Melissococcus plutonius Can Be Effectively and Economically Detected Using Hive Debris and Conventional PCR
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Bacterial Isolates
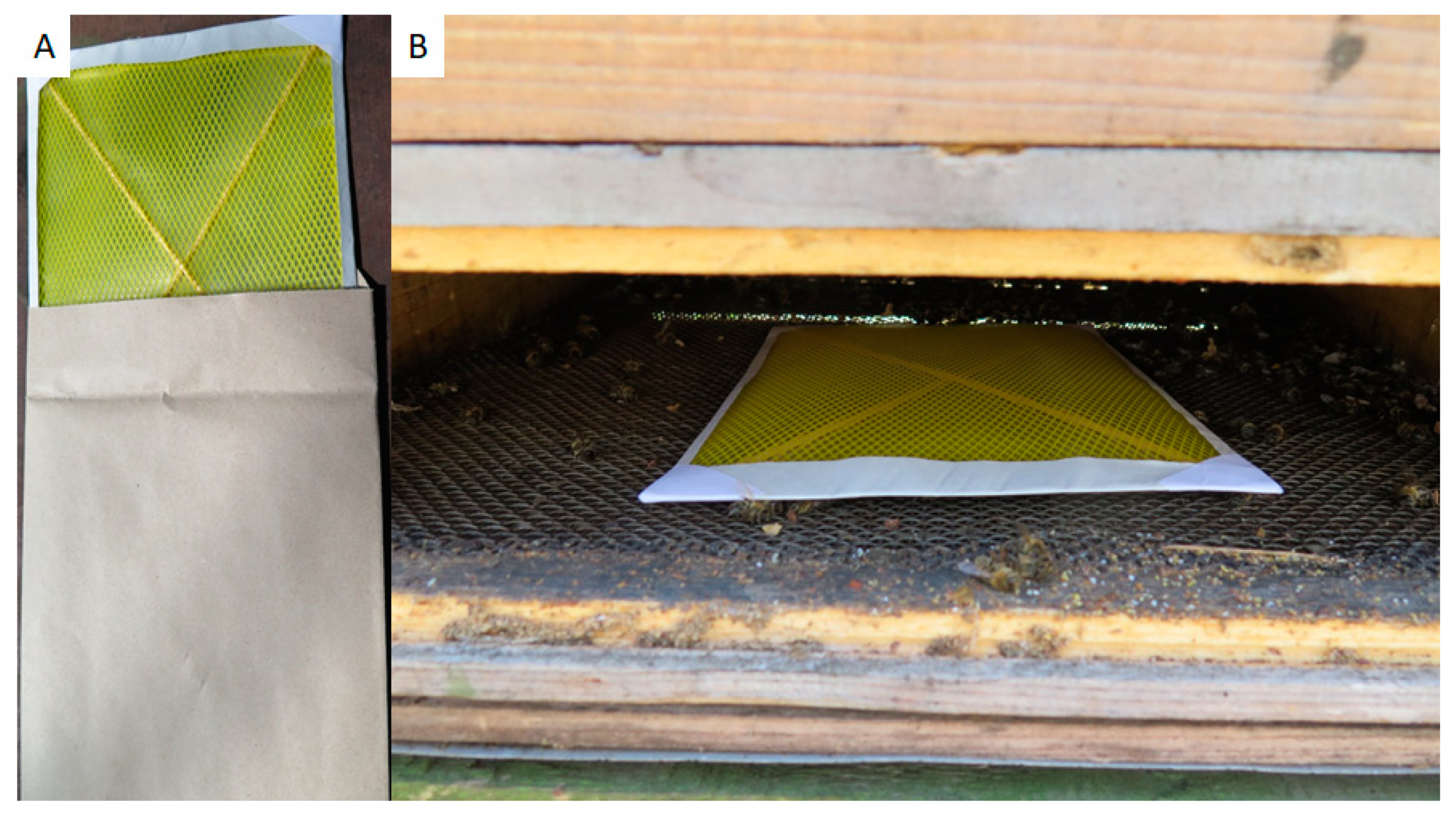
2.2. Spiked Debris and Honey for PCR Method Validation
2.3. Collecting Honey, Debris, and Bees from Colonies
2.4. Isolation of DNA from Hive Debris
2.5. Isolation of DNA from Honey
2.6. Isolation of DNA from Honey Bee Workers
2.7. Polymerase Chain Reaction (PCR)
2.8. qPCR from Adult Honey Bees
2.9. Statistics
3. Results
3.1. Primer Specificity
3.2. Reliability of PCR Detection from Honey and Hive Debris
3.3. Comparison of PCR Confirmations Using Different Sample Types
3.4. Sensitivity and Specificity
3.5. Predictive Values of the Positive and Negative Tests Based on the Prevalence of M. plutonius in Hypothetical Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Forsgren, E. European foulbrood in honey bees. J. Invertebr. Pathol. 2010, 103 (Suppl. 1), S5–S9. [Google Scholar] [CrossRef]
- de Graaf, D.C.; Alippi, A.M.; Antúnez, K.; Aronstein, K.A.; Budge, G.; De Koker, D.; De Smet, L.; Dingman, D.W.; Evans, J.D.; Foster, L.J.; et al. Standard methods for american foulbrood research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef] [Green Version]
- World Organisation for Animal Health. European foulbrood of honey bees (infection of honey bees with Melissococcus plutonius). In Oie Terrestrial Manual; WOAH: Paris, France, 2018. [Google Scholar]
- World Organisation for Animal Health. American foulbrood of honey bees (infection of honey bees with Paenibacillus larvae). In Oie Terrestrial Manual; WOAH: Paris, France, 2018. [Google Scholar]
- Forsgren, E.; Locke, B.; Sircoulomb, F.; Schäfer, M.O. Bacterial diseases in honeybees. Curr. Clin. Microbiol. Rep. 2018, 5, 18–25. [Google Scholar] [CrossRef]
- Boncristiani, H.; Ellis, J.D.; Bustamante, T.; Graham, J.; Jack, C.; Kimmel, C.B.; Mortensen, A.; Schmehl, D.R. World honey bee health: The global distribution of western honey bee (Apis mellifera L.) pests and pathogens. Bee World 2021, 98, 2–6. [Google Scholar] [CrossRef]
