A Bizarre Planthopper Nymph (Hemiptera: Fulgoroidea) from Mid-Cretaceous Kachin Amber
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
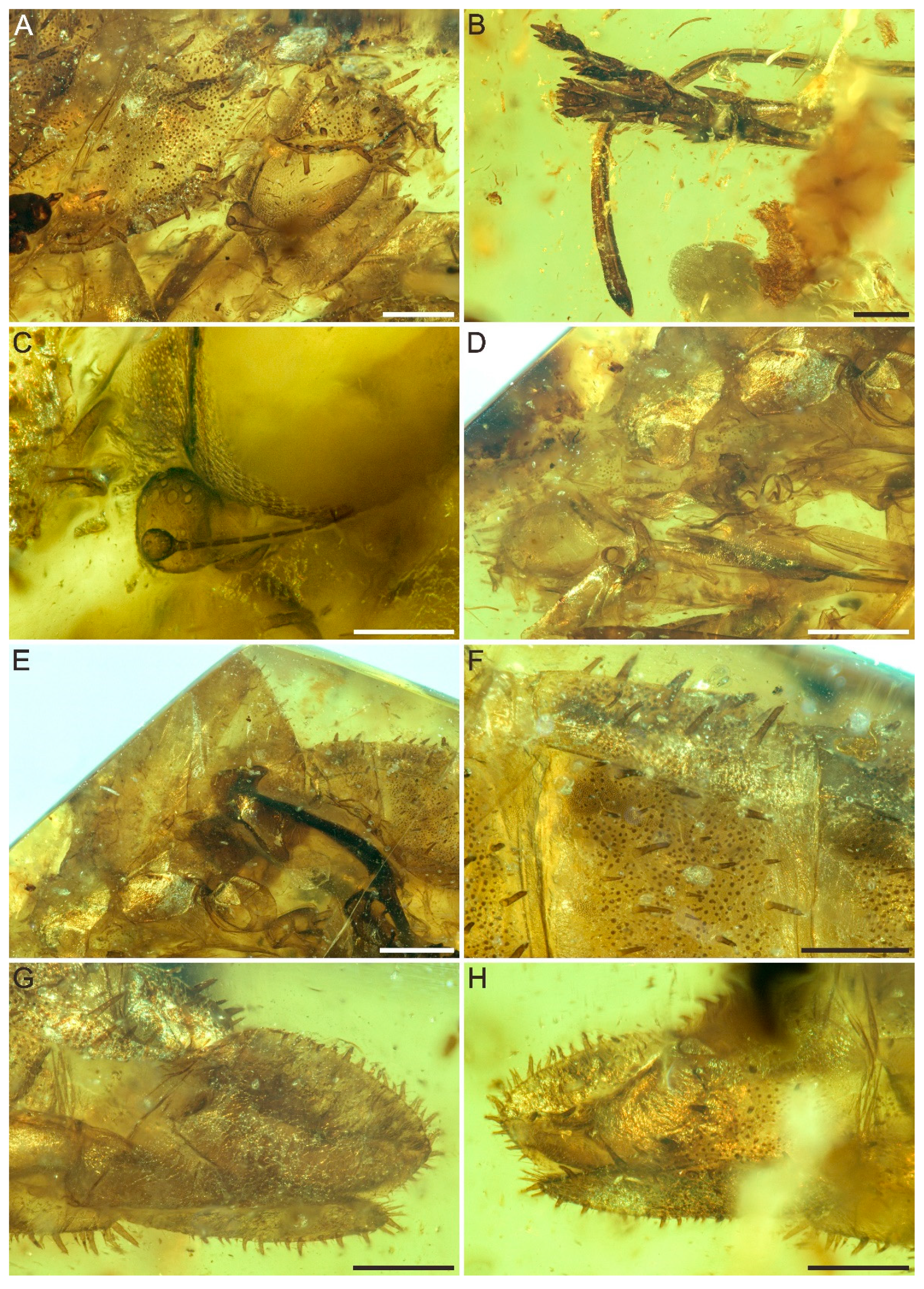
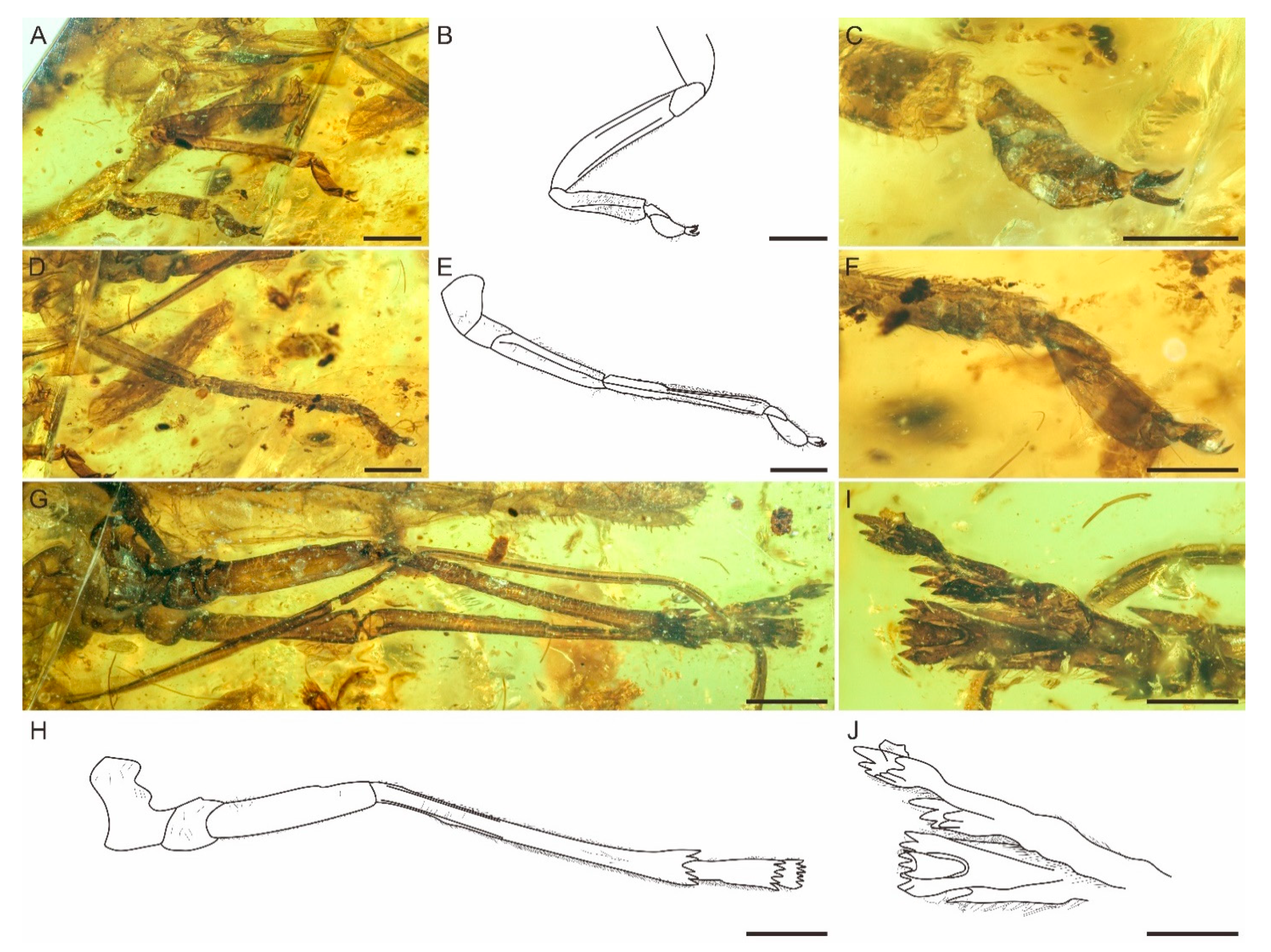
3. Systematic Palaeontology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimaldi, D.A.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005; p. 770. [Google Scholar]
- Szwedo, J. The unity, diversity and conformity of bugs (Hemiptera) through time. Earth Environ. Sci. Trans. R. Soc. Edinb. 2018, 107, 109–128. [Google Scholar] [CrossRef]
- Brysz, A.M.; Szwedo, J. Jeweled Achilidae–a new look at their systematics and relations with other Fulgoroidea (Hemiptera). Monogr. Upper Sil. Mus. 2019, 10, 93–130. [Google Scholar] [CrossRef]
- Szwedo, J.; Bourgoin, T.; Lefebvre, F. Fossil Planthoppers (Hemiptera: Fulgoromorpha) of the World: An Annotated Catalogue with Notes on Hemiptera Classification; Museum and Insitute of Zoology, Polish Academy of Sciences: Warsaw, Poland, 2004; pp. 1–208. [Google Scholar]
- Luo, C.; Jiang, T.; Szwedo, J.; Wang, B.; Xiao, C. A new planthopper family Katlasidae fam. nov. (Hemiptera: Fulgoromorpha: Fulgoroidea) from mid-Cretaceous Kachin amber. Cretac. Res. 2020, 115, 104532. [Google Scholar] [CrossRef]
