. Res. 2019, 168, 230–240. [Google Scholar] [CrossRef]
- Sakauchi, K.; Taira, W.; Toki, M.; Iraha, Y.; Otaki, J.M. Overwintering states of the pale grass blue butterfly Zizeeria maha (Lepidoptera: Lycaenidae) at the time of the Fukushima nuclear accident in March 2011. Insects 2019, 10, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otaki, J.M. The pale grass blue butterfly as an indicator for the biological effect of the Fukushima Daiichi Nuclear Power Plant accident. In Low-Dose Radiation Effects on Animals and Ecosystems; Fukumoto, M., Ed.; Springer: Singapore, 2020; pp. 239–247. [Google Scholar] [CrossRef] [Green Version]
- Sakauchi, K.; Taira, W.; Hiyama, A.; Imanaka, T.; Otaki, J.M. The pale grass blue butterfly in ex-evacuation zones 5.5 years after the Fukushima nuclear accident: Contributions of initial high-dose exposure to transgenerational effects. J. Asia-Pac. Entomol. 2020, 23, 242–252. [Google Scholar] [CrossRef]
- Hiyama, A.; Iwata, M.; Otaki, J.M. Rearing the pale grass blue Zizeeria maha (Lepidoptera, Lycaenidae): Toward the establishment of a lycaenid model system for butterfly physiology and genetics. Entomol. Sci. 2010, 13, 293–302. [Google Scholar] [CrossRef]
- Shirôzu, T. The Standard of Butterflies in Japan; Gakken: Tokyo, Japan, 2006. (In Japanese) [Google Scholar]
- Yata, O. Iconographia Insectorum Japonicorum Colore Naturali Edita. Volumen I (Lepidoptera); Hokuryukan: Tokyo, Japan, 2007. (In Japanese) [Google Scholar]
- Oda, H.; Kitazoe, N. Observational Encyclopedia of Lycaenid Butterflies; Kaisei-Sha: Tokyo, Japan, 2002. (In Japanese) [Google Scholar]
- Groom, Q.J.; Van Der Straeten, J.; Hoste, I. The origin of Oxalis corniculata L. Peer J. 2019, 7, e6384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiyama, A.; Otaki, J.M. Dispersibility of the pale grass blue butterfly Zizeeria maha (Lepidoptera: Lycaenidae) revealed by one-individual tracking in the field: Quantitative comparisons between subspecies and between sexes. Insects 2020, 11, 122. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Matsuyama, S.; Yamaji, K. Oxalic acid as a larval feeding stimulant for the pale grass blue butterfly Zizeeria maha (Lepidoptera: Lycaenidae). Appl. Entomol. Zool. 2015, 51, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Education, Culture, Spots, Science and Technology, Japan. In Japan Food Standard Nutrient Contents Tables 2015, 7th ed.; MEXT: Tokyo, Japan, 2015; Available online: https://www.mext.go.jp/a_menu/syokuhinseibun/1365297.htm (accessed on 15 July 2020). (In Japanese)
- Japan Radioisotope Association (JRA). Radioisotope Pocket Data Book, 11th ed.; Japan Radioisotope Association: Tokyo, Japan, 2011. (In Japanese) [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 22 December 2020).
