Bio-Insecticide of Thymus vulgaris and Ocimum basilicum Extract from Cell Suspensions and Their Inhibitory Effect against Serine, Cysteine, and Metalloproteinases of the Red Palm Weevil (Rhynchophorus ferrugineus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Media
2.3. Plant Material
2.4. Callus Initiation of T. Vulgaris and O. basilicum Using Different Plant Growth Regulators with Biotic Elicitor (V. dahliae)
2.5. Initiation of the Cell Suspension of T. vulgaris and O. basilicum and Their Measure Growth Kinetics (Cell Weight)
2.6. Determination of the Total Volatile Phenolic Content (TVPC)
2.7. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
2.8. Assessment Contact–Insecticide and Antifeedant Activity of the Extracted Secondary Metabolites against R. ferrugineus
- No. of prick marks = [((P1 + P2)/2) * Z]/4
- P1 = maximum numbers of pricks in a window area (2 × 2 cm2);
- P2 = minimum number of pricks in a window area (2 × 2 cm2);
- Z = area of sugarcane piece (~32.5 cm2) in each replicate.
2.9. Determination Effects of T. vulgaris and O. basilicum Extract from Cell Suspension and Pure Compounds on Total Proteolytic Enzymes Activity of R. ferrugineus Larvae
2.10. Determination Effects of T. vulgaris and O. basilicum Extract and Pure Compounds from Cell Suspension on Serine Proteinase Specific Activity Assays
2.11. Determination Effects of T. vulgaris and O. basilicum Extract from Cell Suspension and Pure Compounds on Metalloproteinase Specific Activity Assays
2.12. Determination Effects of T. vulgaris and O. basilicum Extract from Cell Suspension and Pure Compounds on Cysteine Proteinase Specific Activity Assays
2.13. Statistical Design
3. Results
3.1. Cell Suspension and Callus Initiation and Maintenance
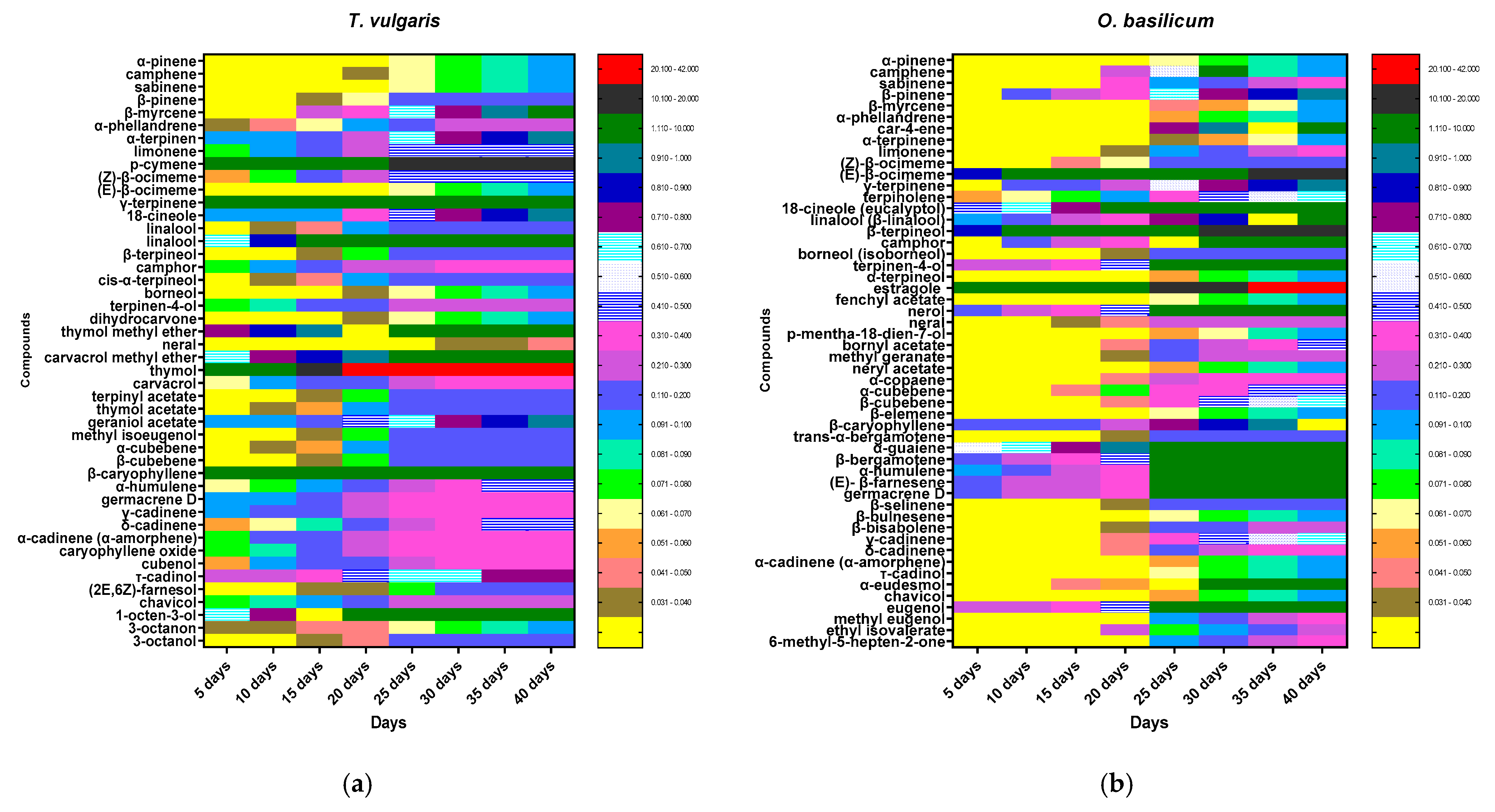
3.2. Chemical Content Analyses and Chemical Composition from Volatile Extract in O. basilicum and T. vulgaris
3.3. Insecticidal and Antifeedant Activity of Extract against Red Palm Weevil (R. ferrugineus) Adults and Larvae
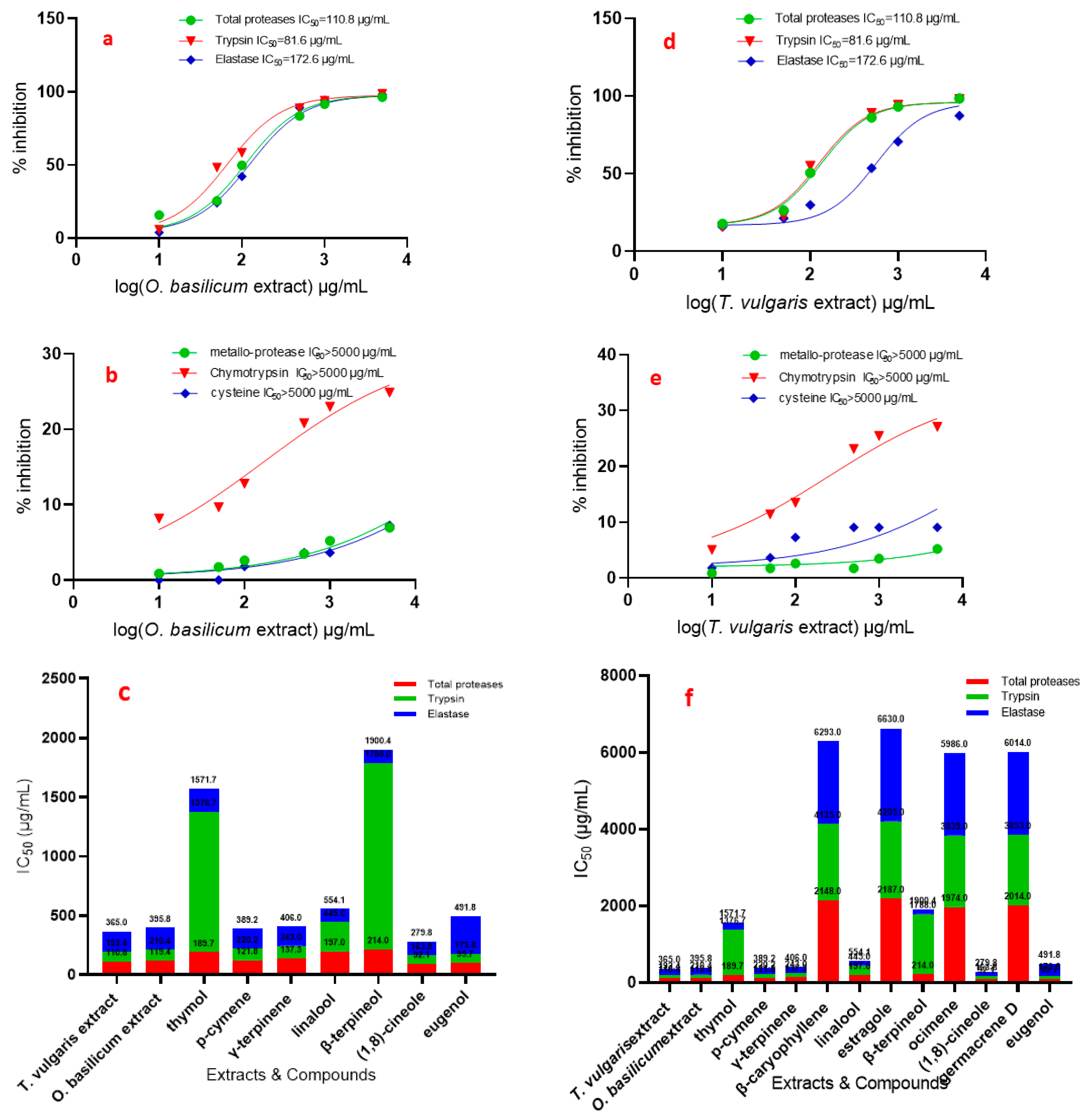
3.4. Effects of T. vulgaris and O. basilicum Extract from Cell Suspension on Serine, Cysteine, and Metalloproteinase Specific Activity Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Hadrami, A.; Al-Khayri, J.M. Socioeconomic and traditional importance of date palm. Emir. J. Food Agric. 2012, 24, 371. [Google Scholar]
- Dembilio, Ó.; Jaques, J.A. Biology and management of red palm weevil. In Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges; Springer: Heidelberg, Germany, 2015; pp. 13–36. [Google Scholar]