- Bourgoin, T. FLOW (Fulgoromorpha Lists on the Web): A World Knowledge Base Dedicated to Fulgoromorpha. Version 8, Updated [5–03–2021]. Available online: https://hemiptera-databases.org/flow/ (accessed on 6 March 2021).
- Emeljanov, A.F. Larval characters and their ontogenetic development in Fulgoroidea (Homoptera, Cicadina). Zoosyst. Ross. 2000, 9, 101–121. [Google Scholar]
- Szwedo, J. Nymphs of a new family Neazoniidae fam. n. (Hemiptera: Fulgoromorpha: Fulgoroidea) from the Lower Cretaceous Lebanese amber. Afr. Invertebr. 2007, 48, 127–143. [Google Scholar]
- Szwedo, J. First discovery of Neazoniidae (Insecta, Hemiptera, Fulgoromorpha) in the Early Cretaceous amber of Archingeay, SW France. Geodiversitas 2009, 31, 105–117. [Google Scholar] [CrossRef]
- Jell, P.A. Late Triassic homopterous nymph from Dinmore, Ipswich basin. Mem. Qld. Mus. 1993, 33, 1–360. [Google Scholar]
- Shcherbakov, D.E. An extraordinary new family of Cretaceous planthoppers (Homoptera: Fulgoroidea). Russ. Entomol. J. 2007, 16, 139–154. [Google Scholar]
- Emeljanov, A.F.; Shcherbakov, D.E. A new genus and species of Dictyopharidae (Homoptera) from Rovno and Baltic amber based on nymphs. ZooKeys 2011, 130, 175–184. [Google Scholar] [CrossRef]
