Elucidating the Fitness of a Dead-End Trap Crop Strategy against the Tomato Fruitworm, Helicoverpa armigera
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Insect
2.3. Multiple-Choice Experiment
2.4. Two-Choice Experiment
2.5. No-Choice Experiment
2.6. Effect of S. viarum Plant Volatiles on Oviposition
2.7. Trichome Density and Types
2.8. Quantification of Acylsugar Content
2.9. Quantification of Total Phenolic Content (TPC)
2.10. Feeding Experiment for the Larval Survival and Growth Analysis
2.11. Oviposition on Tomato and S. viarum Mixtures
2.12. Statistical Analysis
3. Results
3.1. Multiple-Choice Experiment
3.2. Two-Choice Experiment
3.3. No-Choice Experiment
3.4. Effect of S. viarum Plant Volatiles on Oviposition
3.5. Trichome Density and Types
3.6. Total Acylsugar Content
3.7. Total Phenolic Content (TPC)
3.8. Larval Survival and Pupation on Different Accessions of S. viarum Leaves
3.9. Correlation Analysis Between H. armigera and Plant Parameters
3.10. Oviposition on Tomato and S. viarum Mixtures
4. Discussion
4.1. S. viarum, an Oviposition Attractant for H. armigera
4.2. Unsuitability of S. viarum for the Larval Survival and Development of H. armigera
4.3. Morpho-Chemical Composition Vs. H. armigera Performance on S. viarum and Its Natural Host Tomato
4.4. S. viarum as a Dead-End Trap Crop and Implications for H. armigera Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rakha, M.; Bouba, N.; Ramasamy, S.; Regnard, J.-L.; Hanson, P. Evaluation of wild tomato accessions (Solanum spp.) for resistance to two-spotted spider mite (Tetranychus urticae Koch) based on trichome type and acylsugar content. Genet. Resour. Crop Evol. 2017, 64, 1011–1022. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. Food and Agriculture Organization of the United Nations, Rome, Italy. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 5 February 2021).
- Srinivasan, R.; Uthamasamy, S. Does host plant chemistry influence feeding and ovipositional behavior of fruitborer in tomato? Int. J. Veg. Sci. 2005, 11, 97–116. [Google Scholar] [CrossRef]
- Sharma, H. Crop Protection Compendium: Helicoverpa Armigera; Electronic Compendium for Crop Protection; Commonwealth Agricultural Bureau International: Wallingford, UK, 2001. [Google Scholar]
- Srinivasan, R.; Talekar, N.S.; Uthamasamy, S. Feeding Stimulants in Solanum viarum Dunal for Tomato Fruit Borer (Helicoverpa armigera Hübner). Form. Entomol. 2005, 25, 95–102. [Google Scholar] [CrossRef]
- Talekar, N.S.; Opena, R.; Hanson, P. Helicoverpa armigera management: A review of AVRDC’s research on host plant resistance in tomato. Crop. Prot. 2006, 25, 461–467. [Google Scholar] [CrossRef]
- Martin, T.; Ochou, O.G.; Vaissayre, M.; Fournier, D. Organophosphorus insecticides synergize pyrethroids in the resistant strain of cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) from West Africa. J. Econ. Entomol. 2003, 96, 468–474. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, A.; King, A.; Walker, A.; El-Nayir, H. Resistance to synthetic pyrethroids in the bollworm, Heliothis armigera from Andhra Pradesh, India. Pestic. Sci. 1989, 27, 65–76. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R.; Reichelt, M.; Gershenzon, J.; Heckel, D.G. Using plant chemistry and insect preference to study the potential of Barbarea (Brassicaceae) as a dead-end trap crop for diamondback moth (Lepidoptera: Plutellidae). Phytochemistry 2014, 98, 137–144. [Google Scholar] [CrossRef]
- Shelton, A.M.; Nault, B.A. Dead-end trap cropping: A technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop. Prot. 2004, 23, 497–503. [Google Scholar] [CrossRef]
- Zedler, B.; Srinivasan, R.; Su, F.-C. Assessing the potential of spider plant (Cleome gynandra L.) as a trap crop for the management of specialist feeders on vegetable brassicas. J. Asia Pac. Entomol. 2016, 19, 477–485. [Google Scholar] [CrossRef]
- Shelton, A.M.; Badenes-Perez, F.R. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemashenpagam, N.; Selvaraj, T. Effect of arbuscular mycorrhizal (AM) fungus and plant growth-promoting rhizomicroorganisms (PGPR’s) on medicinal plant Solanum viarum seedlings. J. Environ. Biol. 2011, 32, 579–583. [Google Scholar] [PubMed]
- Mullahey, J.J. Tropical soda apple (Solanum viarum Dunal), a biological pollutant threatening Florida. Castanea 1996, 61, 255–260. Available online: www.jstor.org/stable/4033679 (accessed on 15 February 2020).