- Andrea Cecchini, P.H.; Dietemann, V.; Charrière, J.-D.; Grossar, D. The Influence of European Foulbrood on the Mortality of Adult Honeybees. In Proceedings of the 7th European Conference of Apidology, Cluj-Napoca, Romania, 7–9 September 2016. [Google Scholar]
- Bailey, L. Melissococcus pluton, the cause of European foulbrood of honey bees (Apis spp.). J. Appl. Bacteriol. 1983, 55, 65–69. [Google Scholar] [CrossRef]
- Alippi, A.M. Bacterial diseases of honey bees. In Bee Health and Veterinarians; Ritter, W., Ed.; World Organisation for Animal Health: Paris, France, 2014. [Google Scholar]
- Grossar, D.; Kilchenmann, V.; Forsgren, E.; Charriere, J.D.; Gauthier, L.; Chapuisat, M.; Dietemann, V. Putative determinants of virulence in Melissococcus plutonius, the bacterial agent causing european foulbrood in honey bees. Virulence 2020, 11, 554–567. [Google Scholar] [CrossRef]
- Arai, R.; Tominaga, K.; Wu, M.; Okura, M.; Ito, K.; Okamura, N.; Onishi, H.; Osaki, M.; Sugimura, Y.; Yoshiyama, M.; et al. Diversity of Melissococcus plutonius from honeybee larvae in japan and experimental reproduction of european foulbrood with cultured atypical isolates. PLoS ONE 2012, 7, e33708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Okumura, K.; Harada, M.; Okamoto, M.; Okura, M.; Takamatsu, D. Different impacts of pmp19 on the virulence of Melissococcus plutonius strains with different genetic backgrounds. Environ. Microbiol. 2020, 22, 2756–2770. [Google Scholar] [CrossRef]
- Lewkowski, O.; Erler, S. Virulence of Melissococcus plutonius and secondary invaders associated with European foulbrood disease of the honey bee. Microbiol. Open 2019, 8, e00649. [Google Scholar] [CrossRef]
- Takamatsu, D.; Sato, M.; Yoshiyama, M. Infection of Melissococcus plutonius clonal complex 12 strain in european honeybee larvae is essentially confined to the digestive tract. J. Vet. Med. Sci. 2016, 78, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erler, S.; Lewkowski, O.; Poehlein, A.; Forsgren, E. The curious case of achromobacter eurydice, a gram-variable pleomorphic bacterium associated with european foulbrood disease in honeybees. Microb. Ecol. 2017, 75, 1–6. [Google Scholar] [CrossRef]
- CABI. European Foulbrood. Available online: https://www.cabi.org/isc/datasheet/109547#tooverview (accessed on 12 January 2021).