- Emeljanov, A.; Shcherbakov, D.E. A new genus of Dictyopharidae (Homoptera) from Bitterfeld amber based on a nymph. Far East. Entomol. 2020, 403, 1–12. [Google Scholar] [CrossRef]
- Emeljanov, A.F.; Shcherbakov, D.E. The longest-nosed Mesozoic Fulgoroidea (Homoptera): A new family from mid-Cretaceous Burmese amber. Far East. Entomol. 2018, 354, 1–14. [Google Scholar] [CrossRef]
- Westerweel, J.; Roperch, P.; Licht, A.; Dupont-Nivet, G.; Win, Z.; Poblete, F.; Ruffet, G.; Swe, H.H.; Thi, M.K.; Aung, D.W. Burma Terrane part of the Trans-Tethyan arc during collision with India according to palaeomagnetic data. Nat. Geosci. 2019, 12, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Kelly, R.; Mu, L.; Ross, A.; Kennedy, J.; Broly, P.; Xia, F.; Zhang, H.; Wang, B.; Dilcher, D. An ammonite trapped in Burmese amber. Proc. Nati. Acad. Sci. USA 2019, 116, 11345–11350. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.J. Burmese (Myanmar) amber checklist and bibliography 2018. Palaeoentomology 2019, 2, 22–84. [Google Scholar] [CrossRef]
- Ross, A.J. Burmese (Myanmar) Amber Taxa, On-Line Supplement v.2020.1. p. 25. Available online: http://www.nms.ac.uk/explore/stories/natural-world/burmese-amber/ (accessed on 12 February 2021).