- Wu, S.-B.; Meyer, R.S.; Whitaker, B.D.; Litt, A.; Kennelly, E.J. Antioxidant glucosylated caffeoylquinic acid derivatives in the invasive tropical soda apple, Solanum viarum. J. Nat. Prod. 2012, 75, 2246–2250. [Google Scholar] [CrossRef] [PubMed]
- Diongue, A.; Lai, P.-Y.; Chang, Y.-F.; Lin, C. Effects of Solanum viarum cuticular leaf extracts and its fractions on the oviposition of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Form. Entomol. 2005, 25, 23–32. [Google Scholar] [CrossRef]
- Mullahey, J.J.; Nee, M.; Wunderlin, R.P.; Delaney, K.R. Tropical soda apple (Solanum viarum): A new weed threat in subtropical regions. Weed Technol. 1993, 7, 783–786. [Google Scholar] [CrossRef]
- Mahadev, M.D.N.; Panathula, C.S.; Naidu, C. Impact of different carbohydrates and their concentrations on in vitro regeneration of Solanum viarum (Dunal)—An important anticancer medicinal plant. Am. J. Plant Sci. 2014, 5, 200–204. [Google Scholar] [CrossRef] [Green Version]
- AVRDC. AVRDC Report 1999. Asian Vegetable Research and Development Center, Tainan, Taiwan, 2000, Report No.: 0258-3089. Available online: http://worldveg.tind.io/record/57806/files/Annual%20Report (accessed on 15 February 2020).
- Talekar, N.S.; Hau, T.B.H.; Chang, W.C. Solanum viarum, a trap crop for Helicoverpa armigera. Insect Environ. 1999, 5, 142. [Google Scholar]
- Diongue, A.; Lai, P.-Y.; Lin, C. Application of headspace solid-phase microextraction and gas chromatography-mass spectrometry in the study of volatile chemicals from Solanum viarum dunal. Asian J. Chem. 2007, 19, 2002–2014. [Google Scholar]
- Diongue, A.; Lai, P.-Y.; Lin, C.; Hwang, J.S. Bioassays of the ovipositional responses of the tomato fruitworm, Helicoverpa armigera (Lepidoptera: Noctuidae), to Solanum viarum leaf extracts. Plant Prot. Bull. 2006, 47, 281–292. [Google Scholar]
- Georgievska, L. Isolation and Identification of Chemical Stimulants from Solanum viarum (Dunal) and Their Effects of the Oviposition of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Master’s Thesis, Institute of Tropical Agriculture, National Pingtung University of Science and Technology, Taiwan, 2001; p. 136. Available online: https://hdl.handle.net/11296/axa8x8 (accessed on 15 February 2020).
- Srinivasan, R.; Su, F.-C.; Huang, C.C. Oviposition dynamics and larval development of Helicoverpa armigera on a highly preferred unsuitable host plant, Solanum viarum. Entomol. Exp. Appl. 2013, 147, 217–224. [Google Scholar] [CrossRef]