- Aitchison, J. The statistical analysis of compositional data. J. R. Statist. Soc. B 1982, 44, 139–177. [Google Scholar] [CrossRef]
- Aitchison, J. The Statistical Analysis of Compositional Data; Blackburn Press: Caldwell, NJ, USA, 2003. [Google Scholar]
- Ohta, T.; Arai, H. Problems in compositional data analysis and their solutions. J. Geol. Soc. Jpn. 2006, 112, 173–187. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Hijazi, R.H.; Jernigan, R.W. Modeling compositional data using dirichlet regression models. J. Appl. Probab. Stat. 2009, 4, 77–91. [Google Scholar]
- Maier, M.J. DirichletReg: Dirichlet regression for compositional data in R. Res. Rep. Ser. Inst. Stat. Math. 2014, 125, 1–25. [Google Scholar]
- Bréchignac, F.; Oughton, D.; Mays, C.; Barnthouse, L.; Beasley, J.C.; Bonisoli-Alquati, A.; Bradshaw, C.; Brown, J.; Dray, S.; Geras’kin, S.; et al. Addressing ecological effects of radiation on populations and ecosystems to improve protection of the environment against radiation: Agreed statements from a Consensus Symposium. J. Environ. Radioact. 2016, 158–159, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnier-Laplace, J.; Geras’kin, S.; Della-Vedova, C.; Beaugelin-Seiller, K.; Hinton, T.G.; Real, A.; Oudalova, A. Are radiosensitivity data derived from natural field conditions consistent with data from controlled exposures? A case study of Chernobyl wildlife chronically exposed to low dose rates. J. Environ. Radioact. 2013, 121, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Beaugelin-Seiller, K.; Della-Vedova, C.; Garnier-Laplace, J. Is non-human species radiosensitivity in the lab a good indicator of that in the field? Making the comparison more robust. J. Environ. Radioact. 2020, 211, 105870. [Google Scholar] [CrossRef]
- Kumar, V.; Khare, T.; Shaikh, S.; Wani, S.H. Compatible solutes and abiotic stress tolerance in plants. In Metabolic Adaptations in Plants during Abiotic Stress; Ramakrishna, A., Gill, S.S., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 213–220. [Google Scholar]
- Gorham, J. Sodium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 569–583. [Google Scholar]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A functional plant nutrient. Critic. Rev. Plant Sci. 2003, 22, 391–416. [Google Scholar]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikia, P.; Deka, D.C. Mineral content of some wild green leafy vegetables of North-East India. J. Chem. Pharm. Res. 2013, 5, 117–121. [Google Scholar]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yang, G. Signal function studies of ROS, especially RBOH-dependent ROS, in plant growth, development and environmental stress. J. Plant Growth Regul. 2020, 39, 157–171. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Fukunaga, A.; Suga, Y.; Murakami, K.; Ikeda, J.; Hori, K. Effects of changes in N and cations composition and concentration on antioxidant activity of spinach under hydroponic culture. Jpn. Soc. Soil Sci. Plant Nutr. 2006, 77, 423–427. (In Japanese) [Google Scholar]
- Masaoka, Y. Relationship between ascorbic acid concentration and potassium in leaf vegetables. Agric. Sci. 2007, 589, 1–5. (In Japanese) [Google Scholar]
- Michell, A.R. Physiological aspects of the requirement for sodium in mammals. Nutr. Res. Rev. 1989, 2, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Matsubayashi, H.; Lagan, P.; Majalap, N.; Tangah, J.; Sukor, J.R.A.; Kitayama, K. Importance of natural licks for the mammals in Bornean island tropical rain forests. Ecol. Res. 2007, 22, 742–748. [Google Scholar] [CrossRef]
- Iwata, Y.; Nakashima, Y.; Tsuchida, S.; Nguema, P.P.M.; Ando, C.; Ushida, K.; Yamagiwa, J. Decaying toxic wood as sodium supplement for herbivorous mammals in Gabon. J. Vet. Med. Sci. 2015, 77, 1247–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, C.E.; Walker-Simmons, M.; Makus, D.; Zuroske, G.; Graham, J.; Ryan, C.A. Regulation of synthesis and accumulation of proteinase inhibitors in leaves of wounded tomato plants. In Plant Resistance to Insects. ACS Symposium Series 208; Hedin, P.A., Ed.; American Chemical Society: Washington, DC, USA, 1983; pp. 103–122. [Google Scholar] [CrossRef]
