RNAi-Mediated Silencing of the Chitinase 5 Gene for Fall Webworm (Hyphantria cunea) Can Inhibit Larval Molting Depending on the Timing of dsRNA Injection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Cloning and Sequencing of the HcCht5 cDNA
2.3. Deduced Amino Acid Sequence Analysis of HcCht5
2.4. Tissue-Specific and Developmental Expression Analysis of HcCht5
2.5. RT-qPCR
2.6. dsRNA Synthesis and RNAi of HcCht5
2.7. RNA-Seq and Analysis
2.8. Statistical Analysis
3. Results
3.1. Sequence Analysis of HcCht5
3.2. Tissue-Specific and Developmental Expression of HcCht5
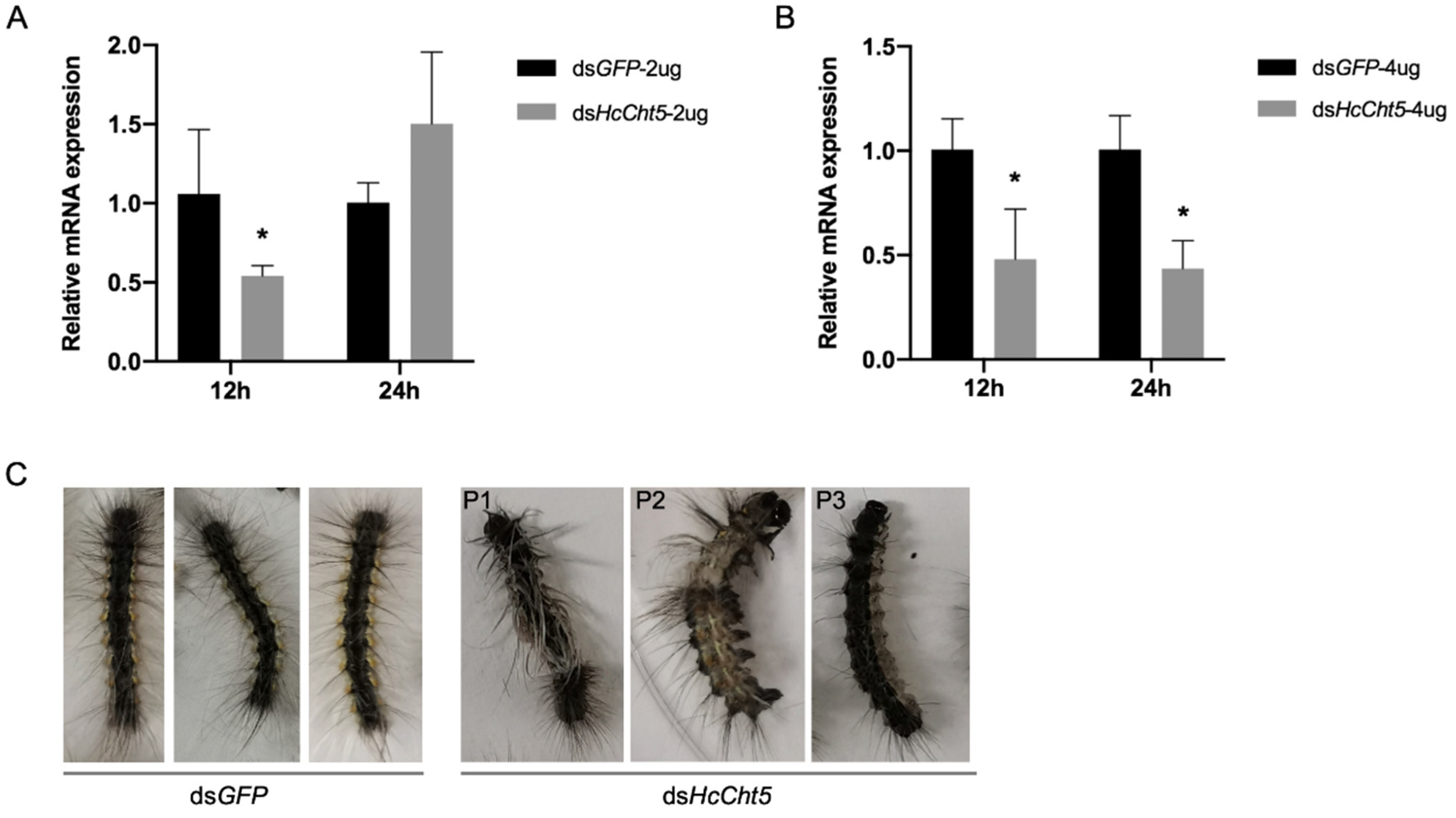
3.3. RNAi of HcCht5
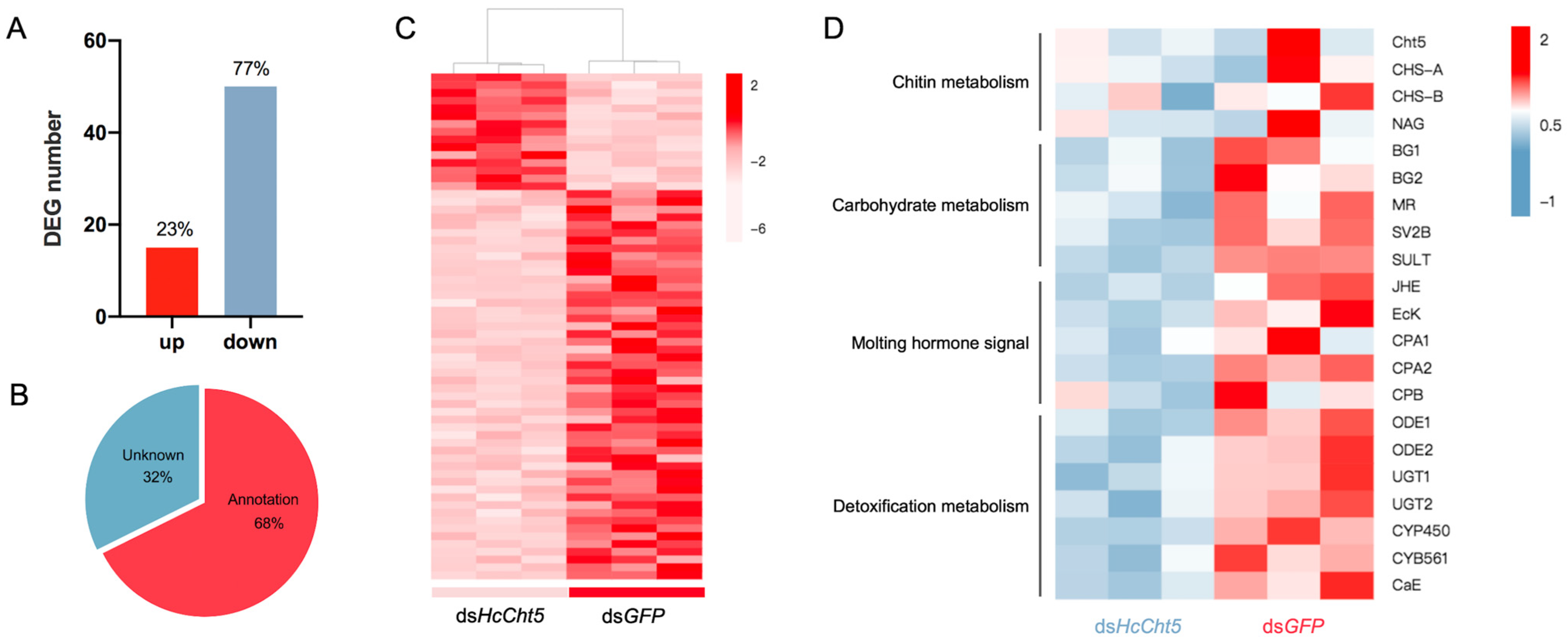
3.4. Differentially Expressed Gene (DEG) Analysis after HcCht5 RNAi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arakane, Y.; Muthukrishnan, S. Insect chitinase and chitinase-like proteins. Cell. Mol. Life Sci. 2010, 67, 201–216. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Zhu, Q.; Arakane, Y.; Beeman, R.W.; Kramer, K.J.; Muthukrishnan, S. Functional specialization among insect chitinase family genes revealed by RNA interference. Proc. Natl. Acad. Sci. USA 2008, 105, 6650–6655. [Google Scholar] [CrossRef] [Green Version]
- Kramer, K.J.; Corpuz, L.; Choi, H.K.; Muthukrishnan, S. Sequence of a cDNA and expression of the gene encoding epidermal and gut chitinases of Manduca-sexta. Insect Biochem. Mol. 1993, 23, 691–701. [Google Scholar] [CrossRef]
- Shinoda, T.; Kobayashi, J.; Matsui, M.; Chinzei, Y. Cloning and functional expression of a chitinase cDNA from the common cutworm, Spodoptera litura, using a recombinant baculovirus lacking the virus-encoded chitinase gene. Insect Biochem. Mol. 2001, 31, 521–532. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Cheng, X.; Ladd, T.; Lingohr, E.J.; Krell, P.J.; Arif, B.M.; Retnakaran, A.; Feng, Q. A molt-associated chitinase cDNA from the spruce budworm, Choristoneura fumiferana. Insect Biochem. Mol. 2002, 32, 1813–1823. [Google Scholar] [CrossRef]
- Ahmad, T.; Rajagopal, R.; Bhatnagar, R.K. Molecular characterization of chitinase from polyphagous pest Helicoverpa armigera. Biochem. Biophys. Res. Commun. 2003, 310, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, J.; Yao, Q.; Pan, Z.; Chen, J.; Zhang, W. Functional analysis of two chitinase genes during the pupation and eclosion stages of the beet armyworm Spodoptera exigua by RNA interference. Arch. Insect Biochem. 2012, 79, 220–234. [Google Scholar] [CrossRef]