- Srinivasan, R.; Uthamasamy, S.; Talekar, N.S. Characterization of oviposition attractants of Helicoverpa armigera in two solanaceous plants, Solanum viarum, and Lycopersicon esculentum. Curr. Sci. 2006, 90, 846–850. Available online: www.jstor.org/stable/24089200 (accessed on 20 March 2020).
- Jayanthi, P.D.K.; Ravindra, M.A.; Kempraj, V.; Roy, T.K.; Shivashankara, K.S.; Singh, T.H. Morphological diversity of trichomes and phytochemicals in wild and cultivated eggplant species. Indian J. Hortic. 2018, 75, 265–272. [Google Scholar] [CrossRef]
- Yang, O.S.C.; Chu, Y.I. The comparison of the development of the tobacco cutworm (Spodoptera litura (F)) reared with natural and artificial diets. Chin. J. Entomol. 1988, 8, 143–150. [Google Scholar]
- Luckwill, L.C. The Genus Lycopersicon: A Historical, Biological, and Taxonomic Survey of the Wild and Cultivated Tomatoes. University Studies; Aberdeen University Press: Aberdeen, UK, 1943; Volume 120. [Google Scholar]
- Savory, E.A. Modification of the PGO Assay for Use in Acylsugar Quantification; Cornell University: Ithaca, NY, USA, 2004; p. 36. [Google Scholar]
- Blainski, A.; Lopes, G.C.; de Mello, J.C. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.N.; Kim, M.R.; Cho, S.M.; Kim, S.Y.; Kim, J.B.; Cho, Y.S. Antioxidant activities and determination of phenolic compounds isolated from oriental plums (Soldam, Oishiwase, and Formosa). Nutr. Res. Pract. 2012, 6, 277–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jallow, M.F.; Zalucki, M.P. Relationship between oviposition preference and offspring performance in Australian Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2003, 42, 343–348. [Google Scholar] [CrossRef]
- Liu, Z.; Scheirs, J.; Heckel, D.G. Trade-offs of host use between generalist and specialist Helicoverpa sibling species: Adult oviposition and larval performance. Oecologia 2012, 168, 459–469. [Google Scholar] [CrossRef]
- Pöykkö, H. Females and larvae of a geometrid moth, Cleorodes lichenaria, prefer a lichen host that assures shortest larval period. Environ. Entomol. 2006, 35, 1669–1676. [Google Scholar] [CrossRef] [Green Version]
- Refsnider, J.M.; Janzen, F.J. Putting eggs in one basket: Ecological and evolutionary hypotheses for variation in oviposition-site choice. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 39–57. [Google Scholar] [CrossRef]
