The Diversity and Dynamics of Fungi in Dryocosmus kuriphilus Community
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Total DNA Extraction and PCR Amplification
2.3. Library Construction and High-Throughput Sequencing
2.4. Bioinformatics Analysis
2.5. Statistical Analysis
3. Results
3.1. The Fungal Community Composition of D. kuriphilus Adults, Associated Insect Galls and the Galled Twigs of C. mollissima
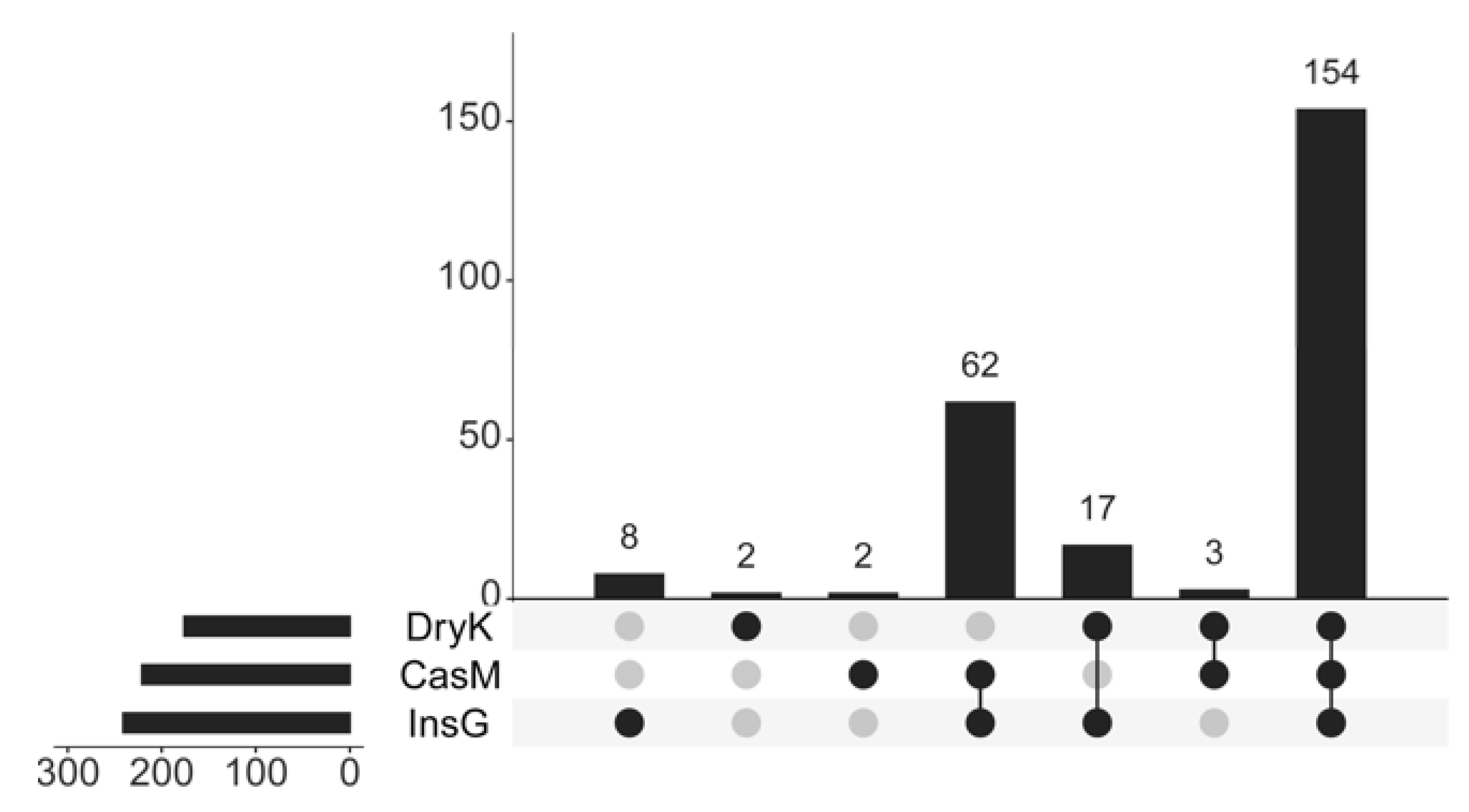
3.2. The Unique and Common Fungi of D. kuriphilus Adults, Associated Insect Galls and the Galled Twigs of C. mollissima
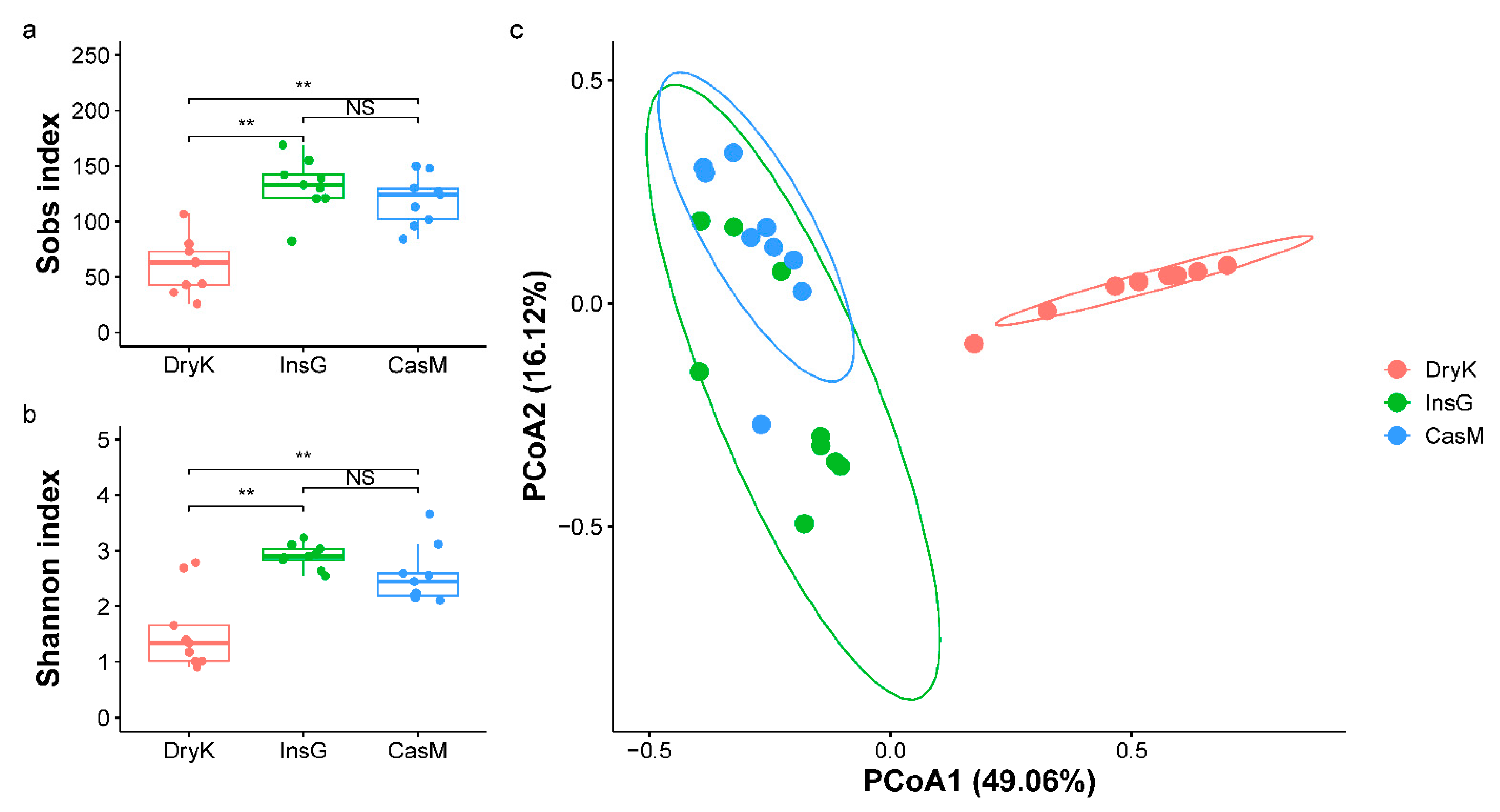
3.3. The Richness and α-Diversity at the Species Level of the Fungal Communities of D. kuriphilus Adults, Associated Insect Galls and the Galled Twigs of C. mollissima
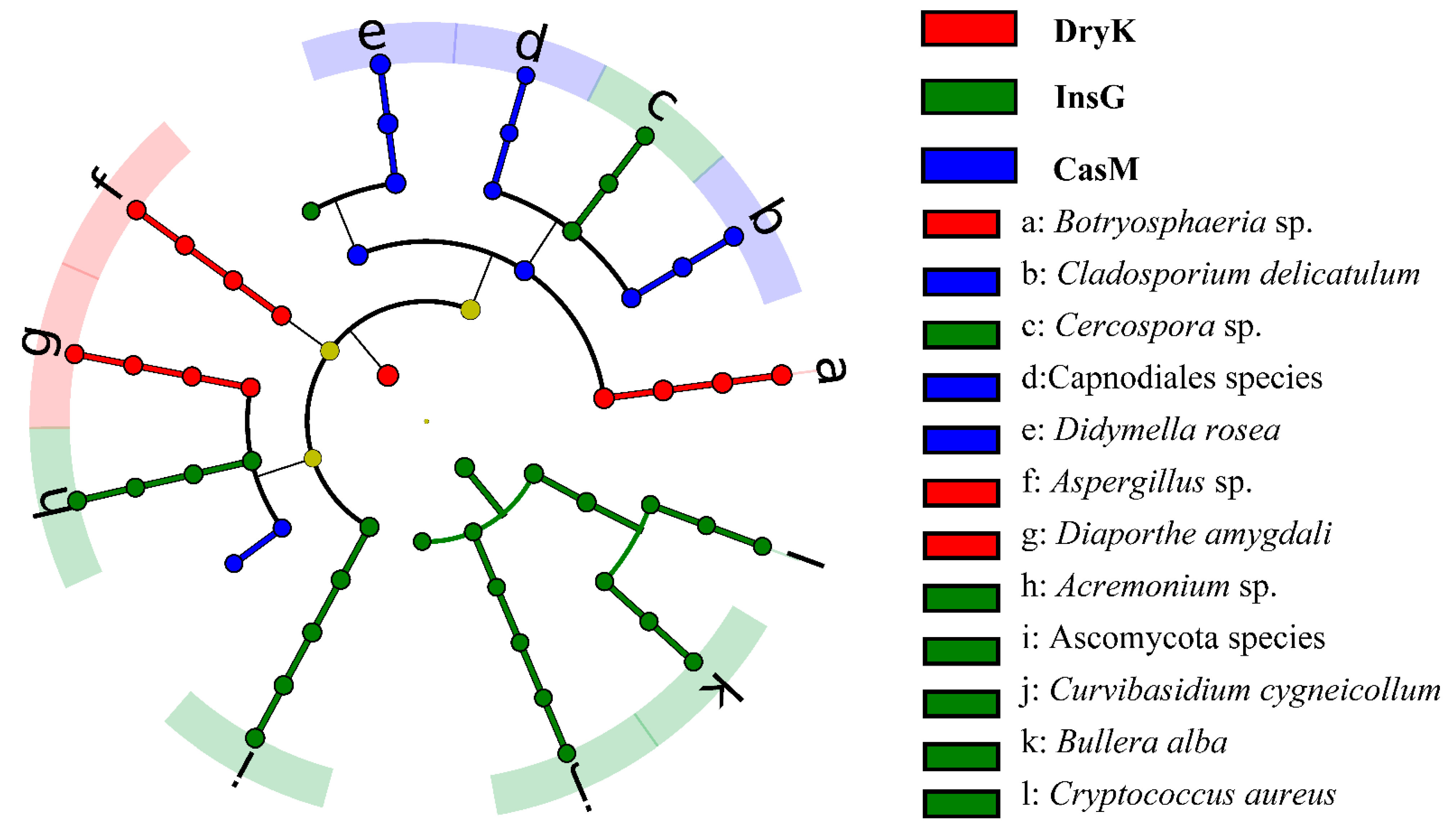
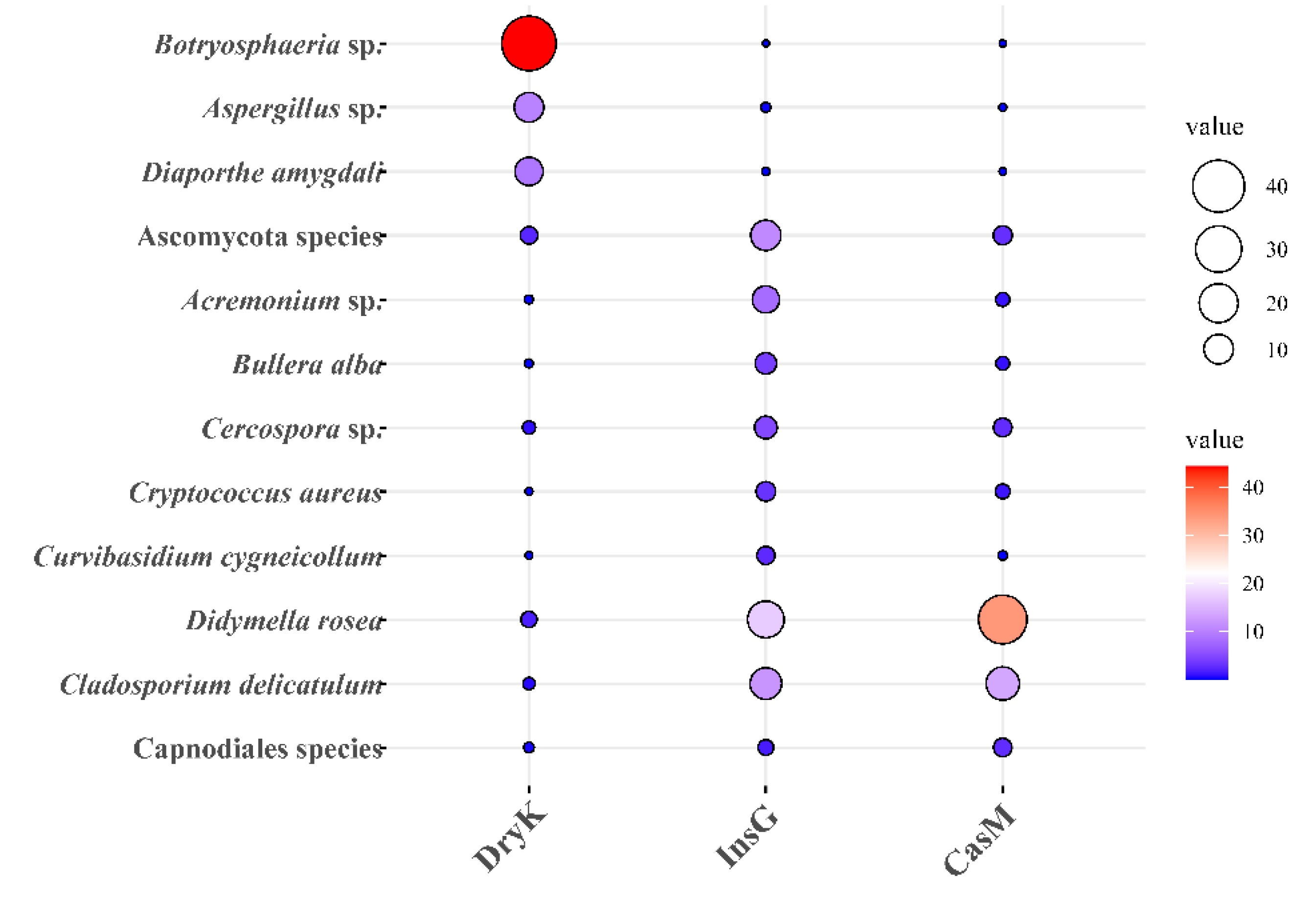
3.4. The Predominant Fungal Species of D. kuriphilus Adults, Associated Insect Galls and the Galled Twigs of C. mollissima
4. Discussion
4.1. The Possibility of Fungal Horizontal Transmission among D. kuriphilus Adults, Associated Insect Galls and the Galled Twigs of C. mollissima
4.2. The Differences in Fungal Community Structure among D. kuriphilus Adults, Associated Insect Galls and the Galled Twigs of C. mollissima
4.3. D. kuriphilus Adults as Potential Vectors of PLANT Pathogens
4.4. The Predominant Fungi in D. kuriphilus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giron, D.; Huguet, E.; Stone, G.N.; Body, M. Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J. Insect Physiol. 2016, 84, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.O.; Pitzschke, A. Plants make galls to accommodate foreigners: Some are friends, most are foes. New Phytol. 2020, 225, 1852–1872. [Google Scholar] [CrossRef]
- Oates, C.N.; Denby, K.J.; Myburg, A.A.; Slippers, B.; Naidoo, S. Insect gallers and their plant hosts: From omics data to systems biology. Int. J. Mol. Sci. 2016, 17, 1891. [Google Scholar] [CrossRef] [Green Version]
- Dodueva, I.E.; Lebedeva, M.A.; Kuznetsova, K.A.; Gancheva, M.S.; Paponova, S.S.; Lutova, L.L. Plant tumors: A hundred years of study. Planta 2020, 251, 82. [Google Scholar] [CrossRef] [PubMed]
- Espíritosanto, M.M.; Fernandes, G.W. How many species of gall-inducing insects are there on earth, and where are they? Ann. Entomol. Soc. Am. 2007, 100, 95–99. [Google Scholar]
- Sevarika, M.; Stacconi, M.V.R.; Romani, R.J.I. Fine morphology of antennal and ovipositor sensory structures of the gall chestnut wasp, Dryocosmus kuriphilus. Insects 2021, 12, 231. [Google Scholar] [CrossRef]