- El-Far, A.; Shaheen, H.; Abdel-Daim, M.; Al Jaouni, S.; Mousa, S. Date palm (Phoenix dactylifera): Protection and remedy food. Curr. Trends Nutraceuticals 2016, 1, 9. [Google Scholar]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Jabr, A.M.; Al-Ayied, H.Y. Managing invasive populations of red palm weevil: A worldwide perspective. J. Food Agric. Environ. 2013, 11, 456–463. [Google Scholar]
- Al-Saqer, S.M.; Hassan, G.M. Artificial neural networks based red palm weevil (Rynchophorus ferrugineous, Olivier) recognition system. Am. J. Agric. Biol. Sci. 2011, 6, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Al-Nujiban, A.A.; Aldosari, S.A.; Al Suhaibani, A.M.; Abdel-Azim, M.M.; Ibrahim, S.M.M.; Shukla, P. Effect of date palm cultivar on fecundity and development of Rhynchophorus ferrugineus. Bull. Insectol. 2015, 68, 199–206. [Google Scholar]
- Milosavljević, I.; El-Shafie, H.A.; Faleiro, J.R.; Hoddle, C.D.; Lewis, M.; Hoddle, M.S. Palmageddon: The wasting of ornamental palms by invasive palm weevils, Rhynchophorus spp. J. Pest. Sci. 2019, 92, 143–156. [Google Scholar] [CrossRef]
- Shehawy, A.A.; Ibrahim, M.T.; Aboutaleb, E.S.; Qari, S.H. Bioactivity and biochemical efficacy of chitinase and Justicia brandegeana extract against Red Palm Weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae). Food Sci. Nutr. 2020, 8, 4625–4636. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.M.; Miller, T.A.; Durvasula, R.; Fedoroff, N. Bridging the knowledge gaps for development of basic components of Red palm weevil IPM. In Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges; Springer: Heidelberg, Germany, 2015; pp. 37–62. [Google Scholar]
- Faleiro, J. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci. 2006, 26, 135–154. [Google Scholar]
- Downer, A.J.; Uchida, J.Y.; Hodel, D.R.; Elliott, M.L. Lethal palm diseases common in the United States. HortTechnology 2009, 19, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Kontodimas, D.; Soroker, V.; Pontikakos, C.; Suma, P.; Beaudoin-Ollivier, L.; Karamaouna, F.; Riolo, P. Visual identification and characterization of Rhynchophorus ferrugineus and Paysandisia archon infestation. Handb. Major Palm Pests Biol. Manag. 2016, 187–208. [Google Scholar]
- Wattanapongsiri, A. A Revision of the Genera Rhynchophorus and Dynamis (Coleoptera: Curculionidae). Ph.D. Thesis, Oregon State University Corvallis, Corvallis, OR, USA, 1966. [Google Scholar]
- Žďárek, J.; Howard, F.W.; Moore, D.; Giblin-Davis, R.M.; Abad, R.G. Insects on Palms. (Ecological Studies 142.). Biol. Plant. 2002, 45, 196. [Google Scholar] [CrossRef]
- Fiaboe, K.; Peterson, A.T.; Kairo, M.; Roda, A. Predicting the potential worldwide distribution of the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) using ecological niche modeling. Fla. Entomol. 2012, 95, 659–673. [Google Scholar] [CrossRef]