- Resetarits, W.J., Jr. Oviposition site choice and life history evolution. Am. Zool. 1996, 36, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Janz, N.; Nylin, S. The role of female search behaviour in determining host plant range in plant feeding insects: A test of the information processing hypothesis. Proc. R. Soc. Lond. 1997, 264, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.N. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. 1988, 47, 3–14. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Zhou, D.-S.; Gao, S.-X.; Zhao, X.-C.; Tang, Q.-B.; Wang, C.-Z.; Van Loon, J.J. Higher plasticity in feeding preference of a generalist than a specialist: Experiments with two closely related Helicoverpa species. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Rojas, J.C.; Kolomiets, M.V.; Bernal, J.S. Nonsensical choices? Fall armyworm moths choose seemingly best or worst hosts for their larvae, but neonate larvae make their own choices. PLoS ONE 2018, 13, e0197628. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R.; Nault, B.A.; Shelton, A.M. Dynamics of diamondback moth oviposition in the presence of a highly preferred non-suitable host. Entomol. Exp. Appl. 2006, 120, 23–31. [Google Scholar] [CrossRef]
- Firempong, S.; Zalucki, M. Host Plant-Selection by Helicoverpa armigera (Hubner) (Lepidoptera, Noctuidae)-Role of Certain Plant Attributes. Aust. J. Zool. 1989, 37, 675–683. [Google Scholar] [CrossRef]
- Hance, T.; Van Baaren, J.; Vernon, P.; Boivin, G. Impact of extreme temperatures on parasitoids in a climate change perspective. Annu. Rev. Entomol. 2007, 52, 107–126. [Google Scholar] [CrossRef]
- Noor-ul-Ane, M.; Kim, D.-S.; Zalucki, M.P. Fecundity and egg laying in Helicoverpa armigera (Lepidoptera: Noctuidae): Model development and field validation. J. Econ. Entomol. 2018, 111, 2208–2216. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R.; Nault, B.A.; Shelton, A.M. Manipulating the attractiveness and suitability of hosts for diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 2005, 98, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, M.; Molet, T. CPHST Pest Datasheet for Helicoverpa armigera. USDA-APHIS-PPQ-CPHST. 2014. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/owb/downloads/owb-factsheet.pdf (accessed on 12 April 2019).