- Ross, A.J. Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2019. Palaeoentomology 2020, 3, 103–118. [Google Scholar] [CrossRef]
- Ross, A.J. Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2020. Palaeoentomology 2021, 4, 57–76. [Google Scholar] [CrossRef]
- Szwedo, J.; Nel, A. The Cretaceous insects: A promising state of the art. Cretac. Res. 2015, 52, 628–630. [Google Scholar] [CrossRef]
- Lloyd, G.T.; Davis, K.E.; Pisani, D.; Tarver, J.E.; Ruta, M.; Sakamoto, M.; Hone, D.W.E.; Jennings, R.; Benton, M.J. Dinosaurs and the Cretaceous terrestrial revolution. P. Roy. Soc. B-Biol. Sci. 2008, 275, 2483–2490. [Google Scholar] [CrossRef] [Green Version]
- Genise, J.F.; Bellosi, E.S.; Sarzetti, L.C.; Krause, J.M.; Dinghi, P.A.; Sánchez, M.V.; Umazano, A.M.; Puerta, P.; Cantil, L.F.; Jicha, B.R. 100 Ma sweat bee nests: Early and rapid co-diversification of crown bees and flowering plants. PLoS ONE 2020, 15, e0227789. [Google Scholar] [CrossRef] [Green Version]
- Peris, D.; Labandeira, C.C.; Barrón, E.; Delclòs, X.; Rust, J.; Wang, B. Generalist pollen-feeding beetles during the mid-Cretaceous. iScience 2020, 23, 100913. [Google Scholar] [CrossRef] [Green Version]
- Rojas, A.; Calatayud, J.; Kowalewski, M.; Neuman, M.; Rosvall, M. A multiscale view of the Phanerozoic fossil record reveals the three major biotic transitions. Commun. Biol. 2021, 4, 309. [Google Scholar] [CrossRef]
- Shi, G.; Grimaldi, D.A.; Harlow, G.E.; Wang, J.; Wang, J.; Yang, M.; Lei, W.; Li, Q.; Li, X. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretac. Res. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Ride, W.D.L.; Cogger, H.G.; Dupuis, C.; Kraus, O.; Mineli, A.; Thompson, F.C.; Tubbs, P.K. International Code of Zoological Nomenclature; International Commission on Zoological Nomenclature, The Natural History Museum: London, UK, 1999; p. 126. [Google Scholar]
- Szwedo, J.; Wang, B.; Soszynska-Maj, A.; Azar, D.; Ross, A. International Palaeoentomological Society Statement. Palaeoentomology 2020, 3, 221–222. [Google Scholar] [CrossRef]
- Baranov, V.; Wang, Y.; Gašparič, R.; Wedmann, S.; Haug, J. Eco-morphological diversity of larvae of soldier flies and their closest relatives in deep time. PeerJ 2020, 8, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Haug, G.; Haug, C.; Pazinato, P.; Braig, F.; Perrichot, V.; Gröhn, C.; Müller, P.; Haug, J. The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time. Palaeontol. Electron. 2020, 23, a39. [Google Scholar] [CrossRef]
- Haug, J.; Schädel, M.; Baranov, V.; Haug, C. An unusual 100-million-year old holometabolan larva with a piercing mouth cone. PeerJ 2020, 8, e8661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haug, J.T.; Haug, C. A 100 million-year-old armoured caterpillar supports the early diversification of moths and butterflies. Gondwana Res. 2021, 93, 101–105. [Google Scholar] [CrossRef]