- Pejchar, P. V Česku byl po 20 Letech Opět Prokázán Případ Hniloby Včelího Plodu; State Veterinary Administration: Prague, Czech Republic, 2015; Available online: https://www.svscr.cz/v_cesku_byl_po_20_letech_opet_prokazan_pripad/ (accessed on 12 January 2021).
- Nakamura, K.; Yamazaki, Y.; Shiraishi, A.; Kobayashi, S.; Harada, M.; Yoshiyama, M.; Osaki, M.; Okura, M.; Takamatsu, D. Virulence differences among Melissococcus plutonius strains with different genetic backgrounds in Apis mellifera larvae under an improved experimental condition. Sci. Rep. 2016, 6, 33329. [Google Scholar] [CrossRef] [Green Version]
- Forsgren, E.; Budge, G.E.; Charrière, J.-D.; Hornitzky, M.A.Z. Standard methods for European foulbrood research. J. Apic. Res. 2013, 52, 1–14. [Google Scholar] [CrossRef]
- Schafer, M.O.; Genersch, E.; Funfhaus, A.; Poppinga, L.; Formella, N.; Bettin, B.; Karger, A. Rapid identification of differentially virulent genotypes of Paenibacillus larvae, the causative organism of American foulbrood of honey bees, by whole cell Maldi-Tof Mass Spectrometry. Vet. Microbiol. 2014, 170, 291–297. [Google Scholar] [CrossRef]
- Sopko, B.; Zitek, J.; Nesvorna, M.; Markovic, M.; Kamler, M.; Titera, D.; Erban, T.; Hubert, J. Detection and quantification of Melissococcus plutonius in honey bee workers exposed to European foulbrood in czechia through conventional PCR, qPCR, and barcode sequencing. J. Apic. Res. 2019, 59, 503–514. [Google Scholar] [CrossRef]
- Polachova, V.; Pastucha, M.; Mikusova, Z.; Mickert, M.J.; Hlavacek, A.; Gorris, H.H.; Skladal, P.; Farka, Z. Click-conjugated photon-upconversion nanoparticles in an immunoassay for honeybee pathogen Melissococcus Plutonius. Nanoscale 2019, 11, 8343–8351. [Google Scholar] [CrossRef]
- Farka, Z.; Jurik, T.; Kovar, D.; Trnkova, L.; Skladal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, Z.; Farka, Z.; Pastucha, M.; Poláchová, V.; Obořilová, R.; Skládal, P. Amperometric immunosensor for rapid detection of honeybee pathogen Melissococcus Plutonius. Electroanalysis 2019, 31, 1969–1976. [Google Scholar] [CrossRef]
- Tomkies, V.; Flint, J.; Johnson, G.; Waite, R.; Wilkins, S.; Danks, C.; Watkins, M.; Cuthbertson, A.G.S.; Carpana, E.; Marris, G.; et al. Development and validation of a novel field test kit for European foulbrood. Apidologie 2008, 40, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Owen, R. Role of human action in the spread of honey bee (hymenoptera: Apidae) pathogens. J. Econ. Èntomol. 2017, 110, 797–801. [Google Scholar] [CrossRef]
- Jacques, A.; Laurent, M.; Consortium, E.; Ribiere-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.P. A pan-european epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE 2017, 12, e0172591. [Google Scholar] [CrossRef] [Green Version]
- Budge, G.E.; Barrett, B.; Jones, B.; Pietravalle, S.; Marris, G.; Chantawannakul, P.; Thwaites, R.; Hall, J.; Cuthbertson, A.G.; Brown, M.A. The occurrence of Melissococcus plutonius in healthy colonies of Apis mellifera and the efficacy of European foulbrood control measures. J. Invertebr. Pathol. 2010, 105, 164–170. [Google Scholar] [CrossRef] [PubMed]
- von Buren, R.S.; Oehen, B.; Kuhn, N.J.; Erler, S. High-resolution maps of swiss apiaries and their applicability to study spatial distribution of bacterial honey bee brood diseases. PeerJ 2019, 7, e6393. [Google Scholar] [CrossRef]