- Battisti, A.; Benvegnù, I.; Colombari, F.; Haack, R.A. Invasion by the chestnut gall wasp in Italy causes significant yield loss in Castanea sativa nut production. Agric. For. Entomol. 2014, 16, 75–79. [Google Scholar] [CrossRef]
- Cooper, W.R.; Rieske, L.K. Community associates of an exotic gallmaker, Dryocosmus kuriphilus (Hymenoptera: Cynipidae), in Eastern North America. Ann. Entomol. Soc. Am. 2007, 100, 236–244. [Google Scholar] [CrossRef]
- Avtzis, D.N.; Melika, G.; Matošević, D.; Coyle, D.R. The Asian chestnut gall wasp Dryocosmus kuriphilus: A global invader and a successful case of classical biological control. J. Pest Sci. 2019, 92, 107–115. [Google Scholar] [CrossRef]
- Sartor, C.; Dini, F.; Marinoni, D.T.; Mellano, M.G.; Beccaro, G.L.; Alma, A.; Quacchia, A.; Botta, R. Impact of the Asian wasp Dryocosmus kuriphilus (Yasumatsu) on cultivated chestnut: Yield loss and cultivar susceptibility. Sci. Hortic. 2015, 197, 454–460. [Google Scholar] [CrossRef]
- Bernardo, U.; Iodice, L.; Sasso, R.; Tutore, V.A.; Cascone, P.; Guerrieri, E. Biology and monitoring of Dryocosmus kuriphilus on Castanea sativa in Southern Italy. Agric. Entomol. 2013, 15, 65–76. [Google Scholar] [CrossRef]
- Gehring, E.; Bellosi, B.; Reynaud, N.; Conedera, M. Chestnut tree damage evolution due to Dryocosmus kuriphilus attacks. J. Pest Sci. 2020, 93, 103–115. [Google Scholar] [CrossRef]
- Haiden, S.; Hoffmann, J.; Cramer, M. Benefits of photosynthesis for insects in galls. Oecologia 2012, 170, 987–997. [Google Scholar] [CrossRef]
- Biedermann, P.H.W.; Vega, F.E. Ecology and evolution of insect-fungus mutualisms. Annu. Rev. Entomol. 2020, 65, 431–455. [Google Scholar] [CrossRef] [Green Version]
- Raman, A.; Suryanarayanan, T.S. Fungus–plant interaction influences plant-feeding insects. Fungal Ecol. 2017, 29, 123–132. [Google Scholar] [CrossRef]
- Wilson, D. Fungal endophytes which invade insect galls: Insect pathogens, benign saprophytes, or fungal inquilines? Oecologia 1995, 103, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Magro, P.; Speranza, S.; Stacchiotti, M.; Martignoni, D.; Paparatti, B. Gnomoniopsis associated with necrosis of leaves and chestnut galls induced by Dryocosmus kuriphilus. Plant Pathol. 2010, 59, 1171. [Google Scholar] [CrossRef]
- Raman, A.; Wheatley, W.; Popay, A. Endophytic fungus-vascular plant-insect interactions. Environ. Entomol. 2012, 41, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.N.; Schönrogge, K.; Atkinson, R.J.; Bellido, D.; Pujade-Villar, J. The population biology of oak gall wasps (Hymenoptera: Cynipidae). Annu. Rev. Entomol. 2002, 47, 633–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amatuzzi, R.F.; Cardoso, N.; Poltronieri, A.S.; Poitevin, C.G.; Dalzoto, P.; Zawadeneak, M.A.; Pimentel, I.C. Potential of endophytic fungi as biocontrol agents of Duponchelia fovealis (Zeller) (Lepidoptera: Crambidae). Braz. J. Biol. 2018, 38, 429–435. [Google Scholar]