- Burguiere, L.; Marion-Poll, F.; Cork, A. Electrophysiological responses of female Helicoverpa armigera (Hübner) (Lepidoptera; Noctuidae) to synthetic host odours. J. Insect. Physiol. 2001, 47, 509–514. [Google Scholar] [CrossRef]
- Steinbauer, M.J. Oviposition preference and neonate performance of Mnesampela privata in relation to heterophylly in Eucalyptus dunnii and E. globulus. Agric. For. Entomol. 2002, 4, 245–253. [Google Scholar] [CrossRef]
- Nayak, B.G.; Patil, S.K. Variation of solasodine in Solanum viarum Dunal with maturity stages. Karnataka J. Agric. Sci. 2001, 14, 185–186. [Google Scholar]
- Satyabrata, M.; Ram, C.; Geetha, K.; Kunal, M. Production technology of some important medicinal and aromatic crops developed under the all India coordinated research project. Indian J. Arecanut Spices Med. Plants 2000, 2, 88–98. [Google Scholar]
- Ben-Israel, I.; Yu, G.; Austin, M.B.; Bhuiyan, N.; Auldridge, M.; Nguyen, T.; Schauvinhold, I.; Noel, J.P.; Pichersky, E.; Fridman, E. Multiple biochemical and morphological factors underlie the production of methylketones in tomato trichomes. Plant Physiol. 2009, 151, 1952–1964. [Google Scholar] [CrossRef] [Green Version]
- Juvik, J.A.; Babka, B.A.; Timmermann, E.A. Influence of trichome exudates from species of Lycopersicon on oviposition behavior of Heliothis zea (Boddie). J. Chem. Ecol. 1988, 14, 1261–1278. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Hussain, B.; Buhroo, A.A.; Ignacimuthu, S.; Sharma, H.C. Effect of plant secondary metabolites on legume pod borer, Helicoverpa armigera. J. Pest Sci. 2013, 86, 399–408. [Google Scholar] [CrossRef]
- Boulogne, I.; Petit, P.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and antifungal chemicals produced by plants: A review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Rakha, M.; Shaheen, F.A.; Srinivasan, R. Resistance of certain wild tomato (Solanum spp.) accessions to Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) based on choice and no-choice bioassays. Fla. Entomol. 2019, 102, 544–548. [Google Scholar] [CrossRef]
- van den Oever-van den Elsen, F.; Lucatti, A.F.; van Heusden, S.; Broekgaarden, C.; Mumm, R.; Dicke, M.; Vosman, B. Quantitative resistance against Bemisia tabaci in Solanum pennellii: Genetics and metabolomics. J. Integr. Plant Biol. 2016, 58, 397–412. [Google Scholar] [CrossRef] [Green Version]