- Rugman-Jones, P.F.; Hoddle, C.D.; Hoddle, M.S.; Stouthamer, R. The lesser of two weevils: Molecular-genetics of pest palm weevil populations confirm Rhynchophorus vulneratus (Panzer 1798) as a valid species distinct from R. ferrugineus (Olivier 1790), and reveal the global extent of both. PLoS ONE 2013, 8, e78379. [Google Scholar] [CrossRef] [PubMed]
- Hoddle, M.; Hoddle, C. Palmageddon: The invasion of California by the South American palm weevil is underway. CAPCA Advis. 2017, 20, 40–44. [Google Scholar]
- Ahmed, F.; Hussein, K.; Gad, M. Biological activity of four plant oils, against the red palm weevil, Rhynchophorus ferrugineus (Oliver),(Coleoptera: Curculionidae). J. Biosci. Appl. Res. 2015, 1, 213–222. [Google Scholar] [CrossRef]
- Abdel-Raheem, M.; ALghamdi, H.A.; Reyad, N.F. Nano essential oils against the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae). Entomol. Res. 2020, 50, 215–220. [Google Scholar] [CrossRef]
- Cangelosi, B.; Clematis, F.; Monroy, F.; Roversi, P.F.; Troiano, R.; Curir, P.; Lanzotti, V. Filiferol, a chalconoid analogue from Washingtonia filifera possibly involved in the defence against the Red Palm Weevil Rhynchophorus ferrugineus Olivier. Phytochemistry 2015, 115, 216–221. [Google Scholar] [CrossRef]
- Orfali, R.; Binsuwaileh, A.; Al-Ala’a, H.A.; Bane-Gamea, S.; Zaidan, N.; Abdelazim, M.; Ismael, M.A.; Perveen, S.; Majrashi, N.; Alluhayb, K. Production of a biopesticide on host and Non-Host serine protease inhibitors for red palm weevil in palm trees. Saudi J. Biol. Sci. 2020, 27, 2803–2808. [Google Scholar] [CrossRef]
- Rodríguez-Sifuentes, L.; Marszalekfer, J.E.; Chuck-Hernández, C.; Serna-Saldívar, S.O. Legumes Protease Inhibitors as Biopesticides and Their Defense Mechanisms against Biotic Factors. Int. J. Mol. Sci. 2020, 21, 3322. [Google Scholar] [CrossRef]
- Saad, M.M.; Gouda, N.A.; Abdelgaleil, S.A. Bioherbicidal activity of terpenes and phenylpropenes against Echinochloa crus-galli. J. Environ. Sci. Health Part B 2019, 54, 954–963. [Google Scholar] [CrossRef]
- Guarino, S.; Colazza, S.; Peri, E.; Bue, P.L.; Germanà, M.P.; Kuznetsova, T.; Gindin, G.; Soroker, V. Behaviour-modifying compounds for management of the red palm weevil (Rhynchophorus ferrugineus Oliver). Pest. Manag. Sci. 2015, 71, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Guarino, S.; Peri, E.; Bue, P.L.; Germanà, M.P.; Colazza, S.; Anshelevich, L.; Ravid, U.; Soroker, V. Assessment of synthetic chemicals for disruption of Rhynchophorus ferrugineus response to attractant-baited traps in an urban environment. Phytoparasitica 2013, 41, 79–88. [Google Scholar] [CrossRef]