- LaBonte, N.R.; Jacobs, J.; Ebrahimi, A.; Lawson, S.; Woeste, K. Data mining for discovery of endophytic and epiphytic fungal diversity in short-read genomic data from deciduous trees. Fungal Ecol. 2018, 35, 1–9. [Google Scholar] [CrossRef]
- Kolp, M.; Double, M.L.; Fulbright, D.W.; Macdonald, W.L.; Jarosz, A.M. Spatial and temporal dynamics of the fungal community of chestnut blight cankers on American chestnut (Castanea dentata) in Michigan and Wisconsin. Fungal Ecol. 2020, 45, 1–11. [Google Scholar] [CrossRef]
- Gaffuri, F.; Maresi, G.; Pedrazzoli, F.; Longa, C.M.O.; Boriani, M.; Molinari, M.; Tantardini, A.; Sieber, T. Colletotrichum acutatum associated with Dryocosmus kuriphilus galls on Castanea Sativa. For. Pathol. 2015, 45, 169–171. [Google Scholar] [CrossRef]
- Fernandez-Conradi, P.; Fort, T.; Castagneyrol, B.; Jactel, H.; Robin, C. Fungal endophyte communities differ between chestnut galls and surrounding foliar tissues. Fungal Ecol. 2019, 42, 1–8. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Daniel, R.; María, C.; Julio Javier, D.; Maria Mercedes Fernandez, F. Fungal community of necrotic and healthy galls in chestnut trees colonized by Dryocosmus kuriphilus (Hymenoptera, Cynipidae). Iforest Biogeosci. For. 2019, 12, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Prospero, S.; Forster, B. Chestnut gall wasp (Dryocosmus kuriphilus) infestations: New opportunities for the chestnut blight fungus Cryphonectria parasitica? New Dis. Rep. 2011, 23, 35. [Google Scholar] [CrossRef] [Green Version]
- Lione, G.; Giordano, L.; Ferracini, C.; Alma, A.; Gonthier, P. Testing ecological interactions between Gnomoniopsis castaneae and Dryocosmus kuriphilus. Acta Oecol. 2016, 77, 10–17. [Google Scholar] [CrossRef]
- Vannini, A.; Vettraino, A.; Martignoni, D.; Morales-Rodriguez, C.; Contarini, M.; Caccia, R.; Paparatti, B.; Speranza, S. Does Gnomoniopsis castanea contribute to the natural biological control of chestnut gall wasp? Fungal Biol. 2017, 121, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Addario, E.; Turchetti, T. Parasitic fungi on Dryocosmus kuriphilus in Castanea sativa necrotic galls. Bull. Insectol. 2011, 64, 269–273. [Google Scholar]
- Morales-Rodriguez, C.; Sferrazza, I.; Aleandri, M.; Dalla Valle, M.; Mazzetto, T.; Speranza, S.; Contarini, M.; Vannini, A. Fungal community associated with adults of the chestnut gall wasp Dryocosmus kuriphilus after emergence from galls: Taxonomy and functional ecology. Fungal Biol. 2019, 123, 905–912. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package ‘vegan’. Community Ecol. Package 2013, 2, 1–295. [Google Scholar]