- Vosman, B.; Van’t Westende, W.P.C.; Henken, B.; van Eekelen, H.D.; de Vos, R.C.H.; Voorrips, R.E. Broad spectrum insect resistance and metabolites in close relatives of the cultivated tomato. Euphytica 2018, 214, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakha, M.; Hanson, P.; Ramasamy, S. Identification of resistance to Bemisia tabaci Genn. in closely related wild relatives of cultivated tomato based on trichome type analysis and choice and no-choice assays. Genet. Resour. Crop Evol. 2017, 64, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Rakha, M.; Zekeya, N.; Sevgan, S.; Musembi, M.; Ramasamy, S.; Hanson, P. Screening recently identified whitefly/spider mite-resistant wild tomato accessions for resistance to Tuta absoluta. Plant Breed. 2017, 136, 562–568. [Google Scholar] [CrossRef] [Green Version]
- Weinhold, A.; Baldwin, I.T. Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc. Natl. Acad. Sci. USA 2011, 108, 7855–7859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shafie, H.A.F. Integrated Insect Pest Management. In Pests Control and Acarology; Haouas, D., Hufnagel, L., Eds.; IntechOpen Limited: London, UK, 2018; p. 81827. [Google Scholar] [CrossRef] [Green Version]
- Badenes-Perez, F.R.; Reichelt, M.; Heckel, D.G. Can sulfur fertilisation improve the effectiveness of trap crops for diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae)? Pest Manag. Sci. 2010, 66, 832–838. [Google Scholar] [CrossRef]
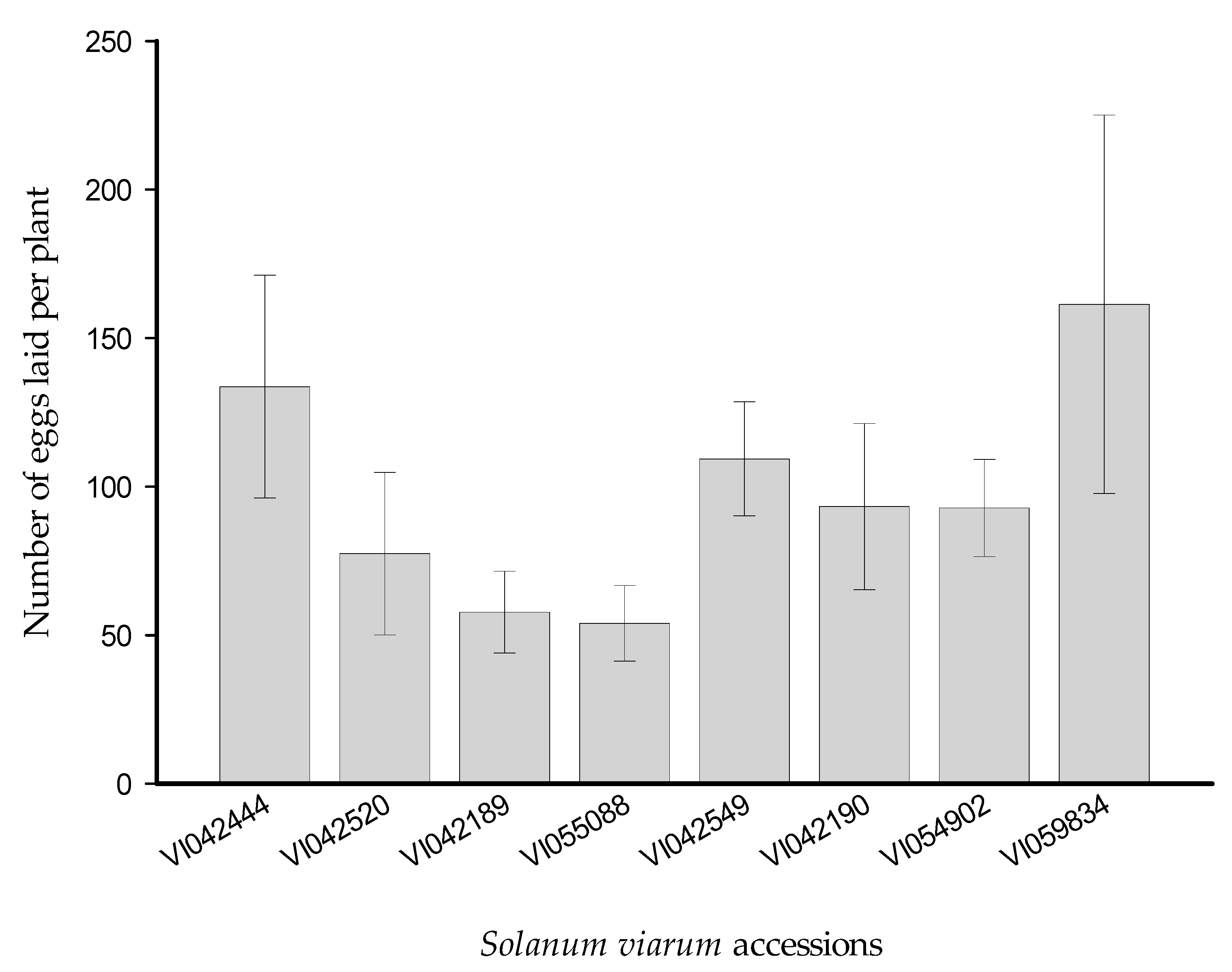

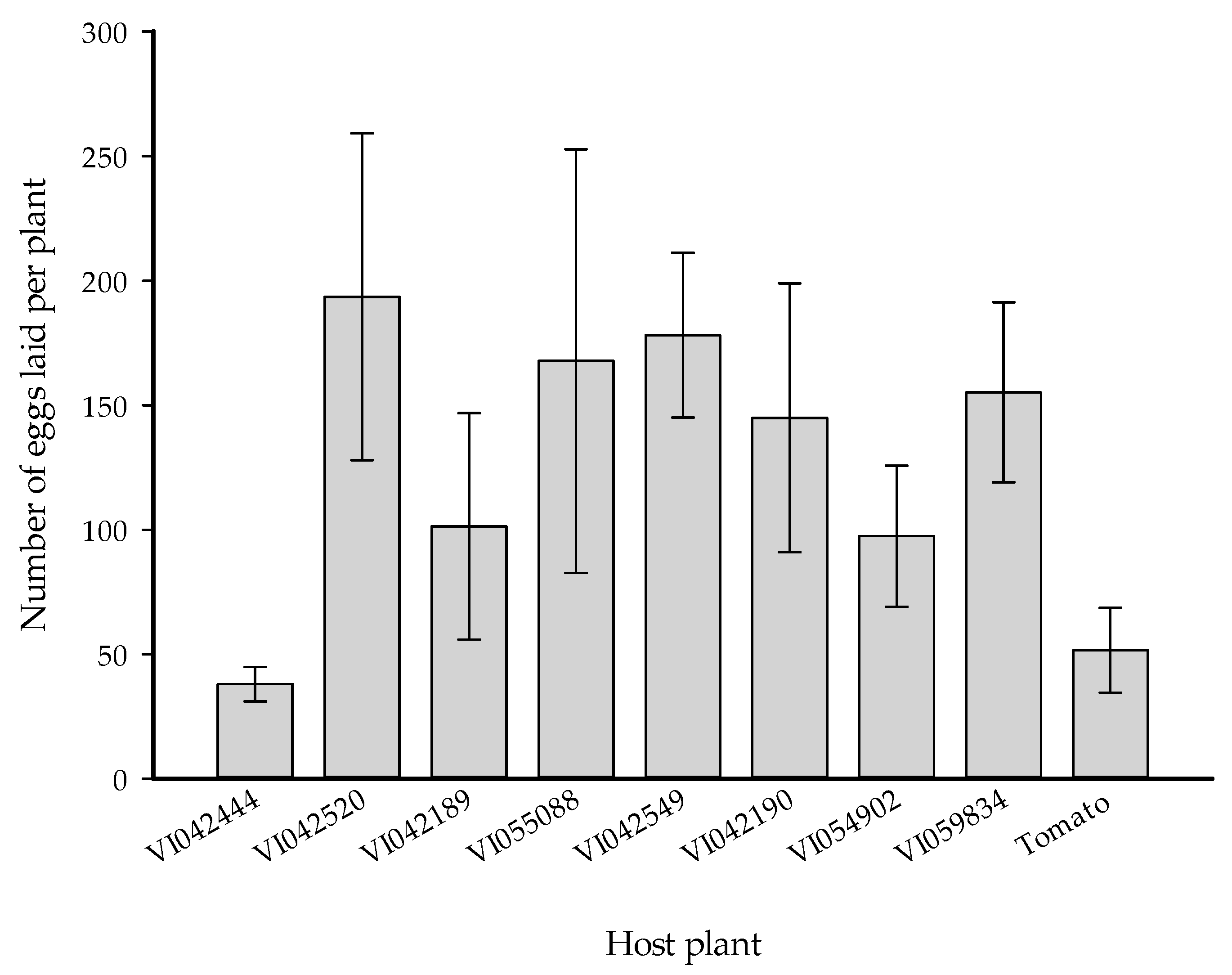
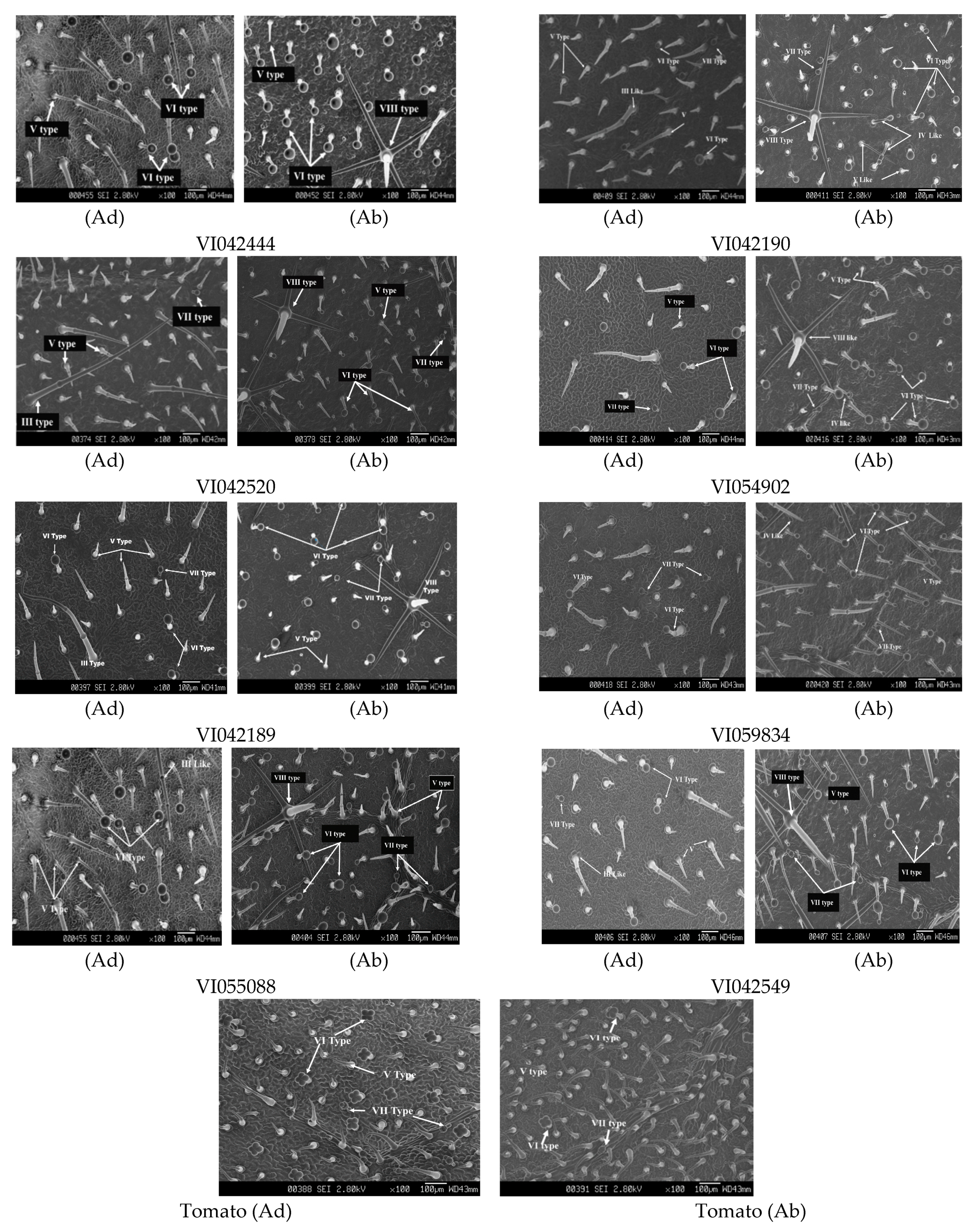
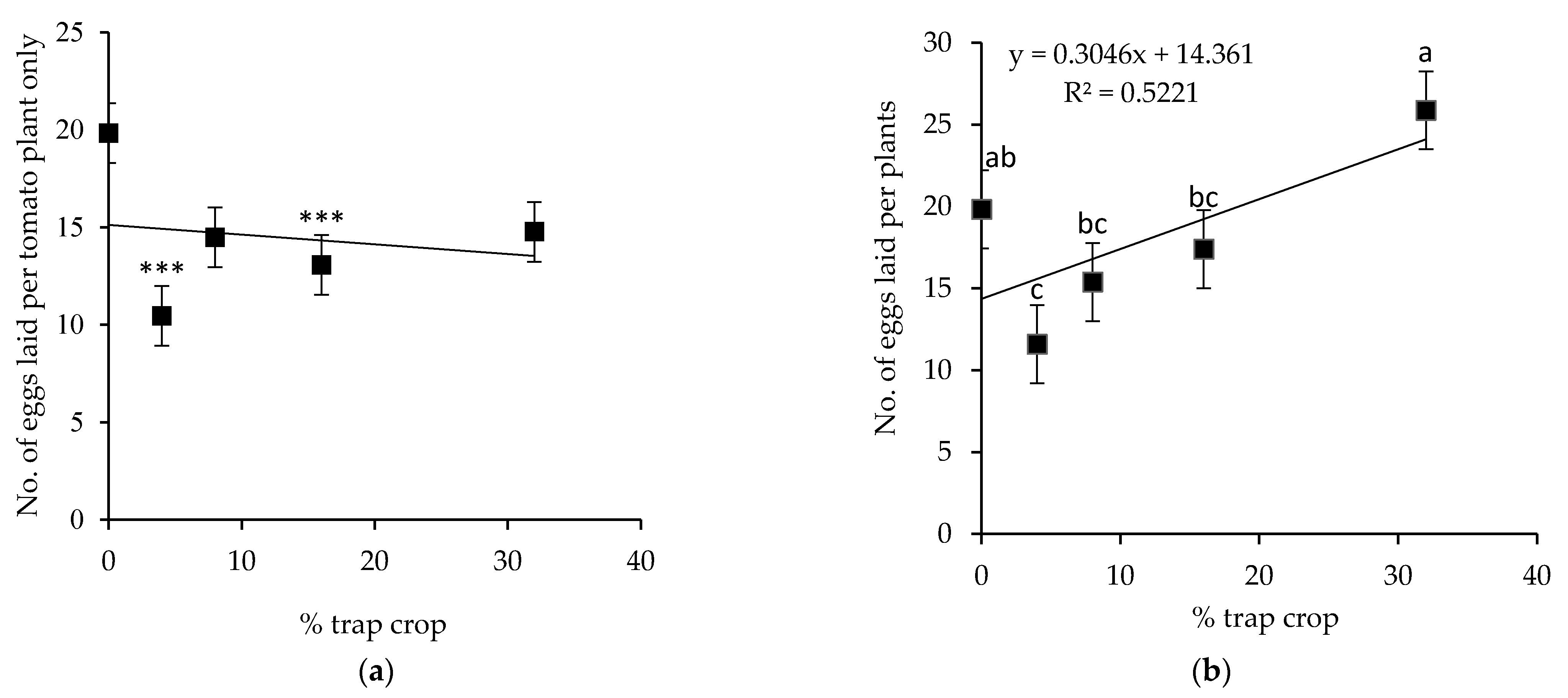
| Plant Species | Number of Eggs Laid Per Plant (Mean ± SEM) | t-Value | p-Value | Significance |
|---|---|---|---|---|
| S. viarum VI042444 | 104.00 ± 66.18 | 0.4454 | 0.6791 | ns |
| (8.28 ± 2.99) | ||||
| Tomato | 55.60 ± 19.67 | |||
| (7.04 ± 1.28) | ||||
| S. viarum VI042520 | 182.80 ± 101.59 | 1.0232 | 0.3641 | ns |
| (11.16 ± 3.83) | ||||
| Tomato | 50.50 ± 18.45 | |||
| (6.75 ± 1.18) | ||||
| S. viarum VI042189 | 362.00 ± 102.89 | 4.8531 | 0.0083 | ** |
| (18.07 ± 3.00) | ||||
| Tomato | 6.60 ± 3.84 | |||
| (2.23 ± 0.73) | ||||
| S. viarum VI055088 | 580.00 ± 243.56 | 3.5657 | 0.0235 | * |
| (21.19 ± 5.75) | ||||
| Tomato | 29.80 ± 23.83 | |||
| (4.23 ± 1.76) | ||||
| S. viarum VI042549 | 180.40 ± 107.13 | 1.9046 | 0.1296 | ns |
| (11.54 ± 3.45) | ||||
| Tomato | 17.80 ± 11.16 | |||
| (3.47 ± 1.25) | ||||
| S. viarum VI042190 | 330.60 ± 165.82 | 3.2449 | 0.0315 | * |