- AlJabr, A.M.; Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H. Toxicity of plant secondary metabolites modulating detoxification genes expression for natural red palm weevil pesticide development. Molecules 2017, 22, 169. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Rizwan-Ul-Haq, M.; AlJabr, A.M.; Al-Ayedh, H. Lethality of sesquiterpenes reprogramming red palm weevil detoxification mechanism for natural novel biopesticide development. Molecules 2019, 24, 1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Saudi Rosmarinus officinalis and Ocimum basilicum L. Polyphenols and Biological Activities. Processes 2020, 8, 446. [Google Scholar] [CrossRef] [Green Version]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Albohy, A.; Zahran, E.M.; Abdelmohsen, U.R.; Salem, M.A.; Al-Warhi, T.; Al-Sanea, M.M.; Abelyan, N.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A. Multitarget in silico studies of Ocimum menthiifolium, family Lamiaceae against SARS-CoV-2 supported by molecular dynamics simulation. J. Biomol. Struct. Dyn. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Abdelmohsen, U.R.; Kolkeila, A.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Anti-epileptic potential, metabolic profiling and in silico studies of the aqueous fraction from Ocimum menthiifolium benth, family Lamiaceae. Nat. Prod. Res. 2020, 1–5. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Diversity, phytochemical and medicinal potential of the genus Ocimum L. (Lamiaceae). Phytochem. Rev. 2020, 19, 907–953. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Ayoub, A.T.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Metabolic profiling, histopathological anti-ulcer study, molecular docking and molecular dynamics of ursolic acid isolated from Ocimum forskolei Benth. (family Lamiaceae). S. Afr. J. Bot. 2020, 131, 311–319. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Athar, M.T.; Al-Faraidy, A.A.; Almuhaiza, M.S. Chemical composition of essential oil of Thymus vulgaris collected from Saudi Arabian market. Asian Pac. J. Trop. Biomed. 2017, 7, 147–150. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padalia, R.; Verma, R.; Chauhan, A.; Chanotiya, C. Changes in aroma profiles of 11 Indian Ocimum taxa during plant ontogeny. Acta Physiol. Plant. 2013, 35, 2567–2587. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant. Cell Tissue Organ. Cult. (PCTOC) 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Açıkgöz, M.A. Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind. Crops Prod. 2020, 148, 112278. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific Oxford: Oxford, UK, 1994; Volume 83. [Google Scholar]
- Shukla, P.; Vidyasagar, P.; Aldosari, S.A.; Abdel-Azim, M. Antifeedant activity of three essential oils against the red palm weevil, Rhynchophorus ferrugineus. Bull. Insectol. 2012, 65, 71–76. [Google Scholar]