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Driss, J.O.E. Chestnut Rots: Disease Incidence and Molecular Identification of Causal Agents. Master’s Thesis, Université Librede Tunis, Tunis, Tunisia, 2019. [Google Scholar]
- Vinale, F.; Ruocco, M.; Manganiello, G.; Guerrieri, E.; Bernardo, U.; Mazzei, P.; Piccolo, A.; Sannino, F.; Caira, S.; Woo, S.L. Metabolites produced by Gnomoniopsis castanea associated with necrosis of chestnut galls. Chem. Biol. Technol. Agric. 2014, 1, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Seddaiu, S.; Cerboneschi, A.; Sechi, C.; Mello, A. Gnomoniopsis castaneae associated with Dryocosmus kuriphilus galls in chestnut stands in Sardinia (Italy). Iforest Biogeosci. For. 2017, 10, 440–445. [Google Scholar] [CrossRef]
- Egan, S.P.; Hood, G.R.; Martinson, E.O.; Ott, J.R. Cynipid gall wasps. Curr. Biol. 2018, 28, R1370–R1374. [Google Scholar] [CrossRef] [Green Version]
- Fay, P.A.; Hartnett, D.C.; Knapp, A.K. Increased photosynthesis and water potentials in Silphium integrifolium galled by cynipid wasps. Oecologia 1993, 93, 114–120. [Google Scholar] [CrossRef]
- Reale, L.; Tedeschini, E.; Rondoni, G.; Ricci, C.; Bin, F.; Frenguelli, G.; Ferranti, F. Histological investigation on gall development induced by a worldwide invasive pest, Dryocosmus kuriphilus, on Castanea sativa. Plant Biosyst. 2016, 150, 35–42. [Google Scholar] [CrossRef]
- Sachs, I.B. Penetration and degradation of cell walls in oaks infected with Ceratocystis fagacearum. Phytopathology 1970, 60, 1399. [Google Scholar] [CrossRef]
- Juzwik, J.; Harrington, T.C.; Macdonald, W.L.; Appel, D.N. The origin of Ceratocystis fagacearum, the oak wilt fungus. Annu. Rev. Phytopathol. 2008, 46, 13–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, E.; Bellosi, B.; Quacchia, A.; Conedera, M. Assessing the impact of Dryocosmus kuriphilus on the chestnut tree: Branch architecture matters. J. Pest Sci. 2018, 91, 189–202. [Google Scholar] [CrossRef]
- Álvarez, R.; Encina, A.; Pérez Hidalgo, N. Histological aspects of three Pistacia terebinthus galls induced by three different aphids: Paracletus cimiciformis, Forda marginata and Forda formicaria. Plant Sci. 2009, 176, 303–314. [Google Scholar] [CrossRef]
- De Oliveira, D.C.; da Silva Carneiro, R.G.; Magalhães, T.A.; dos Santos Isaias, R.M. Cytological and histochemical gradients on two Copaifera langsdorffii Desf. (Fabaceae)—Cecidomyiidae gall systems. Protoplasma 2011, 248, 829–837. [Google Scholar] [CrossRef]
- Lawson, S.P.; Christian, N.; Abbot, P. Comparative analysis of the biodiversity of fungal endophytes in insect-induced galls and surrounding foliar tissue. Fungal Divers. 2014, 66, 89–97. [Google Scholar] [CrossRef]
