Response of Different Insect Groups to Various Wavelengths of Light under Field Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
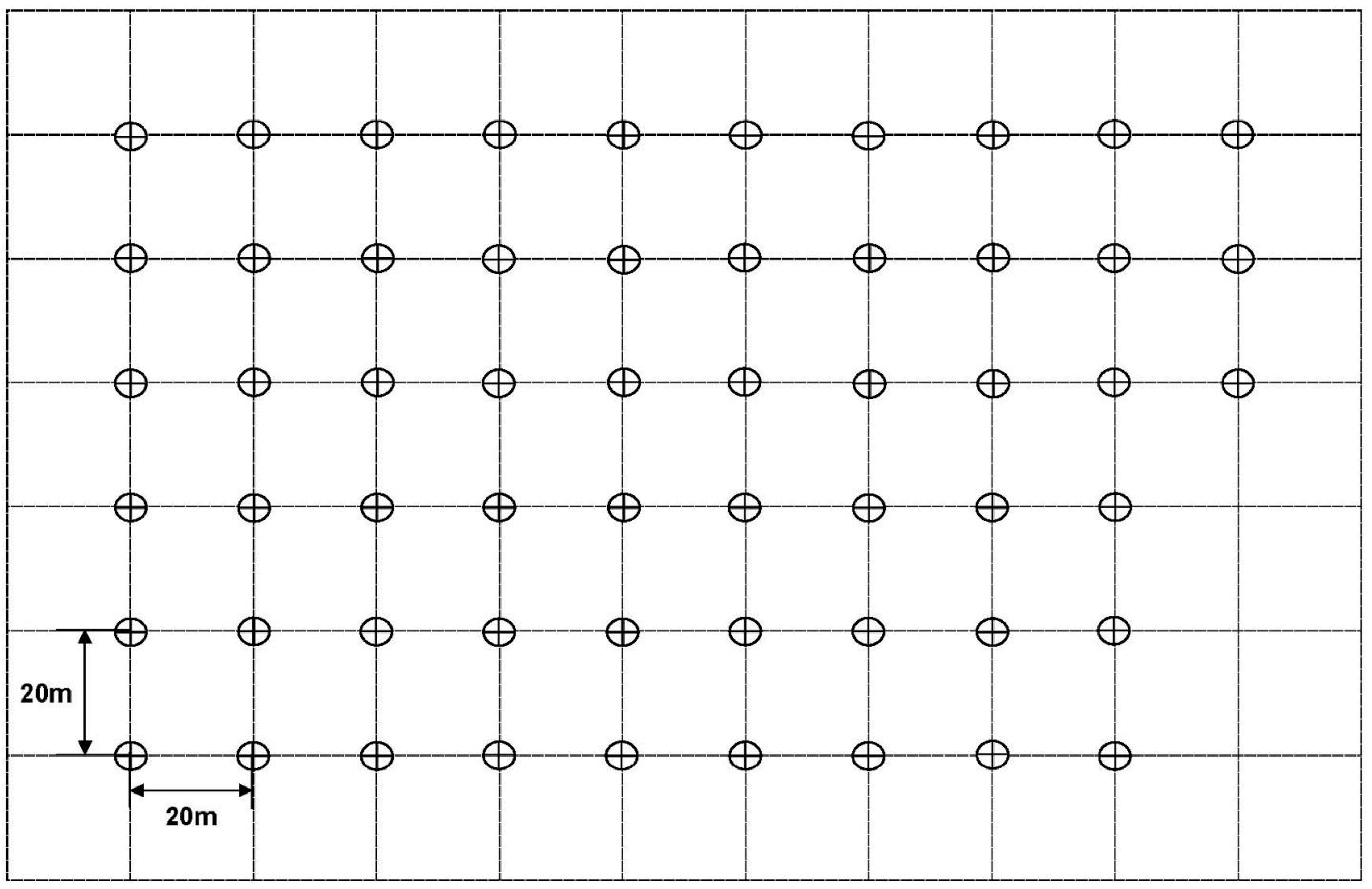
2.1. Field Trials
2.2. Statistical Analysis
3. Results
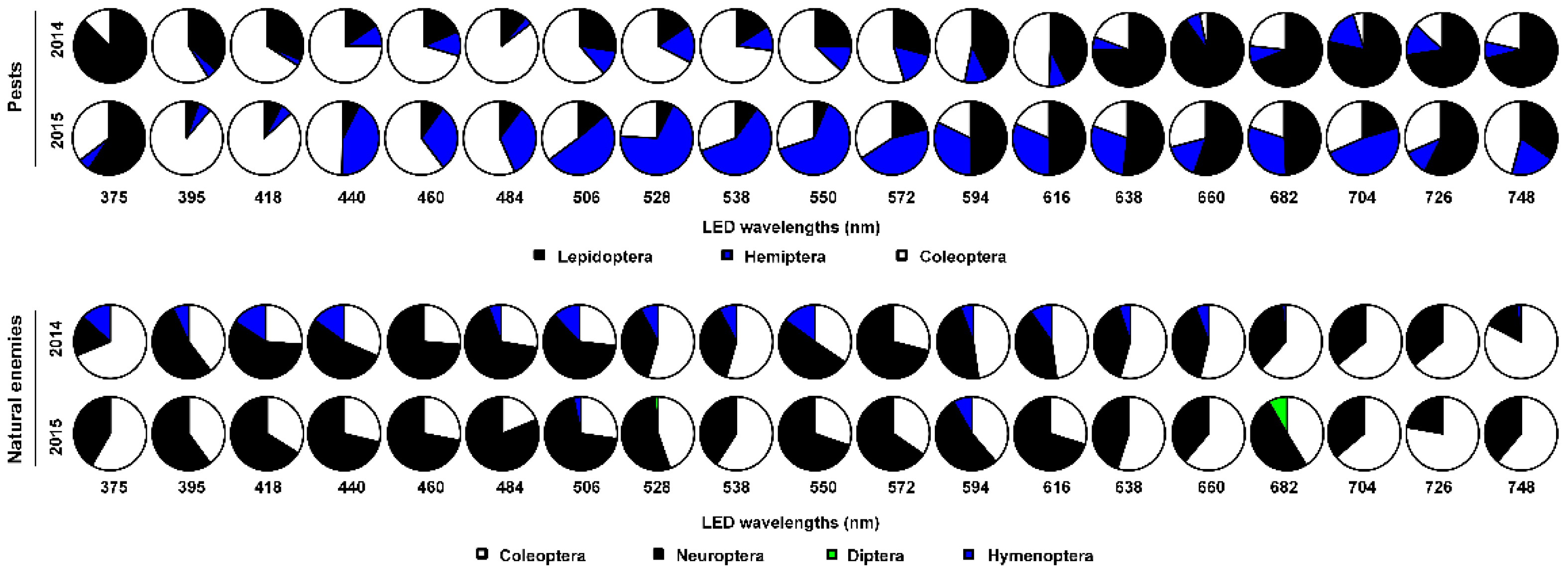
3.1. Insect Abundances of Pests and Natural Enemies in Different Orders
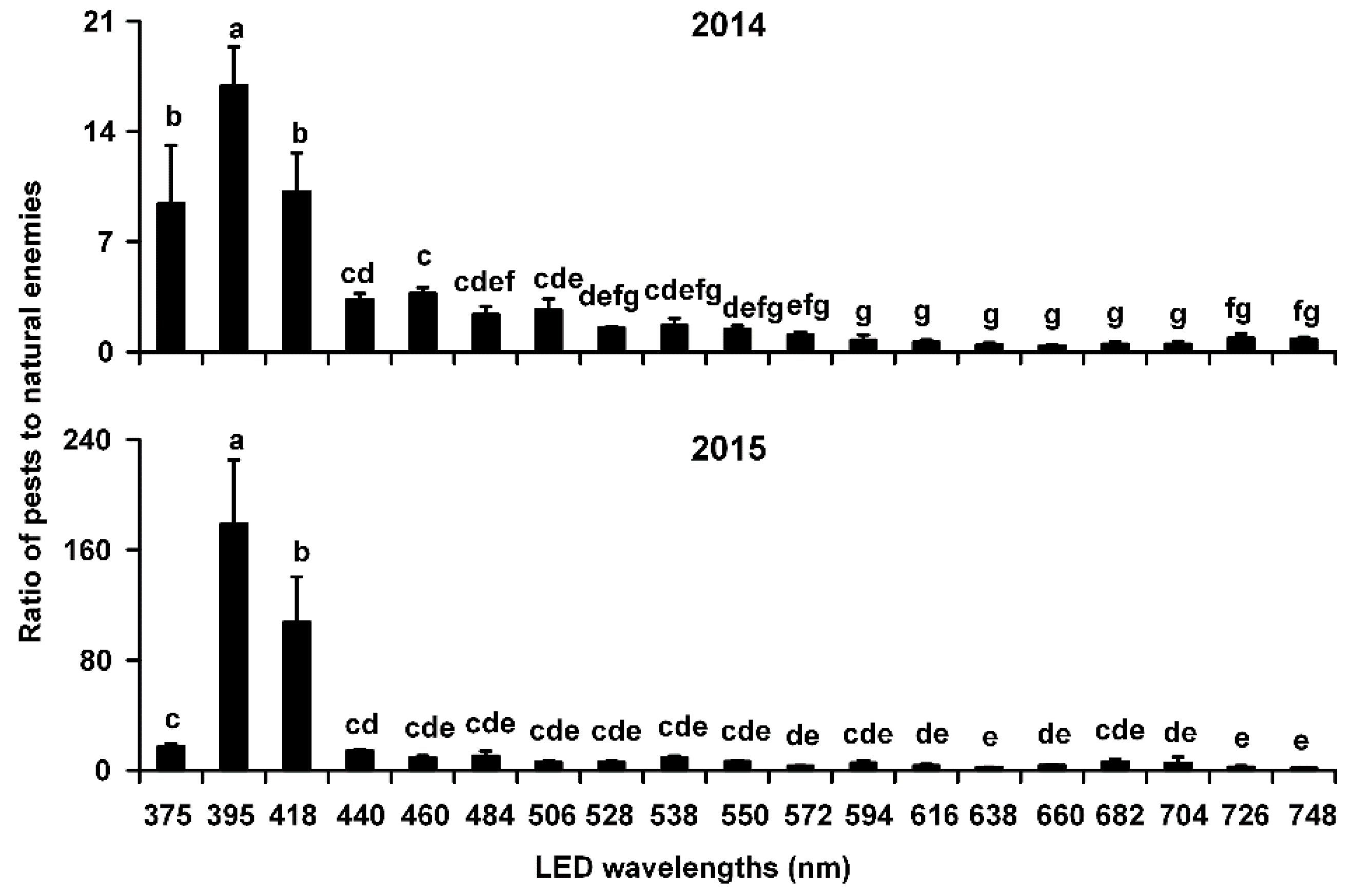
3.2. Ratio of Pests to Natural Enemies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapman, R.R. The Insects: Structure and Function, 4th ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Briscoe, A.D.; Chittka, L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef]
- Song, X.Y.; Zhang, G.X.; Li, X.J. Application of frequency-vibrancy pest-killing lamp in controlling agricultural pests. Crops 2005, 1, 30–31. [Google Scholar]
- Cowan, T.; Gries, G. Ultraviolet and violet light: Attractive orientation cues for the Indian meal moth, Plodia interpunctella. Entomol. Exp. Appl. 2009, 131, 148–158. [Google Scholar] [CrossRef]
- Fan, X.Y.; Li, Y.Z.; Wang, X.L.; Lin, L.J.; Yu, H.L.; Guan, J.C. Experiment on attraction of scarab adults (Scarabaeidae) to a black light lamp in the Chinese olive (Canarium album) plantation. Guangdong Forest Sci. Tech. 2009, 25, 31–34. [Google Scholar]
- Shimoda, M.; Honda, K.-I. Insect reactions to light and its applications to pest management. Appl. Entomol. Zoöl. 2013, 48, 413–421. [Google Scholar] [CrossRef]
- Sang, W.; Huang, Q.Y.; Wang, X.P.; Guo, S.H.; Lei, C.L. Progress in research on insect phototaxis and future prospects for pest light-trap technology in China. Chin. J. Appl. Entomol. 2019, 56, 907–916. [Google Scholar]
- Beck, J.; Linsemair, K.E. Feasibility of light-trapping in community research on moths: Attraction radius of light, completeness of samples, nightly flight times and seasonality of Southeast-Asian hawkmoths (Lepidoptera: Sphingidae). J. Res. Lepid. 2006, 39, 18–37. [Google Scholar]