- Fries, I.; Camazine, S. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie 2001, 32, 199–214. [Google Scholar] [CrossRef] [Green Version]
- Bzdil, J. Detection of Paenibacillus larvae spores in the debris and wax of honey bee by the tween 80 method. Acta Vet. BRNO 2007, 76, 643–648. [Google Scholar] [CrossRef]
- Ryba, S.; Titera, D.; Haklova, M.; Stopka, P. A PCR method of detecting American foulbrood (Paenibacillus larvae) in winter beehive wax debris. Vet. Microbiol. 2009, 139, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Ryba, S.; Kindlmann, P.; Titera, D.; Haklova, M.; Stopka, P. A new low-cost procedure for detecting nucleic acids in low-incidence samples: A case study of detecting spores of Paenibacillus larvae from bee debris. J. Econ. Entomol. 2012, 105, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Amadoro, C.; Ruberto, A.; Ricchiuti, L. Evaluation of quantitative PCR (qPCR) Paenibacillus larvae targeted assays and definition of optimal conditions for its detection/quantification in honey and hive debris. Insects 2018, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Ward, L.; Brown, M.; Neumann, P.; Wilkins, S.; Pettis, J.; Boonham, N. A DNA method for screening hive debris for the presence of small hive beetle (Aethina tumida). Apidologie 2007, 38, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Ponting, S.; Tomkies, V.; Stainton, K. Rapid identification of the invasive small hive beetle (Aethina tumida) using lamp. Pest Manag. Sci. 2021, 77, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Govan, V.A.; Brozel, V.; Allsopp, M.H.; Davison, S. A pcr detection method for rapid identification of Melissococcus pluton in honeybee larvae. Appl. Environ. Microbiol. 1998, 64, 1983–1985. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.D.; Schwarz, R.S.; Chen, Y.P.; Budge, G.; Cornman, R.S.; De la Rua, P.; de Miranda, J.R.; Foret, S.; Foster, L.; Gauthier, L.; et al. Standard methods for molecular research in Apis Mellifera. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Roetschi, A.; Berthoud, H.; Kuhn, R.; Imdorf, A. Infection rate based on quantitative real-time PCR of Melissococcus plutonius, the causal agent of European foulbrood, in honeybee colonies before and after apiary sanitation. Apidologie 2008, 39, 362–371. [Google Scholar] [CrossRef]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Dainat, B.; Grossar, D.; Ecoffey, B.; Haldemann, C. Triplex real-time PCR method for the qualitative detection of European and american foulbrood in honeybee. J. Microbiol. Methods 2018, 146, 61–63. [Google Scholar] [CrossRef]
- Metz, C.E. Basic principles of roc analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Pagano, M.; Gauvreau, K. Principles of Biostatistics, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2018. [Google Scholar]
- vanEngelsdorp, D.; Lengerich, E.; Spleen, A.; Dainat, B.; Cresswell, J.; Baylis, K.; Nguyen, B.K.; Soroker, V.; Underwood, R.; Human, H.; et al. Standard epidemiological methods to understand and improve Apis mellifera health. J. Apic. Res. 2013, 52, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Erban, T.; Ledvinka, O.; Kamler, M.; Hortova, B.; Nesvorna, M.; Tyl, J.; Titera, D.; Markovic, M.; Hubert, J. Bacterial community associated with worker honey bees (Apis mellifera) affected by European foulbrood. PeerJ 2017, 5, e3816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alippi, A.M.; Lopez, A.C.; Aguilar, O.M. A PCR-based method that permits specific detection of Paenibacillus larvae subsp. Larvae, the cause of American foulbrood of honey bees, at the subspecies level. Lett. Appl. Microbiol. 2004, 39, 25–33. [Google Scholar] [CrossRef]