- Washburn, G.; Van Bael, S.A. Fungal diversity in galls of baldcypress trees. Fungal Ecol. 2017, 29, 85–89. [Google Scholar] [CrossRef]
- Allison, S.D.; Schultz, J.C. Biochemical responses of chestnut oak to a galling cynipid. J. Chem. Ecol. 2005, 31, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Ikai, N.; Hijii, N. Manipulation of tannins in oaks by galling cynipids. J. Res. 2007, 12, 316–319. [Google Scholar] [CrossRef]
- Koncz, N.K.; Szabó, L.J.; Máthé, C.; Jámbrik, K.; M-Hamvas, M.; Koncz, N.K.; Jámbrik, K.; M-Hamvas, M. Histological study of quercus galls of Neuroterus quercusbaccarum (Linnaeus, 1758) (Hymenoptera: Cynipidae). Acta Biol. Szeged 2011, 55, 247–253. [Google Scholar]
- Li, X.-M.; Yang, X.-H. Comparison of the contents or activities of nutritional and defensive substances between the larval galls and host plants of Dryocosmus kuriphilus. Life Sci. Res. 2019, 23, 214–218. [Google Scholar]
- Yang, X.-H.; Li, X.-M.; Zhu, D.-H. Alteration of free amino acid concentrations in insect galls induced by Andricus mukaigawae (Hymenoptera; Cynipidae). Ecol. Entomol. 2020, 45, 1–10. [Google Scholar] [CrossRef]
- Cornell, H.V. The secondary chemistry and complex morphology of galls formed by the Cynipinae (Hymenoptera): Why and how? Am. Midl. Nat. 1983, 110, 225–234. [Google Scholar] [CrossRef]
- Hearn, J.; Blaxter, M.; Schönrogge, K.; Nieves-Aldrey, J.-L.; Pujade-Villar, J.; Huguet, E.; Drezen, J.-M.; Shorthouse, J.D.; Stone, G.N. Genomic dissection of an extended phenotype: Oak galling by a cynipid gall wasp. PLoS Genet. 2019, 15, e1008398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Miyauchi, S.; Rancon, A.; Drula, E.; Hage, H.; Chaduli, D.; Favel, A.; Grisel, S.; Henrissat, B.; Herpoël-Gimbert, I.; Ruiz-Dueñas, F.J.; et al. Integrative visual omics of the white-rot fungus Polyporus brumalis exposes the biotechnological potential of its oxidative enzymes for delignifying raw plant biomass. Biotechnol. Biofuels 2018, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Van Baarlen, P.; Van Belkum, A.; Summerbell, R.C.; Crous, P.W.; Thomma, B.P.H.J. Molecular mechanisms of pathogenicity: How do pathogenic microorganisms develop cross-kingdom host jumps? FEMS Microbiol. Rev. 2007, 31, 239–277. [Google Scholar] [CrossRef] [Green Version]
- Tack, A.J.M.; Gripenberg, S.; Roslin, T. Cross-kingdom interactions matter: Fungal-mediated interactions structure an insect community on oak. Ecol. Lett. 2012, 15, 177–185. [Google Scholar] [CrossRef]