- Prokopy, R.J.; Owens, E.D. Visual Detection of Plants by Herbivorous Insects. Annu. Rev. Entomol. 1983, 28, 337–364. [Google Scholar] [CrossRef]
- Eguchi, E.; Watanabe, K.; Hariyama, T.; Yamamoto, K. A comparison of electrophysiologically determined spectral responses in 35 species of Lepidoptera. J. Insect Physiol. 1982, 28, 675–682. [Google Scholar] [CrossRef]
- Gilbert, C. Form and function of stemmata in larvae of holometabolous insects. Annu. Rev. Entomol. 1994, 39, 323–349. [Google Scholar] [CrossRef]
- Lin, J.T.; Hwang, P.C.; Tung, L.C. Visual organization and spectral sensitivity of larval eyes in the moth Trabala vishnou, Lefebur (Lepidoptera: Lasiocampidae). Zool. Stud. 2002, 41, 366–375. [Google Scholar]
- Castrejon, F.; Rojas, J.C. Behavioral responses of larvae and adults of Estigmene acrea (Lepidoptera: Arctiidae) to light of dif-ferent wavelengths. Fla. Entomol. 2010, 93, 505–509. [Google Scholar]
- Ramamurthy, V.V.; Akhtar, M.S.; Patankar, N.V.; Menon, P.; Kumar, R.; Singh, S.K.; Ayri, S.; Parveen, S.; Mittal, V. Efficiency of different light source in light traps in monitoring insect diversity. Munis Entomol. Zool. 2010, 5, 109–114. [Google Scholar]
- Fu, G.X.; Li, W.Z.; Wu, S.Y.; Yuan, G.H.; Wang, Y.H.; An, J.J.; Chai, X.L. Bioassays on phototactic responses of Myzus persicae (Homoptera: Aphididae) to different monochromatic lights. Acta Entomol. Sin. 2009, 52, 1171–1176. [Google Scholar]
- Somers-Yeates, R.; Hodgson, D.; McGregor, P.K.; Spalding, A.; Ffrench-Constant, R.H. Shedding light on moths: Shorter wavelengths attract noctuids more than geometrids. Biol. Lett. 2013, 9, 20130376. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L. Field Evaluation of Light-Emitting Diodes with Different Wave Lengths as Traps of Five Agricultural Pests; Yangzhou University: Yangzhou, China, 2020. [Google Scholar]