- Luo, Z.-X. Transformation and diversification in early mammal evolution. Nature 2007, 450, 1011–1019. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Z.; Dudley, R.; Mackem, S.; Chuong, C.-M.; Erickson, G.M.; Varricchio, D.J. An integrative approach to understanding bird origins. Science 2014, 346, 1253293. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Xia, F.; Engel, M.S.; Perrichot, V.; Shi, G.; Zhang, H.; Chen, J.; Jarzembowski, E.A.; Wappler, T.; Rust, J. Debris-carrying camouflage among diverse lineages of Cretaceous insects. Sci. Adv. 2016, 2, e1501918. [Google Scholar] [CrossRef] [Green Version]
- Imada, Y. Moss mimesis par excellence: Integrating previous and new data on the life history and larval ecomorphology of long-bodied craneflies (Diptera: Cylindrotomidae: Cylindrotominae). Zool. J. Linn. Soc. 2020, zlaa177. [Google Scholar] [CrossRef]
- Gnezdilov, V.M.; Wilson, M.R. A new genus and a new species of the tribe Mithymnini (Hemiptera: Fulgoromorpha: Nogodinidae) from Namibia, with sternal sensory pits in the adult. Zootaxa 2007, 1453, 55–62. [Google Scholar] [CrossRef]
- Handlirsch, A. Neue Untersuchungen über die fossilen Insekten, Teil 2. Ann. Nat. Mus. Wien. 1939, 49, 1–240. [Google Scholar]
- Bode, A. Die Insektenfauna des ostniedersächsischen oberen Lias. Paleontogr. Abt. A 1953, 103, 1–375. [Google Scholar]
- Shcherbakov, D.E. First record of the Cretaceous family Mimarachnidae (Homoptera: Fulgoroidea) in amber. Russ. Entomol. J. 2017, 26, 389–392. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, D.; Yao, Y. A new genus and species of Mimarachnidae (Hemiptera: Fulgoromorpha: Fulgoroidea) from mid-Cretaceous Burmese amber. Cretac. Res. 2018, 90, 168–173. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, D. New data on fossil mimarachnids (Hemiptera, Fulgoromorpha, Fulgoroidea) in mid-Cretaceous Burmese amber. Palaeoentomology 2020, 3, 317–331. [Google Scholar] [CrossRef]
- Shcherbakov, D.E.; Popov, Y.A. Order Hemiptera Linné, 1758. The bugs, cicadas, plantlice, scale insects, etc. In History of Insects; Rasnitsyn, A.P., Quicke, D.L.J., Eds.; Kluwer: Dordrecht, The Netherlands, 2002; pp. 143–157. [Google Scholar]
- Szwedo, J. A new tribe of Dictyopharidae planthoppers from Eocene Baltic amber (Hemiptera: Fulgoromorpha: Fulgoroidea), with a brief review of the fossil record of the family. Palaeodiversity 2008, 1, 75–85. [Google Scholar]
- Zhang, X.; Ren, D.; Yao, Y. A new family Jubisentidae fam. nov. (Hemiptera: Fulgoromorpha: Fulgoroidea) from the mid-Cretaceous Burmese amber. Cretac. Res. 2019, 94, 1–7. [Google Scholar] [CrossRef]
- Shcherbakov, D.E. The earliest fully brachypterous auchenorrhynchan from Cretaceous Burmese amber (Homoptera: Fulgoroidea: Jubisentidae). Russ. Entomol. J. 2020, 29, 6–11. [Google Scholar] [CrossRef]
- Hamilton, K.G.A. Insects from the Santana Formation, Lower Cretaceous, of Brazil. B. Am. Mus. Nat. Hist. 1990, 195, 82–122. [Google Scholar]
- Peñalver, E.; Szwedo, J. Perforissidae (Hemiptera: Fulgoroidea) from the Lower Cretaceous San Just amber (Eastern Spain). Alavesia 2010, 3, 97–103. [Google Scholar]
- Szwedo, J.; Azar, D.; Nohra, Y. First record of Perforissidae from the Early Cretaceous Lebanese amber (Hemiptera: Fulgoromorpha: Fulgoroidea), insect evolution in an amberiferous and stone alphabet. In Insect Evolution in an Amberiferous and Stone Alphabet; Proceedings of the 6th International Congress on Fossil Insects, Arthropods and Amber, Byblos, Lebanon, 14–18 April 2013; Brill: Leiden-Boston, The Netherlands, 2013; pp. 145–163. [Google Scholar]