- St Leger, R.J.; Screen, S.E.; Shams-Pirzadeh, B. Lack of host specialization in Aspergillus flavus. Appl. Environ. Microbiol. 2000, 66, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Gomes, R.R.; Glienke, C.; Videira, S.I.R.; Lombard, L.; Groenewald, J.Z.; Crous, P.W. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 2013, 31, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Marsberg, A.; Kemler, M.; Jami, F.; Nagel, J.H.; Postma-Smidt, A.; Naidoo, S.; Wingfield, M.J.; Crous, P.W.; Spatafora, J.W.; Hesse, C.N.; et al. Botryosphaeria dothidea: A latent pathogen of global importance to woody plant health. Mol. Plant Pathol. 2017, 18, 477–488. [Google Scholar] [CrossRef]
- Overy, D.P.; Seifert, K.A.; Savard, M.E.; Frisvad, J.C. Spoilage fungi and their mycotoxins in commercially marketed chestnuts. Int. J. Food Microbiol. 2003, 88, 69–77. [Google Scholar] [CrossRef]
- Donis-González, I.R.; Guyer, D.E.; Fulbright, D.W. Quantification and identification of microorganisms found on shell and kernel of fresh edible chestnuts in Michigan. J. Sci. Food Agric. 2016, 96, 4514–4522. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Yu, C.; Wang, C. First report of shoot canker on chestnut caused by Diaporthe nobilis in shandong province of China. Plant Dis. 2018, 102, 2376. [Google Scholar] [CrossRef]
- Meyer, J.B.; Laure, G.; Simone, P. Interaction between two invasive organisms on the European chestnut: Does the chestnut blight fungus benefit from the presence of the gall wasp? FEMS Microbiol. Ecol. 2015, 91, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimowska, B.; Okoń, S.; Becchimanzi, A.; Krol, E.D.; Nicoletti, R. Phylogenetic characterization of Botryosphaeria strains associated with Asphondylia galls on species of Lamiaceae. Diversity 2020, 12, 41. [Google Scholar] [CrossRef] [Green Version]
- Panzavolta, T.; Panichi, A.; Bracalini, M.; Croci, F.; Ginetti, B.; Ragazzi, A.; Tiberi, R.; Moricca, S. Dispersal and propagule pressure of Botryosphaeriaceae species in a declining oak stand is affected by insect vectors. Forests 2017, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Panzavolta, T.; Panichi, A.; Bracalini, M.; Croci, F.; Benigno, A.; Ragazzi, A.; Tiberi, R.; Moricca, S. Tree pathogens and their insect-mediated transport: Implications for oak tree die-off in a natural park area. Glob. Ecol. Conserv. 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Raman, A. Insect-induced plant galls of India: Unresolved questions. Curr. Sci. 2007, 92, 748–757. [Google Scholar]