- Lowry, O.; Rosebrough, N.J.; Farr, A.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Olga, L.; Ibrahim, M.; Candas, N.; Koller, N.; Bauer, L.; Bulla, L. Changes in proteases activity and cry 3Aa toxin binding in the Colorado potato beetle: Implications for insect resistance to Bacillus thuringiensis toxins. Insect Biochem. Mol. Biol. 2002, 32, 567–577. [Google Scholar]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Cvikrova, M.; Hrubcova, M.; Eder, J.; Binarova, P. Changes in the levels of endogenous phenolics, aromatic monoamines, phenylalanine ammonia-lyase, peroxidase and auxin oxidase activities during initiation of alfalfa embryogenic and nonembryogenic calli. Plant. Physiol. Biochem. (Paris) 1996, 34, 853–861. [Google Scholar]
- Ross, D.C.; Brown, T.M. Inhibition of larval growth in Spodoptera frugiperda by sublethal dietary concentrations of insecticides. J. Agric. Food Chem. 1982, 30, 193–196. [Google Scholar] [CrossRef]
- Lewis, N.G.; Yamamoto, E. Lignin: Occurrence, biogenesis and biodegradation. Annu. Rev. Plant Biol. 1990, 41, 455–496. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Robbins, M.P.; Dixon, R.A. Metabolic changes in elicitor-treated bean cells: Enzymic responses associated with rapid changes in cell wall components. Eur. J. Biochem. 1985, 148, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Kefeli, V.I.; Kalevitch, M.V.; Borsari, B. Phenolic cycle in plants and environment. J. Cell Mol. Biol. 2003, 2, 13–18. [Google Scholar]
- López Arnaldos, T.; Muñoz, R.; Ferrer, M.A.; Calderón, A.A. Changes in phenol content during strawberry (Fragaria× ananassa, cv. Chandler) callus culture. Physiol. Plant. 2001, 113, 315–322. [Google Scholar] [CrossRef]
- Mato, M.; Rua, M.; Ferro, A. Changes in levels of peroxidases and phenolics during root formation in Vitis cultured in vitro. Physiol. Plant. 1988, 72, 84–88. [Google Scholar] [CrossRef]
- Pickens, C.L.; Airavaara, M.; Theberge, F.; Fanous, S.; Hope, B.T.; Shaham, Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011, 34, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Sankar, P.D. Comparison of major secondary metabolites quantified in elicited cell cultures, non-elicited cell cultures, callus cultures and field grown plants of Ocimum. Int. J. Pharm. Pharm. Sci. 2014, 6, 102–106. [Google Scholar]
- Michaud, N.R.; Fabian, J.R.; Mathes, K.D.; Morrison, D.K. 14-3-3 is not essential for Raf-1 function: Identification of Raf-1 proteins that are biologically activated in a 14-3-3-and Ras-independent manner. Mol. Cell. Biol. 1995, 15, 3390–3397. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Child, M.A.; Bogyo, M. Proteases as regulators of pathogenesis: Examples from the Apicomplexa. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2012, 1824, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Callus and Cell Suspension | TVPC (mg of Gallic Acid/g DW) | |