- Orozco-Cardenas, M.; McGurl, B.; Ryan, C. Expression of an antisense prosystemin gene in tomato plants reduces resistance toward Manduca sexta larvae. Proc. Natl. Acad. Sci. USA 1993, 90, 8273–8276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogan, M.; Paxton, J. Natural inducers of plant resistance to insects. In Plant Resistance to Insects. ACS Symposium Series 208; Hedin, P.A., Ed.; American Chemical Society: Washington, DC, USA, 1983; pp. 153–171. [Google Scholar] [CrossRef]
- Stipanovic, R.D. Function and chemistry of plant trichomes and glands in insect resistance: Protective chemicals in plant epidermal glands and appendages. In Plant Resistance to Insects. ACS Symposium Series 208; Hedin, P.A., Ed.; American Chemical Society: Washington, DC, USA, 1983; pp. 69–100. [Google Scholar] [CrossRef]
- Snell-Rood, E.C.; Espeset, A.; Boser, C.J.; White, W.A.; Smykalski, R. Anthropogenic changes in sodium affect neural and muscle development in butterflies. Proc. Natl. Acad. Sci. USA 2014, 111, 10221–10226. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, T.S.; Shephard, A.M.; Kalinowski, C.R.; Kobiela, M.E.; Snell-Rood, E.C. Butterflies do not alter oviposition or larval foraging in response to anthropogenic increases in sodium. Anim. Behav. 2019, 154, 121–129. [Google Scholar] [CrossRef]
- Pivnick, K.A.; McNeil, J.N. Puddling in butterflies: Sodium affects reproductive success in Thymelicus lineola. Physiol. Entomol. 1987, 12, 461–472. [Google Scholar] [CrossRef]
- Boggs, C.L.; Dau, B. Resource specialization in puddling Lepidoptera. Environ. Entomol. 2004, 33, 1020–1024. [Google Scholar] [CrossRef] [Green Version]
- Mitra, C.; Reynoso, E.; Davidowitz, G.; Papaj, D. Effects of sodium puddling on male mating success, courtship and flight in a swallowtail butterfly. Anim. Behav. 2016, 114, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Mevi-Schütz, J.; Erhardt, A. Larval nutrition affects female nectar amino acid preference in the map butterfly (Araschnia levana). Ecology 2003, 84, 2788–2794. [Google Scholar] [CrossRef]
- Wetzel, W.C.; Kharouba, H.M.; Robinson, M.; Holyoak, M.; Karban, R. Variability in plant nutrients reduces insect herbivore performance. Nature 2016, 539, 425–427. [Google Scholar] [CrossRef]
- Møller, A.P.; Barnier, F.; Mousseau, T.A. Ecosystems effects 25 years after Chernobyl: Pollinators, fruit set and recruitment. Oecologia 2012, 170, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Mousseau, T.A. Reduced colonization by soil invertebrates to irradiated decomposing wood in Chernobyl. Sci. Total Environ. 2018, 645, 773–779. [Google Scholar] [CrossRef]
- Mappes, T.; Boratyński, Z.; Kivisaari, K.; Lavrinienko, A.; Milinevsky, G.; Mousseau, T.A.; Møller, A.P.; Tukalenko, E.; Watts, P.C. Ecological mechanisms can modify radiation effects in a key forest mammal of Chernobyl. Ecosphere 2019, 10, e02667. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakauchi, K.; Taira, W.; Toki, M.; Tsuhako, M.; Umetsu, K.; Otaki, J.M. Nutrient Imbalance of the Host Plant for Larvae of the Pale Grass Blue Butterfly May Mediate the Field Effect of Low-Dose Radiation Exposure in Fukushima: Dose-Dependent Changes in the Sodium Content. Insects 2021, 12, 149. https://doi.org/10.3390/insects12020149
Sakauchi K, Taira W, Toki M, Tsuhako M, Umetsu K, Otaki JM. Nutrient Imbalance of the Host Plant for Larvae of the Pale Grass Blue Butterfly May Mediate the Field Effect of Low-Dose Radiation Exposure in Fukushima: Dose-Dependent Changes in the Sodium Content. Insects. 2021; 12(2):149. https://doi.org/10.3390/insects12020149
Chicago/Turabian StyleSakauchi, Ko, Wataru Taira, Mariko Toki, Masakazu Tsuhako, Kazuo Umetsu, and Joji M. Otaki. 2021. "Nutrient Imbalance of the Host Plant for Larvae of the Pale Grass Blue Butterfly May Mediate the Field Effect of Low-Dose Radiation Exposure in Fukushima: Dose-Dependent Changes in the Sodium Content" Insects 12, no. 2: 149. https://doi.org/10.3390/insects12020149
APA StyleSakauchi, K., Taira, W., Toki, M., Tsuhako, M., Umetsu, K., & Otaki, J. M. (2021). Nutrient Imbalance of the Host Plant for Larvae of the Pale Grass Blue Butterfly May Mediate the Field Effect of Low-Dose Radiation Exposure in Fukushima: Dose-Dependent Changes in the Sodium Content. Insects, 12(2), 149. https://doi.org/10.3390/insects12020149