- Taper, M.; Case, T. Interactions between oak tannins and parasite community structure: Unexpected benefits of tannins to cynipid gall-wasps. Oecologia 1987, 71, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Bezos, D.; Diez, J. Fungi associated with necrotic galls of Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in northern Spain. Silva Fenn. 2018, 52, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Heneberg, P.; Bizos, J.; Čmoková, A.; Kolařík, M.; Astapenková, A.; Bogusch, P. Assemblage of filamentous fungi associated with aculeate Hymenopteran brood in reed galls. J. Invertebr. Pathol. 2016, 133, 95–106. [Google Scholar] [CrossRef]
- Karthi, S.; Vaideki, K.; Shivakumar, M.S.; Ponsankar, A.; Thanigaivel, A.; Chellappandian, M.; Vasantha-Srinivasan, P.; Muthu-Pandian, C.K.; Hunter, W.B.; Senthil-Nathan, S. Effect of Aspergillus flavus on the mortality and activity of antioxidant enzymes of Spodoptera litura Fab. (Lepidoptera: Noctuidae) larvae. Pestic. Biochem. Physiol. 2018, 149, 54–60. [Google Scholar] [CrossRef]
- Mukasa, Y.; Kyamanywa, S.; Sserumaga, J.P.; Otim, M.; Tumuhaise, V.; Erbaugh, M.; Egonyu, J.P. An atoxigenic L-strain of Aspergillus flavus (Eurotiales: Trichocomaceae) is pathogenic to the coffee twig borer, Xylosandrus compactus (Coleoptera: Curculionidea: Scolytinae). Environ. Microbiol. Rep. 2019, 11, 508–517. [Google Scholar] [CrossRef]
- Bawin, T.; Seye, F.; Boukraa, S.; Zimmer, J.-Y.; Raharimalala, F.N.; Ndiaye, M.; Compere, P.; Delvigne, F.; Francis, F. Histopathological effects of Aspergillus clavatus (Ascomycota: Trichocomaceae) on larvae of the southern house mosquito, Culex quinquefasciatus (Diptera: Culicidae). Fungal Biol. 2016, 120, 489–499. [Google Scholar] [CrossRef]
- Su, W.; Liu, J.; Bai, P.; Ma, B.; Liu, W. Pathogenic fungi-induced susceptibility is mitigated by mutual Lactobacillus plantarum in the Drosophila melanogaster model. BMC Microbiol. 2019, 19, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratnaweera, P.B.; M Jayasundara, J.M.N.; Herath, H.H.M.S.D.; Williams, D.E.; Rajapaksha, S.U.; Nishantha, K.M.D.W.P.; Silva, E.D.d.; Andersen, R.J. Antifeedant, contact toxicity and oviposition deterrent effects of phyllostine acetate and phyllostine isolated from the endophytic fungus Diaporthe miriciae against Plutella xylostella larvae. Pest Manag. Sci. 2020, 76, 1541–1548. [Google Scholar] [CrossRef]
| Phylum | Class | Order | Family | Genus | Species | OTU | |
|---|---|---|---|---|---|---|---|
| D. kuriphilus adults | 4 | 20 | 46 | 93 | 133 | 176 | 250 |
| Insect galls | 4 | 22 | 56 | 114 | 171 | 241 | 375 |
| Galled twigs | 4 | 21 | 53 | 107 | 164 | 221 | 347 |
| Total | 4 | 22 | 56 | 116 | 177 | 248 | 385 |
| Phylum | Class | Order | Family | Genus | Species | OTU | |
|---|---|---|---|---|---|---|---|
| D. Kuriphilus adults | Ascomycota (97.28) A | Dothideomycetes (51.79) A | Botryosphaeriales (44.91) A | Botryosphaeriaceae (44.91) A | Botryosphaeria (44.90) A | Botryosphaeria sp. (44.90) A | OTU726 (44.90) A |