- Li, Y.L.; Song, H.F.; Zhang, X.Y.; Li, D.Q.; Zhang, T.T.; Ma, E.B.; Zhang, J.Z. Heterologous expression and characterization of two chitinase 5 enzymes from the migratory locust Locusta migratoria. Insect Sci. 2016, 23, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, C.; Long, G.; Yang, H.; Chen, C.; Jin, D. Characterization and functional analysis of chitinase family genes involved in nymph-adult transition of Sogatella furcifera. Insect Sci. 2020, 1–16. [Google Scholar] [CrossRef]
- Tetreau, G.; Cao, X.; Chen, Y.R.; Muthukrishnan, S.; Jiang, H.; Blissard, G.W.; Kanost, M.R.; Wang, P. Overview of chitin metabolism enzymes in Manduca sexta: Identification, domain organization, phylogenetic analysis and gene expression. Insect Biochem. Mol. 2015, 62, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, X.; Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Ma, E.; Zhu, K.Y. Comparative genomic analysis of chitinase and chitinase-like genes in the African malaria mosquito (Anopheles gambiae). PLoS ONE 2011, 6, e19899. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Arakane, Y.; Beeman, R.W.; Kramer, K.J.; Muthukrishnan, S. Characterization of recombinant chitinase-like proteins of Drosophila melanogaster and Tribolium castaneum. Insect Biochem. Mol. 2008, 38, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, J.; Wang, Y.; Liu, X.; Ma, E.; Sun, Y.; Li, S.; Zhu, K.Y.; Zhang, J. Two chitinase 5 genes from Locusta migratoria: Molecular characteristics and functional differentiation. Insect Biochem. Mol. 2015, 58, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; He, S.; Zhu, C.; Wang, T.; Xu, Z.; Zong, S. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest. Manag. Sci. 2019, 75, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Shen, Y.; Cui, J.; Wang, A.; Li, N.; Wang, C.; Cui, B.; Sun, C.; Zhao, X.; Wang, C.; et al. Avermectin loaded carboxymethyl cellulose nanoparticles with stimuli-responsive and controlled release properties. Ind. Crop. Prod. 2020, 152, 112497. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Wei, J.R.; Wang, X.Y. Mass rearing and augmentative releases of the native parasitoid Chouioia cunea for biological control of the introduced fall webworm Hyphantria cunea in China. BioControl 2006, 51, 401–418. [Google Scholar] [CrossRef]
- Su, M.; Fang, Y.; Tao, W.; Yan, G.; Ma Wan, E.; Zhang, Z. Identification and field evaluation of the sex pheromone of an invasive pest, the fall webworm Hyphantria cunea in China. Chin. Sci. Bull. 2008, 53, 555–560. [Google Scholar] [CrossRef]