- Forsgren, E.; Lundhagen, A.C.; Imdorf, A.; Fries, I. Distribution of Melissococcus plutonius in honeybee colonies with and without symptoms of European foulbrood. Microb. Ecol. 2005, 50, 369–374. [Google Scholar] [CrossRef]
- Forsgren, E.; Laugen, A.T. Prognostic value of using bee and hive debris samples for the detection of American foulbrood disease in honey bee colonies. Apidologie 2013, 45, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Bloch, D.A. Comparing two diagnostic tests against the same “gold standard” in the same sample. Biometrics 1997, 53, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Umemneku Chikere, C.M.; Wilson, K.; Graziadio, S.; Vale, L.; Allen, A.J. Diagnostic test evaluation methodology: A systematic review of methods employed to evaluate diagnostic tests in the absence of gold standard—An update. PLoS ONE 2019, 14, e0223832. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.R.; Rocha-Silva, F.; Graciele-Melo, C.; Lafuente, C.R.; Magalhaes, T.; Caligiorne, R.B. Comparison between conventional and real-time PCR assays for diagnosis of visceral leishmaniasis. Biomed Res. Int. 2014, 2014, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Johansson, M.L.; Gao, Y.; Zhang, L.; Haffner, G.D.; MacIsaac, H.J.; Zhan, A. Conventional versus real-time quantitative pcr for rare species detection. Ecol. Evol. 2018, 8, 11799–11807. [Google Scholar] [CrossRef]
- Belloy, L.; Imdorf, A.; Fries, I.; Forsgren, E.; Berthoud, H.; Kuhn, R.; Charrière, J.-D. Spatial distribution of Melissococcus plutonius in adult honey bees collected from apiaries and colonies with and without symptoms of European foulbrood. Apidologie 2007, 38, 136–140. [Google Scholar] [CrossRef]
- Cremer, S.; Armitage, S.A.; Schmid-Hempel, P. Social immunity. Curr. Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef] [Green Version]
- Simone-Finstrom, M. Social immunity and the superorganism: Behavioral defenses protecting honey bee colonies from pathogens and parasites. Bee World 2017, 94, 21–29. [Google Scholar] [CrossRef]
- Spivak, M.; Reuter, G.S. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 2001, 32, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Swanson, J.A.; Torto, B.; Kells, S.A.; Mesce, K.A.; Tumlinson, J.H.; Spivak, M. Odorants that induce hygienic behavior in honeybees: Identification of volatile compounds in chalkbrood-infected honeybee larvae. J. Chem. Ecol. 2009, 35, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Palacio, M.A.; Rodriguez, E.; Goncalves, L.; Bedascarrasbure, E.; Spivak, M. Hygienic behaviors of honey bees in response to brood experimentally pin-killed or infected with Ascosphaera apis. Apidologie 2010, 41, 602–612. [Google Scholar] [CrossRef]
| Technical Replicate | CFU M. plutonius in 1 g Debris | CFU M. plutonius in 1 g Honey | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 × 100 | 6.70 × 102 | 6.70 × 103 | 6.70 × 104 | 6.70 × 105 | 0.00 × 100 | 6.70 × 102 | 6.70 × 103 | 6.70 × 104 | 6.70 × 105 | |