|---|---|---|
| Species | O. basilicum | T. vulgaris |
| Callus without infection | 7.12 ± 0.11 | 4.02 ± 0.13 |
| Callus with infection | 11.02 ± 0.15 | 6.84 ± 0.11 |
| Cell suspension without infection | 14.24 ± 0.12 | 10.04 ± 0.22 |
| Cell suspension with infection | 25.74 ± 0.20 | 15.36 ± 0.24 |
| No. | Compounds | RI (exp) | RI (lit) | T. Vulgaris | O. Basilicum |
|---|---|---|---|---|---|
| Relative Abundance % | |||||
| Monoterpene Hydrocarbons | |||||
| 1 | α-pinene | 937–934 * | 932 | 0.1 ± 0.02 | 0.1 ± 0.02 |
| 2 | camphene | 952–948 * | 946 | 0.1 ± 0.02 | 0.1± 0.02 |
| 3 | sabinene | 973–976 * | 973 | 0.1 ± 0.02 | 0.4 ± 0.06 |
| 4 | β-pinene | 977–978 * | 977 | 0.2 ± 0.02 | 1 ± 0.02 |
| 5 | β-myrcene | 991–992 * | 988 | 1.2 ± 0.2 | 0.1 ± 0.04 |
| 6 | α-phellandrene | 1005 | 1002 | 0.3 ± 0.02 | 0.1 ± 0.04 |
| 7 | car-4-ene | 1009 | 1004 | - | 1.2 ± 0.1 |
| 8 | α-terpinene | 1017–1018 * | 1014 | 1 ± 0.2 | 0.1 ± 0.1 |
| 9 | limonene | 1030 | 1224 | 0.5 ± 0.06 | 0.4 ± 0.1 |
| 10 | p-cymene | 1026–1023 * | 1023 | 17.3 ± 0.4 | - |
| 11 | (Z)-β-ocimeme | 1038–1040 * | 1032 | 0.5 ± 0.06 | 0.2 ± 0.02 |
| 12 | (E)-β-ocimeme | 1049–1050 * | 1044 | 0.1 ± 0.03 | 11.96 ± 0.2 |
| 13 | γ-terpinene | 1060–1061 * | 1067 | 9.1 ± 0.5 | 1.0 ± 0.2 |
| 14 | terpinolene | 1088 | 1086 | - | 0.7 ± 0.1 |
| Total Monoterpene Hydrocarbons Identified % | 30.5 ± 1.55 | 17.63± 1.02 | |||
| Oxygenated Monoterpenes | |||||
| 1 | 1,8-cineole (eucalyptol) | 1031–1026 * | 1031 | 1 ± 0.1 | 7.24 ± 0.4 |
| 2 | linalool oxide | 1094 | 1094 | 0.2 ± 0.04 | - |
| 3 | linalool (β-linalool) | 1099–1100 * | 1095 | 4.0 ± 0.8 | 1.2 ± 0.2 |
| 4 | β-terpineol | 1130 | 1130 | 0.2 ± 0.05 | 12.37 ±0.87 |
| 5 | camphor | 1145–1150 * | 1141 | 0.4 ± 0.03 | 1.4 ± 0.2 |
| 6 | cis-α-terpineol | 1143 | 1143 | 0.2 ± 0.04 | - |
| 7 | borneol (isoborneol) | 1167–1141 * | 1165 | 0.1 ± 0.02 | 0.2 ± 0.05 |
| 9 | terpinen-4-ol | 1177–1182 * | 1174 | 0.3 ± 0.02 | 2 ± 0.3 |
| 10 | dihydrocarvone | 1179 | 1179 | 0.1 ± 0.02 | - |
| 11 | α-terpineol | 1189 | 1186 | - | 0.1 ± 0.04 |
| 12 | estragole | 1199 | 1199 | - | 22.38 ± 0.7 |
| 13 | fenchyl acetate | 1214 | 1214 | - | 0.1 ± 0.02 |
| 14 | nerol | 1228 | 1227 | - | 1.6± 0.2 |
| 15 | thymol methyl ether | 1235–1161 * | 1235 | 1.7 ± 0.2 | - |
| 16 | neral | 1244 | 1244 | 0.05 ± 0.02 | 0.3 ± 0.1 |
| 17 | carvacrol methyl ether | 1248–1165 * | 1245 | 1.7 ± 0.1 | - |
| 18 | p-mentha-1,8-dien-7-ol | 1261 | 1261 | - | 0.1 ± 0.04 |
| 19 | thymol | 1264–1265 * | 1266 | 40.5± 0.86 | - |