- Zhang, L.W.; Kang, K.; Liu, Y.J.; Zhang, J.; Sun, L.; Zhan, C.; Huang, C.C.; Jiang, L.Y.; Ye, K.Y.; Ding, D.G. Evaluation of Beauveria bassiana isolates as potential agents for control of Hyphantria cunea (Lepidoptera Arctiidae). Acta Entomol. Sin. 2016, 59, 111–118. [Google Scholar]
- Deng, Y.; Li, F.; Rieske, L.K.; Sun, L.L.; Sun, S.H. Transcriptome sequencing for identification of diapause-associated genes in fall webworm, Hyphantria cunea Drury. Gene 2018, 668, 229–236. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, S.; Li, X.; Cao, Y.; Liu, X.; Wang, Q.; Liu, Q.; Liu, H.; Hu, X.; Zhou, X.J.; et al. Fall webworm genomes yield insights into rapid adaptation of invasive species. Nat. Ecol. Evol. 2019, 3, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, H.; Wen, M.; Li, J.; Zhou, H.; Wang, J.; Zhou, Y.; Liu, Y.; Du, L.; Kang, H.; et al. Genome of the webworm Hyphantria cunea unveils genetic adaptations supporting its rapid invasion and spread. BMC Genom. 2020, 21, 242. [Google Scholar] [CrossRef]
- Zhu, B.; Shan, J.; Li, R.; Liang, P.; Gao, X. Identification and RNAi-based function analysis of chitinase family genes in diamondback moth, Plutella xylostella. Pest. Manag. Sci. 2019, 75, 1951–1961. [Google Scholar] [CrossRef]
- Omar, M.A.A.; Ao, Y.; Li, M.; He, K.; Xu, L.; Tong, H.; Jiang, M.; Li, F. The functional difference of eight chitinase genes between male and female of the cotton mealybug, Phenacoccus solenopsis. Insect Mol. Biol. 2019, 28, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Shin, S.W.; Bae, K.S.; Kim, S.C.; Park, H.Y. Molecular cloning of chitinase cDNAs from the silkworm, Bombyx mori and the fall webworm, Hyphantria cunea. Insect Biochem. Mol. 1998, 28, 163–171. [Google Scholar] [CrossRef]
- Chenna, R.; Sugawara, H.; Koike, T.; Lopez, R.; Gibson, T.J.; Higgins, D.G.; Thompson, J.D. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003, 31, 3497–3500. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.Z.; Zhang, X.; Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Ma, E.; Zhu, K.Y. Identification and characterization of a novel chitinase-like gene cluster (AgCht5) possibly derived from tandem duplications in the African malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. 2011, 41, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Kramer, K.J.; Muthukrishnan, S. Insect chitinases: Molecular biology and potential use as biopesticides. Insect Biochem. Mol. 1997, 27, 887–900. [Google Scholar] [CrossRef]