| (16.20 ± 4.14) | ||||
| Tomato | 23.00 ± 7.36 | |||
| (4.39 ± 1.02) | ||||
| S. viarum VI054902 | 97.80 ± 45.06 | 1.2157 | 0.2909 | ns |
| (8.56 ± 2.50) | ||||
| Tomato | 55.80 ± 41.07 | |||
| (6.08 ± 2.20) | ||||
| S. viarum VI059834 | 281.80 ± 62.48 | 2.0954 | 0.1042 | ns |
| (16.38 ± 1.87) | ||||
| Tomato | 103.60 ± 41.63 | |||
| (9.00 ± 2.40) |
| Plant | Glandular Trichome Density/mm2 Leaf | Non-Glandular Trichome Density/mm2 Leaf | ||
|---|---|---|---|---|
| Adaxial | Abaxial | Adaxial | Abaxial | |
| S. viarum VI042444 | 3.88 ± 0.15 a | 7.12 ± 0.83 a | 4.90 ± 0.36 | 4.37 ± 0.08 b |
| S. viarum VI042520 | 2.75 ± 0.12 ab | 5.67 ± 0.77 ab | 5.05 ± 0.36 | 4.83 ± 0.16 b |
| S. viarum VI042189 | 2.75 ± 0.23 ab | 5.67 ± 0.15 ab | 4.56 ± 0.30 | 4.93 ± 0.62 b |
| S. viarum VI055088 | 3.05 ± 0.42 ab | 6.23 ± 0.17 a | 5.47 ± 0.85 | 4.14 ± 0.37 b |
| S. viarum VI042549 | 1.88 ± 0.26 b | 3.93 ± 0.17 b | 3.53 ± 0.19 | 3.57 ± 0.27 b |
| S. viarum VI042190 | 2.95 ± 0.36 ab | 6.25 ± 0.30 a | 5.02 ± 0.96 | 4.37 ± 0.33 b |
| S. viarum VI054902 | 2.63 ± 0.25 ab | 5.77 ± 0.48 ab | 5.81 ± 0.92 | 5.52 ± 0.45 b |
| S. viarum VI059834 | 2.87 ± 0.54 ab | 5.52 ± 0.45 ab | 4.47 ± 0.29 | 5.09 ± 0.33 b |
| Tomato (check) | 1.88 ± 0.26 b | 0.60 ± 0.13 c | 6.38 ± 0.75 | 11.15 ± 0.49 a |
| Plant | Total Acyl Sugar (µmol/g of Dry Weight) | Total Phenolic Content (mg/100 g of Dry Weight) |
|---|---|---|
| S. viarum VI042444 | 2.25 ± 0.23 | 2432.70 ± 192.65 ab |
| S. viarum VI042520 | 2.08 ± 0.05 | 2030.60 ± 242.89 b |
| S. viarum VI042189 | 2.04 ± 0.21 | 2568.42 ± 126.61 ab |
| S. viarum VI055088 | 1.79 ± 0.06 | 2384.26 ± 107.31 ab |
| S. viarum VI042549 | 2.26 ± 0.19 | 2298.66 ± 76.13 ab |
| S. viarum VI042190 | 1.81 ± 0.23 | 2674.01 ± 146.47 ab |
| S. viarum VI054902 | 2.45 ± 0.40 | 2825.50 ± 203.46 a |
| S. viarum VI059834 | 2.49 ± 0.12 | 2675.56 ± 136.49 ab |
| Tomato | 1.68 ± 0.11 | 1170.67 ± 76.76 c |
| Treatments | Initial No. of Larvae | Larval Mortality (%) | Larval Development (Days) | Pupal Weight (mg) |
|---|---|---|---|---|
| S. viarum VI042444 | 100 | 55.00 ± 6.64 a | 27.10 ± 0.79 a | 119.14 ± 1.98 c |
| S. viarum VI042520 | 100 | 53.33 ± 4.41 a | 25.73 ± 0.47 a | 102.36 ± 2.55 c |
| S. viarum VI042189 | 100 | 55.00 ± 6.64 a | 27.18 ± 0.56 a | 116.43 ± 6.16 c |
| S. viarum VI055088 | 100 | 51.67 ± 6.67 a | 25.25 ± 0.43 a | 114.75 ± 6.00 c |