- Zhang, X.; Ren, D.; Yao, Y. A new species of Foveopsis Shcherbakov (Hemiptera: Fulgoromorpha: Fulgoroidea: Perforissidae) from mid-Cretaceous Burmese amber. Cretac. Res. 2017, 79, 35–42. [Google Scholar] [CrossRef]
- Luo, C.; Jiang, T.; Szwedo, J.; Wang, B.; Xiao, C. A new genus and species of Perforissidae (Hemiptera: Fulgoromorpha) from mid-Cretaceous Kachin amber. Cretac. Res. 2020, 114, 104518. [Google Scholar] [CrossRef]
- Szwedo, J.; Stroiński, A. An extraordinary tribe of Tropiduchidae from the Eocene Baltic amber (Hemiptera: Fulgoromorpha: Fulgoroidea). Zootaxa 2013, 3647, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Szwedo, J.; Stroiński, A. Who’s that girl? A singular Tropiduchidae planthopper from the Eocene Baltic amber (Hemiptera: Fulgoromorpha). Palaeontol. Electron. 2017, 20, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Bourgoin, T.; Wang, R.-R.; Szwedo, J.; Li, X.-Y.; Chen, X. A new early Miocene fossil genus from Dominican amber extends the Eastern Asia distribution of Paricanini (Hemiptera: Fulgoromorpha: Tropiduchidae) to the Neotropics. Palaeontol. Electron. 2019, 22, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, F.; Bourgoin, T.; Nel, A. New Cixiidae and Achilidae fossils from Middle Eocene Baltic amber (Hemiptera: Fulgoromorpha). Ann. Soc. Entomol. Fr. 2007, 43, 37–43. [Google Scholar] [CrossRef]
- Stroiński, A.; Szwedo, J. Tonacatecutlius gibsoni gen. and sp. nov. from the Oligocene/Miocene Mexican amber (Hemiptera: Fulgoromorpha: Nogodinidae). Ann. Zool. 2000, 50, 341–345. [Google Scholar]
- Szwedo, J.; Stroiński, A. Tainosia quisqueyae gen. and sp. nov. from the Oligocene/Miocene Dominican amber (Hemiptera: Fulgoroidea: Nogodinidae). Genus 2001, 12, 29–34. [Google Scholar]
- Petrulevičius, J.F. A plant hopper (Nogodinidae) from the Upper Palaeocene of Argentina: Systematics and taphonomy. Palaeontology 2005, 48, 299–308. [Google Scholar] [CrossRef]
- Stroiński, A. Hagneia kallea gen. and sp. nov. (Hemiptera: Fulgoromorpha: Ricaniidae) from North Vietnam. Zootaxa 2020, 4861, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.-S.; Xu, G.-H.; Liang, A.-P.; Szwedo, J.; Bourgoin, T. Still greater disparity in basal planthopper lineage: A new planthopper family Yetkhatidae (Hemiptera, Fulgoromorpha, Fulgoroidea) from mid-Cretaceous Myanmar amber. Cretac. Res. 2019, 101, 47–60. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Wang, B.; Jarzembowski, E.A. A Bizarre Planthopper Nymph (Hemiptera: Fulgoroidea) from Mid-Cretaceous Kachin Amber. Insects 2021, 12, 318. https://doi.org/10.3390/insects12040318
Luo C, Wang B, Jarzembowski EA. A Bizarre Planthopper Nymph (Hemiptera: Fulgoroidea) from Mid-Cretaceous Kachin Amber. Insects. 2021; 12(4):318. https://doi.org/10.3390/insects12040318
Chicago/Turabian StyleLuo, Cihang, Bo Wang, and Edmund A. Jarzembowski. 2021. "A Bizarre Planthopper Nymph (Hemiptera: Fulgoroidea) from Mid-Cretaceous Kachin Amber" Insects 12, no. 4: 318. https://doi.org/10.3390/insects12040318