- Funkhouser, J.D.; Aronson, N.N., Jr. Chitinase family GH18: Evolutionary insights from the genomic history of a diverse protein family. BMC Evol. Biol. 2007, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Pan, P.L.; Ye, Y.X.; Yu, B.; Xu, H.J.; Zhang, C.X. Chitinase-like gene family in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2015, 24, 29–40. [Google Scholar] [CrossRef]
- Scott, J.G.; Michel, K.; Bartholomay, L.C.; Siegfried, B.D.; Hunter, W.B.; Smagghe, G.; Zhu, K.Y.; Douglas, A.E. Towards the elements of successful insect RNAi. J. Insect Physiol. 2013, 59, 1212–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.G.; Robinson, K.E.; Fletcher, S.J.; Mitter, N. RNAi-Based functional genomics in hemiptera. Insects 2020, 11, 557. [Google Scholar] [CrossRef]
- Jaubert-Possamai, S.; Le Trionnaire, G.; Bonhomme, J.; Christophides, G.K.; Rispe, C.; Tagu, D. Gene knockdown by RNAi in the pea aphid Acyrthosiphon pisum. BMC Biotechnol. 2007, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Vogel, H.; Heckel, D.G. Comparative analysis of the UDP-glycosyltransferase multigene family in insects. Insect Biochem. Mol. 2012, 42, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Insect Biochem. Mol. 2007, 52, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Wojtasek, H.; Leal, W.S. Degradation of an alkaloid pheromone from the pale-brown chafer, Phyllopertha diversa (Coleoptera: Scarabaeidae), by an insect olfactory cytochrome P450. FEBS Lett. 1999, 458, 333–336. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.X.; Wang, W.L.; Li, M.Y.; Li, S.G.; Liu, S. Identification of putative carboxylesterase and aldehyde oxidase genes from the antennae of the rice leaffolder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). J. Asia-Pac. Entomol. 2017, 20, 907–913. [Google Scholar] [CrossRef]
- Huang, F.F.; Chai, C.L.; Zhang, Z.; Liu, Z.H.; Dai, F.Y.; Lu, C.; Xiang, Z.H. The UDP-glucosyltransferase multigene family in Bombyx mori. BMC Genom. 2008, 9, 563. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, T.L.; Kramer, K.J. Insect cuticle sclerotization. Annu. Rev. Entomol. 1992, 37, 273–302. [Google Scholar] [CrossRef]