- Zhang, G.X.; Zheng, G.; Li, X.J.; Bu, J. Discussion of using frequency trembler grid lamps from angle of protecting biodiversity. Entomol. Knowl. 2004, 41, 532–535. [Google Scholar]
- Duehl, A.J.; Cohnstaedt, L.W.; Arbogast, R.T.; Teal, P. Evaluating light attraction to increase trap efficiency for Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2011, 104, 1430–1435. [Google Scholar] [CrossRef]
- Bian, L.; Sun, X.L.; Gao, Y.; Luo, Z.X.; Jin, S.; Zhang, Z.Q.; Chen, Z.M. Research on the light tropism of insects and the progress in application. Chin. J. Appl. Entomol. 2012, 49, 1677–1686. [Google Scholar]
- Yang, X.M.; Lu, Y.H.; Liang, G.M. Insect phototaxis behavior and light trapping technology. Zhaoming Gongcheng Xuebao 2020, 31, 22–31. [Google Scholar]
- Nakamoto, Y.; Kuba, H. The effectiveness of a green light emitting diode (LED) trap at capturing the West Indian sweet potato weevil, Euscepes postfasciatus (Fairmaire) (Coleoptera: Curculionidae) in a sweet potato field. Appl. Èntomol. Zoöl. 2004, 39, 491–495. [Google Scholar] [CrossRef]
- Kim, M.-G.; Lee, H.-S. Attractive Effects of American Serpentine Leafminer, Liriomyza trifolii (Burgess), to Light-Emitting Diodes. J. Insect Behav. 2013, 27, 127–132. [Google Scholar] [CrossRef]
- Park, J.-H.; Sung, B.-K.; Lee, H.-S. Phototactic behavior 7: Phototactic response of the maize weevil, Sitotroga zeamais motsch (Coleopter: Curculionidae), to light-emitting diodes. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 373–376. [Google Scholar] [CrossRef]
- Xu, X.; Ma, L.; Yue, Z.L.; Chen, H.B. Trapping effect of LED single wavelength insecticidal lamp against tea insect pests. China Plant Prot. 2017, 37, 53–56. [Google Scholar]
- Sang, W.; Cai, F.Y.; Wang, X.P.; Zhang, S.; Huang, Q.Y.; Zhu, F.; Guo, S.H.; Lei, C.L. Application status and prospects of insect trapping lamp in fields. China Plant Prot. 2018, 38, 26–30. [Google Scholar]