| 20 | bornyl acetate | 1285 | 1284 | - | 0.5 ± 0.02 |
| 21 | carvacrol | 1299–1293 * | 1298 | 0.4 ± 0.04 | - |
| 22 | methyl geranate | 1321 | 1319 | - | 0.3 ± 0.03 |
| 23 | terpinyl acetate | 1333 | 1333 | 0.2 ± 0.03 | - |
| 24 | thymol acetate | 1349 | 1352 | 0.2 ± 0.04 | - |
| 25 | neryl acetate | 1364 | 1359 | - | 0.1 ± 0.04 |
| 26 | geraniol acetate | 1370 | 1368 | 1 ± 0.2 | - |
| 27 | methyl isoeugenol | 1492 | 1495 | 0.2 ± 0.02 | - |
| Total Oxygenated Monoterpenes Identified % | 52.45± 2.63 | 49.89± 2.88 | |||
| Sesquiterpene Hydrocarbons | |||||
| 1 | α-copaene | 1376 | 1374 | - | 0.4 ± 0.03 |
| 2 | α-cubebene | 1385–1386 * | 1387 | 0.2 ± 0.04 | 0.5 ± 0.02 |
| 3 | β-cubebene | 1389–1494 * | 1387 | 0.2 ± 0.03 | 0.7 ± 0.05 |
| 4 | β-elemene | 1391 | 1389 | - | 0.1 ± 0.03 |
| 5 | β-caryophyllene | 1424–1433 * | 1424 | 6.12 ± 0.1 | 1.1 ± 0.1 |
| 6 | trans-α-bergamotene | 1435 | 1432 | - | 0.2 ± 0.03 |
| 7 | α-guaiene | 1439 | 1437 | - | 4.8 ± 0.2 |
| 8 | β-bergamotene | 1441 | 1438 | - | 2.3 ± 0.2 |
| 9 | α-humulene | 1455 | 1452 | 0.5 ± 0.05 | 1.52 ± 0.04 |
| 10 | (E)-β-farnesene | 1457 | 1454 | - | 2.3 ± 0.08 |
| 11 | germacrene D | 1481 | 1484 | 0.4 ± 0.02 | 4.2 ± 0.2 |
| 12 | β-selinene | 1486 | 1489 | - | 0.2 ± 0.03 |
| 13 | β-bulnesene | 1505 | 1508 | - | 0.1 ± 0.02 |
| 14 | β-bisabolene | 1509 | 1512 | - | 0.3 ± 0.06 |
| 15 | γ-cadinene | 1513–1527 * | 1513 | 0.4 ± 0.03 | 0.7 ± 0.02 |
| 16 | δ-cadinene | 1525–1535 * | 1522 | 0.5 ± 0.05 | 0.4 ± 0.04 |
| 17 | α-cadinene (α-amorphene) | 1538–1487 * | 1537 | 0.4 ± 0.02 | 0.1 ± 0.02 |
| Total Sesquiterpene Hydrocarbons (SH) Identified % | 8.72 ± 0.34 | 19.92 ± 1.17 | |||
| Oxygenated Sesquiterpenes | |||||
| 1 | caryophyllene oxide | 1509–1512 * | 1507 | 0.4 ± 0.04 | - |
| 2 | cubenol | 1515 | 1514 | 0.4 ± 0.03 | - |
| 3 | τ-cadinol | 1640 | 1638 | 0.8 ± 0.05 | 0.1 ± 0.02 |
| 4 | α-eudesmol | 1653 | 1652 | - | 1.8 ± 0.1 |
| 5 | (2E,6Z)-farnesol | 1715 | 1712 | 0.2 ± 0.02 | - |
| Total Oxygenated Sesquiterpenes (OS) Identified (%) | 1.8 ± 0.14 | 1.9± 0.12 | |||
| Phenylpropanoids | |||||
| 1 | chavicol | 1256–1250 * | 1247 | 0.3 ± 0.05 | 0.1 ± 0.03 |
| 2 | eugenol | 1357 | 1356 | - | 3.7 ± 0.3 |
| 3 | methyl eugenol | 1406 | 1402 | - | 0.4 ± 0.02 |
| Total Phenylpropanoids (PP) Identified (%) | 0.3 ± 0.05 | 4.2 ± 0.35 | |||
| Non-Terpene Derivatives | |||||
| 1 | ethyl isovalerate | 853 | 856 | - | 0.3 ± 0.02 |
| 2 | 1-octen-3-ol | 981 | 981 | 1.32 ± 0.1 | - |
| 3 | 6-methyl-5-hepten-2-one | 985 | 988 | - | 0.4 ± 0.02 |
| 4 | 3-octanon | 989 | 988 | 0.1 ± 0.01 | - |
| 5 | 3-octanol | 996 | 996 | 0.2 ± 0.03 | - |
| Total Non-Terpene Derivatives (NT) Identified (%) | 1.62 ± 0.14 | 0.7 ± 0.04 | |||
| Total Identified (%) | 95.39 | 94.24 | |||