| Insect galls | Ascomycota (79.26) A | Dothideomycetes (49.60) A | Pleosporales (25.55) A | Didymellaceae (18.36) A | Didymella (17.92) A | Didymella rosea (17.89) A | OTU1183 (17.89) A |
| Galled twigs | Ascomycota (88.43) A | Dothideomycetes (66.33) A | Pleosporales (41.69) A | Didymellaceae (35.72) A | Didymella (34.84) A | D. rosea (34.82) A | OTU1183 (34.82) A |
| Source | Unique Fungi | Fungi Common to DryK and InsG | Fungi Common to DryK and CasM | Fungi Common to InsG and CasM | Fungi Common to DryK, InsG and CasM |
|---|---|---|---|---|---|
| D. Kuriphilus adults | 0.14 A | 0.42 A | 0.08 A | - | 99.36 A |
| Insect galls | 0.03 A | 0.40 A | - | 1.54 A | 98.03 A |
| Galled twigs | 0.04 A | - | 0.06 A | 1.20 A | 98.71 A |
| Predominant Fungi | Reported in D. kuriphilus | Reported in Insect Galls | Reported in Galled Twigs |
|---|---|---|---|
| D. kuriphilus group | |||
| Botryosphaeria sp. | No | Fernandez-Conradi et al., 2019 | Fernandez-Conradi et al., 2019. |
| Aspergillus sp. | No | Vannini et al., 2017. | Overy et al., 2003; Donis-González et al., 2016 |
| Diaporthe sp. | No | Fernandez-Conradi et al., 2019. | Zhang et al., 2018; Fernandez-Conradi et al., 2019. |
| Insect galls group | |||
| Ascomycota species | – | – | – |
| Acremonium sp. | No | No | Driss, 2019 |
| Bullera alba | No | No | Driss, 2019 |
| Cercospora sp. | No | Vinale et al., 2014. | Vinale et al., 2014. |
| Cryptococcus aureus | No | Fernandez-Conradi et al., 2019. | LaBonte et al., 2018; Fernandez-Conradi et al., 2019. |
| Curvibasidium cygneicollum | No | Driss, 2019. | Driss, 2019. |
| Galled twigs group | |||
| Didymella rosea | No | No | LaBonte et al., 2018. |
| Cladosporium delicatulum | Fernandez-Conradi et al., 2019 | Seddaiu et al., 2017. | Zhang et al., 2009; LaBonte et al., 2018 |
| Capnodiales species | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.-H.; Li, X.-M.; Zhu, D.-H.; Zeng, Y.; Zhao, L.-Q. The Diversity and Dynamics of Fungi in Dryocosmus kuriphilus Community. Insects 2021, 12, 426. https://doi.org/10.3390/insects12050426
Yang X-H, Li X-M, Zhu D-H, Zeng Y, Zhao L-Q. The Diversity and Dynamics of Fungi in Dryocosmus kuriphilus Community. Insects. 2021; 12(5):426. https://doi.org/10.3390/insects12050426
Chicago/Turabian StyleYang, Xiao-Hui, Xiang-Mei Li, Dao-Hong Zhu, Yang Zeng, and Lv-Quan Zhao. 2021. "The Diversity and Dynamics of Fungi in Dryocosmus kuriphilus Community" Insects 12, no. 5: 426. https://doi.org/10.3390/insects12050426
APA StyleYang, X.-H., Li, X.-M., Zhu, D.-H., Zeng, Y., & Zhao, L.-Q. (2021). The Diversity and Dynamics of Fungi in Dryocosmus kuriphilus Community. Insects, 12(5), 426. https://doi.org/10.3390/insects12050426