- Lu, Y.H.; Jian, G.L.; Wu, K.M. Concise Identification Manual of Main Diseases and Insect Pests of Cotton; China Agriculture Press: Beijing, China, 2013. [Google Scholar]
- Institute of Plant Protection, Hubei Academy of Agricultural Sciences. Atlas of Cotton Pests and Their Natural Enemies; Hubei People’s Publishing Press: Wuhan, China, 1980. [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide, version 9.13; SAS Institute: Cary, NC, USA, 2005. [Google Scholar]
- Pan, H.; Xu, Y.; Liang, G.; Wyckhuys, K.A.; Yang, Y.; Lu, Y. Field evaluation of light-emitting diodes to trap the cotton bollworm, Helicoverpa armigera. Crop. Prot. 2020, 137, 105267. [Google Scholar] [CrossRef]
- Xu, Y.L.; Pan, H.S.; Liang, G.M.; Yang, Y.Z.; Lu, Y.H. Field evaluation of light-emitting diodes with different wavelengths as traps of Anomala corpulenta and Holotrichia parallela. Xinjiang Agri. Sci. 2020, 58, 2028–2033. [Google Scholar]
- Poiani, S.; Dietrich, C.; Barroso, A.; Costa-Leonardo, A. Effects of residential energy-saving lamps on the attraction of nocturnal insects. Light. Res. Technol. 2014, 47, 338–348. [Google Scholar] [CrossRef]
- Van Grunsven, R.H.A.; Donners, M.; Boekee, K.; Tichelaar, I.; Van Geffen, K.G.; Groenendijk, D.; Berendse, F.; Veenendaal, E.M. Spectral composition of light sources and insect phototaxis, with an evaluation of existing spectral response models. J. Insect Conserv. 2014, 18, 225–231. [Google Scholar] [CrossRef]
- Longcore, T.; Aldern, H.L.; Eggers, J.F.; Flores, S.; Franco, L.; Hirshfield-Yamanishi, E.; Petrinec, L.N.; Yan, W.A.; Barroso, A.M. Tuning the white light spectrum of light emitting diode lamps to reduce attraction of nocturnal arthropods. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140125. [Google Scholar] [CrossRef] [PubMed]