| S. viarum VI042549 | 100 | 56.67 ± 1.67 a | 25.1 ± 0.22 a | 119.73 ± 6.06 c |
| S. viarum VI042190 | 100 | 55.00 ± 7.64 a | 25.43 ± 0.26 a | 119.18 ± 0.93 c |
| S. viarum VI054902 | 100 | 60.00 ± 5.77 a | 26.75 ± 0.46 a | 110.13 ± 1.52 c |
| S. viarum VI059834 | 100 | 51.67 ± 7.26 a | 26.38 ± 0.69 a | 112.87 ± 3.99 c |
| Tomato | 100 | 33.33 ± 1.67 ab | 20.71 ± 0.50 b | 175.76 ± 2.77 b |
| Artificial diet | 100 | 21.67 ± 1.67 b | 14.60 ± 0.59 c | 234.59 ± 3.50 a |
| Correlation Between H. armigera Oviposition and Evaluated Plant Parameters. | ||||||
|---|---|---|---|---|---|---|
| Glandular Trichome | Non-Glandular Trichomes | Total Acylsugar Content | Total Phenolic Content | |||
| Adaxial | Abaxial | Adaxial | Abaxial | |||
| No-choice Test | −0.583 | −0.774 * | −0.771 * | −0.288 | −0.583 | −0.774 * |
| Correlation between H. armigera Life Parameters and Evaluated Plant Parameters | ||||||
| Larval mortality (%) | 0.534 | 0.850 ** | −0.696 * | −0.907 ** | 0.528 | 0.882 ** |
| Larval duration (days) | 0.389 | 0.811 ** | −0.676 * | −0.963 ** | 0.448 | 0.830 ** |
| Pupal wt. (mg) | −0.440 | −0.851 ** | 0.499 | 0.895 ** | −0.558 | −0.881 ** |
| Correlation between Trichomes and Evaluated Plant Secondary Metabolites | ||||||
| Total acylsugar content | 0.174 | 0.372 | −0.456 | −0.418 | ||
| Total phenolic content | 0.505 | 0.830 ** | −0.437 | −0.792 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyawali, P.; Hwang, S.-Y.; Sotelo-Cardona, P.; Srinivasan, R. Elucidating the Fitness of a Dead-End Trap Crop Strategy against the Tomato Fruitworm, Helicoverpa armigera. Insects 2021, 12, 506. https://doi.org/10.3390/insects12060506
Gyawali P, Hwang S-Y, Sotelo-Cardona P, Srinivasan R. Elucidating the Fitness of a Dead-End Trap Crop Strategy against the Tomato Fruitworm, Helicoverpa armigera. Insects. 2021; 12(6):506. https://doi.org/10.3390/insects12060506
Chicago/Turabian StyleGyawali, Purushottam, Shaw-Yhi Hwang, Paola Sotelo-Cardona, and Ramasamy Srinivasan. 2021. "Elucidating the Fitness of a Dead-End Trap Crop Strategy against the Tomato Fruitworm, Helicoverpa armigera" Insects 12, no. 6: 506. https://doi.org/10.3390/insects12060506
APA StyleGyawali, P., Hwang, S.-Y., Sotelo-Cardona, P., & Srinivasan, R. (2021). Elucidating the Fitness of a Dead-End Trap Crop Strategy against the Tomato Fruitworm, Helicoverpa armigera. Insects, 12(6), 506. https://doi.org/10.3390/insects12060506