| Extract | Adult | 4th Larvae | ||||||
|---|---|---|---|---|---|---|---|---|
| LC50 (µg/mL) 95% CF | Slope | Chi Square | p | LD50 (µg/Larvae) 95% CF | Slope | Chi Square | p | |
| T. vulgaris | 1032 (891–1223) | 25.4 ± 1.41 | 51.8 | <0.01 | 11.4 (9.97–12.74) | 9.32 ± 0.64 | 47.21 | <0.01 |
| O. basilicum | 1246 (1046–1501) | 2.51 ± 0.21 | 43.7 | <0.01 | 14.6 (12.32–15.94) | 1.24 ± 0.23 | 41.58 | <0.01 |
| Inhibitors | Conc. (mM) | In Vitro (OD/mg Protein min) | |||||
|---|---|---|---|---|---|---|---|
| Total Proteases | Trypsin | Chymotrypsin | Elastase | Metallo-Protease | Cysteine Protease | ||
| Leupeptin A PMSF B TLCK C TPCK D EDTA E Iodoacetic acid F | control | 25.83 ± 0.45 | 4.10 ± 0.11 | 7.35 ± 0.02 | 8.23 ± 0.02 | 1.15 | 0.59 ± 0.05 |
| 0.01 | 0.12 ± 0.05 | 3.90 ± 0.11 | 6.97 ± 0.03 | ND | ND | 0.28 ± 0.04 | |
| 0.05 | 0.08 ± 0.05 | 3.82 ± 0.1 | 6.40 ± 0.02 | ND | ND | 0.24 ± 0.05 | |
| 0.1 | 0.07 ± 0.05 | 3.57 ± 0.1 | 6.18 ± 0.02 | 6.46 ± 0.01 | 0.89 ± 0.11 | 0.19 ± 0.04 | |
| 1 | 0.01 ± 0.05 | 3.41 ± 0.09 | 5.28 ± 0.02 | 5.26 ± 0.03 | 0.80 ± 0.11 | 0.16 ± 0.05 | |
| 10 | ND | 3.08 ± 0.1 | 4.87 ± 0.02 | 4.18 ± 0.03 | 0.68 ± 0.11 | ND | |
| 50 | ND | 2.84 ± 0.09 | 3.64 ± 0.03 | 3.98 ± 0.0 | 0.59 ± 0.1 | ND | |
| 100 | ND | 2.12 ± 0.1 | 2.02 ± 0.02 | ND | 0.42 ± 0.1 | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darrag, H.M.; Alhajhoj, M.R.; Khalil, H.E. Bio-Insecticide of Thymus vulgaris and Ocimum basilicum Extract from Cell Suspensions and Their Inhibitory Effect against Serine, Cysteine, and Metalloproteinases of the Red Palm Weevil (Rhynchophorus ferrugineus). Insects 2021, 12, 405. https://doi.org/10.3390/insects12050405
Darrag HM, Alhajhoj MR, Khalil HE. Bio-Insecticide of Thymus vulgaris and Ocimum basilicum Extract from Cell Suspensions and Their Inhibitory Effect against Serine, Cysteine, and Metalloproteinases of the Red Palm Weevil (Rhynchophorus ferrugineus). Insects. 2021; 12(5):405. https://doi.org/10.3390/insects12050405
Chicago/Turabian StyleDarrag, Hossam Moustafa, Mohammed Refdan Alhajhoj, and Hany Ezzat Khalil. 2021. "Bio-Insecticide of Thymus vulgaris and Ocimum basilicum Extract from Cell Suspensions and Their Inhibitory Effect against Serine, Cysteine, and Metalloproteinases of the Red Palm Weevil (Rhynchophorus ferrugineus)" Insects 12, no. 5: 405. https://doi.org/10.3390/insects12050405
APA StyleDarrag, H. M., Alhajhoj, M. R., & Khalil, H. E. (2021). Bio-Insecticide of Thymus vulgaris and Ocimum basilicum Extract from Cell Suspensions and Their Inhibitory Effect against Serine, Cysteine, and Metalloproteinases of the Red Palm Weevil (Rhynchophorus ferrugineus). Insects, 12(5), 405. https://doi.org/10.3390/insects12050405