- Spears, L.R.; Ramirez, R.A. Learning to love leftovers: Using bycatch to expand our knowledge in entomology. Am. Entomol. 2015, 61, 168–173. [Google Scholar] [CrossRef]
- Zhao, J.W.; He, Y.X.; Weng, Q.Y. Application and research of insect light traps in China. Entomol. J. East China 2008, 17, 76–80. [Google Scholar]
- Wu, S.; Zhang, Y.M.; Guo, X.; Huang, Y.F.; Liu, J.F. Effectiveness of different wavelength LED insect lamp traps in vegetable fields. Chin. J. Appl. Entomol. 2021, 58, 172–180. [Google Scholar]
- Nabli, H.; Bailey, W.C.; Necibi, S. Beneficial Insect Attraction to Light Traps with Different Wavelengths. Biol. Control. 1999, 16, 185–188. [Google Scholar] [CrossRef]
- Mondor, E.B.; Warren, J.L. Unconditioned and conditioned responses to colour in the predatory coccinellid, Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 2000, 97, 463–467. [Google Scholar] [CrossRef]
- Cheng, D.F.; Feng, H.Q.; Wu, K.M. Scanning Insect Radar and Insect Migration Monitoring; Science Press: Beijing, China, 2005. [Google Scholar]


| Category | Orders | Insect Species |
|---|---|---|
| Pests | Lepidoptera | Helicoverpa armigera Hübner |
| Agrotis ypsilon Rottemberg | ||
| Spodotera exigua Hübner | ||
| Scotogramma trifolii (Rottemberg) | ||
| Macdunnoughia crassisigna (Warren) | ||
| Argyrogramma agnata (Staudinger) | ||
| Hemiptera | Apolygus lucorum (Meyer-Dür) | |
| Adelphocoris suturalis Jakovlev | ||
| Adelphocoris fasciaticollis Reuter | ||
| Dolycoris baccarum Linnaeus | ||
| Empoasca biguttula Ishida | ||
| Coleoptera | Holotrichia parallela Motschulsky | |
| Holotrichia oblita Faldermann | ||
| Holitrichia trichophora (Fairmaire) | ||
| Anomala corpulenta Motschulsky | ||
| Monolepta hieroglyphica Motschulsky | ||
| Natural enemies | Coleoptera | Propylea japonica Thunberg |
| Harmonia axyridis Pallas | ||
| Neuroptera | Lacewings | |
| Diptera | Hoverflies | |
| Hymenoptera | Parasitoid wasps |