- Kenney, E.; Yaparla, A.; Hawdon, J.M.; O’Halloran, D.M.; Grayfer, L.; Eleftherianos, I. A putative UDP-glycosyltransferase from Heterorhabditis bacteriophora suppresses antimicrobial peptide gene expression and factors related to ecdysone signaling. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- He, P.; Durand, N.; Dong, S.L. Editorial: Insect olfactory proteins (from gene identification to functional characterization). Front. Physiol. 2019, 10, 1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Lu, A.; Kong, L.; Zhang, Q.; Ling, E. Functional analysis of insect molting fluid proteins on the protection and regulation of ecdysis. J. Asia-Pac. Entomol. 2014, 289, 35891–35906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.Y.; Lu, C.; Li, W.L.; Xiang, Z.H.; Zhang, Z. Annotation and expression of carboxylesterases in the silkworm, Bombyx mori. BMC Genom. 2009, 10, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barski, O.A.; Tipparaju, S.M.; Bhatnagar, A. The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab. Rev. 2008, 40, 553–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Treatment 1 | dsGFP | dsHcCht5 | dsGFP | dsHcCht5 |
|---|---|---|---|---|
| 2 ug/Larva | 4 ug/Larva | |||
| Abnormal rate | ND | 33.3% | ND | 66.7% |
| Mortality rate | ND | 33.3% | ND | 66.7% |
| Treatment 1 | dsGFP | dsHcCht5 | |
|---|---|---|---|
| Abnormal rate | L4D1 | ND | ND |
| L4D2 | ND | ND | |
| L4D3 | ND | ND | |
| L4D4 | ND | 10.0% | |
| L4D5 | ND | 26.7% | |
| Mortality rate | L4D1 | ND | ND |
| L4D2 | ND | ND | |
| L4D3 | ND | ND | |
| L4D4 | ND | 10.0% | |
| L4D5 | ND | 26.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, Y.; Zhang, S.; Kong, X.; Liu, F.; Zhang, Z. RNAi-Mediated Silencing of the Chitinase 5 Gene for Fall Webworm (Hyphantria cunea) Can Inhibit Larval Molting Depending on the Timing of dsRNA Injection. Insects 2021, 12, 406. https://doi.org/10.3390/insects12050406
Zhang X, Wang Y, Zhang S, Kong X, Liu F, Zhang Z. RNAi-Mediated Silencing of the Chitinase 5 Gene for Fall Webworm (Hyphantria cunea) Can Inhibit Larval Molting Depending on the Timing of dsRNA Injection. Insects. 2021; 12(5):406. https://doi.org/10.3390/insects12050406
Chicago/Turabian StyleZhang, Xun, Yue Wang, Sufang Zhang, Xiangbo Kong, Fu Liu, and Zhen Zhang. 2021. "RNAi-Mediated Silencing of the Chitinase 5 Gene for Fall Webworm (Hyphantria cunea) Can Inhibit Larval Molting Depending on the Timing of dsRNA Injection" Insects 12, no. 5: 406. https://doi.org/10.3390/insects12050406
APA StyleZhang, X., Wang, Y., Zhang, S., Kong, X., Liu, F., & Zhang, Z. (2021). RNAi-Mediated Silencing of the Chitinase 5 Gene for Fall Webworm (Hyphantria cunea) Can Inhibit Larval Molting Depending on the Timing of dsRNA Injection. Insects, 12(5), 406. https://doi.org/10.3390/insects12050406