| 1 | negative | positive | positive | positive | n/a | negative | positive | positive | positive | positive |
| 2 | negative | positive | n/a | positive | n/a | negative | positive | positive | positive | positive |
| 3 | negative | negative | positive | positive | positive | negative | negative | positive | positive | positive |
| 4 | negative | negative | positive | positive | positive | negative | positive | positive | positive | positive |
| 5 | negative | positive | positive | positive | positive | negative | positive | positive | positive | positive |
| 6 | negative | negative | positive | positive | positive | negative | n/a | n/a | n/a | n/a |
| Reliability | 50% | 100% | 100% | 100% | 80% | 100% | 100% | 100% | ||
| Status | Description | Honey (n) | Honey (% from Total Analyzed) | Debris (n) | Debris (% from Total Analyzed) | Adult Bees (n) | Adult Bees (% from Total Analyzed) |
|---|---|---|---|---|---|---|---|
| Positive | PCR positive with clinical symptoms | 13 | 38.2 | 15 | 41.7 | 16 | 47.1 |
| False positive | PCR positive without clinical symptoms | 3 | 8.8 | 3 | 8.3 | 3 | 8.8 |
| Negative | PCR negative without clinical symptoms | 16 | 47.1 | 12 | 33.3 | 15 | 44.1 |
| False negative | PCR negative with clinical symptoms | 2 | 5.9 | 6 | 16.7 | 0 | 0.0 |
| Bayes Statistics | Honey (Conventional PCR) | Hive Debris (Conventional PCR) | Adult Bees (qPCR) |
|---|---|---|---|
| Sensitivity | 0.867 | 0.714 | 1.000 |
| Specificity | 0.842 | 0.800 | 0.833 |
| Predictive values of positive test | 0.813 | 0.833 | 0.842 |
| Predictive value of negative test | 0.889 | 0.667 | 1.000 |
| Real accuracy | 0.853 | 0.750 | 0.912 |
| Pessimistic accuracy | 0.659 | 0.614 | 0.738 |
| Hypothetical Prevalence of M. plutonius | Honey (Conventional PCR) | Hive Debris (Conventional PCR) | Adult Bees (qPCR) | |||
|---|---|---|---|---|---|---|
| Positive Test | Negative Test | Positive Test | Negative Test | Positive Test | Negative Test | |
| 0.3 | 0.702 | 0.936 | 0.605 | 0.867 | 0.934 | 1.000 |
| 0.1 | 0.379 | 0.983 | 0.284 | 0.962 | 0.400 | 1.000 |
| 0.01 | 0.053 | 0.998 | 0.035 | 0.996 | 0.048 | 1.000 |
| 0.001 | 0.005 | 1.000 | 0.004 | 1.000 | 0.006 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biová, J.; Charrière, J.-D.; Dostálková, S.; Škrabišová, M.; Petřivalský, M.; Bzdil, J.; Danihlík, J. Melissococcus plutonius Can Be Effectively and Economically Detected Using Hive Debris and Conventional PCR. Insects 2021, 12, 150. https://doi.org/10.3390/insects12020150
Biová J, Charrière J-D, Dostálková S, Škrabišová M, Petřivalský M, Bzdil J, Danihlík J. Melissococcus plutonius Can Be Effectively and Economically Detected Using Hive Debris and Conventional PCR. Insects. 2021; 12(2):150. https://doi.org/10.3390/insects12020150
Chicago/Turabian StyleBiová, Jana, Jean-Daniel Charrière, Silvie Dostálková, Mária Škrabišová, Marek Petřivalský, Jaroslav Bzdil, and Jiří Danihlík. 2021. "Melissococcus plutonius Can Be Effectively and Economically Detected Using Hive Debris and Conventional PCR" Insects 12, no. 2: 150. https://doi.org/10.3390/insects12020150
APA StyleBiová, J., Charrière, J.-D., Dostálková, S., Škrabišová, M., Petřivalský, M., Bzdil, J., & Danihlík, J. (2021). Melissococcus plutonius Can Be Effectively and Economically Detected Using Hive Debris and Conventional PCR. Insects, 12(2), 150. https://doi.org/10.3390/insects12020150