| Year | Category | Groups | Statistical Values |
|---|---|---|---|
| 2014 | Pests | Lepidoptera | F = 13.52, df = 18,38, p < 0.0001 |
| Hemiptera | F = 5.76, df = 18,38, p < 0.0001 | ||
| Coleoptera | F = 26.35, df = 18,38, p < 0.0001 | ||
| Total | F = 23.00, df = 18,38, p < 0.0001 | ||
| Natural enemies | Coleoptera | F = 1.95, df = 18,38, p = 0.0413 | |
| Neuroptera | F = 9.85, df = 18,38, p < 0.0001 | ||
| Diptera | F = 0.94, df = 18,38, p = 0.5363 | ||
| Hymenoptera | F = 1.77, df = 18,38, p = 0.0680 | ||
| Total | F = 7.69, df = 18,38, p < 0.0001 | ||
| 2015 | Pests | Lepidoptera | F = 14.56, df = 18,38, p < 0.0001 |
| Hemiptera | F = 14.90, df = 18,38, p < 0.0001 | ||
| Coleoptera | F = 309.25, df = 18,38, p < 0.0001 | ||
| Total | F = 164.39, df = 18,38, p < 0.0001 | ||
| Natural enemies | Coleoptera | F = 2.71, df = 18,38, p = 0.0048 | |
| Neuroptera | F = 9.57, df = 18,38, p < 0.0001 | ||
| Diptera | F = 0.94, df = 18,38, p = 0.5363 | ||
| Hymenoptera | F = 0.94, df = 18,38, p = 0.5363 | ||
| Total | F = 9.38, df = 18,38, p < 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Liang, G.; Lu, Y. Response of Different Insect Groups to Various Wavelengths of Light under Field Conditions. Insects 2021, 12, 427. https://doi.org/10.3390/insects12050427
Pan H, Liang G, Lu Y. Response of Different Insect Groups to Various Wavelengths of Light under Field Conditions. Insects. 2021; 12(5):427. https://doi.org/10.3390/insects12050427
Chicago/Turabian StylePan, Hongsheng, Gemei Liang, and Yanhui Lu. 2021. "Response of Different Insect Groups to Various Wavelengths of Light under Field Conditions" Insects 12, no. 5: 427. https://doi.org/10.3390/insects12050427
APA StylePan, H., Liang, G., & Lu, Y. (2021). Response of Different Insect Groups to Various Wavelengths of Light under Field Conditions. Insects, 12(5), 427. https://doi.org/10.3390/insects12050427







