Effects of Different Nitrogen Sources and Ratios to Carbon on Larval Development and Bioconversion Efficiency in Food Waste Treatment by Black Soldier Fly Larvae (Hermetia illucens)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Larvae, Food Waste, and Nitrogen Source Preparation
2.2. Experimental Design
2.3. Analysis of Larval Development and Nutrient Composition
2.4. Assessment of the Process Efficiency
2.5. Enzyme Activity and Amino Acid Composition Analysis
2.6. Statistical Analyses
3. Results
3.1. Effects of Nitrogen Source on the Food Waste Treatment
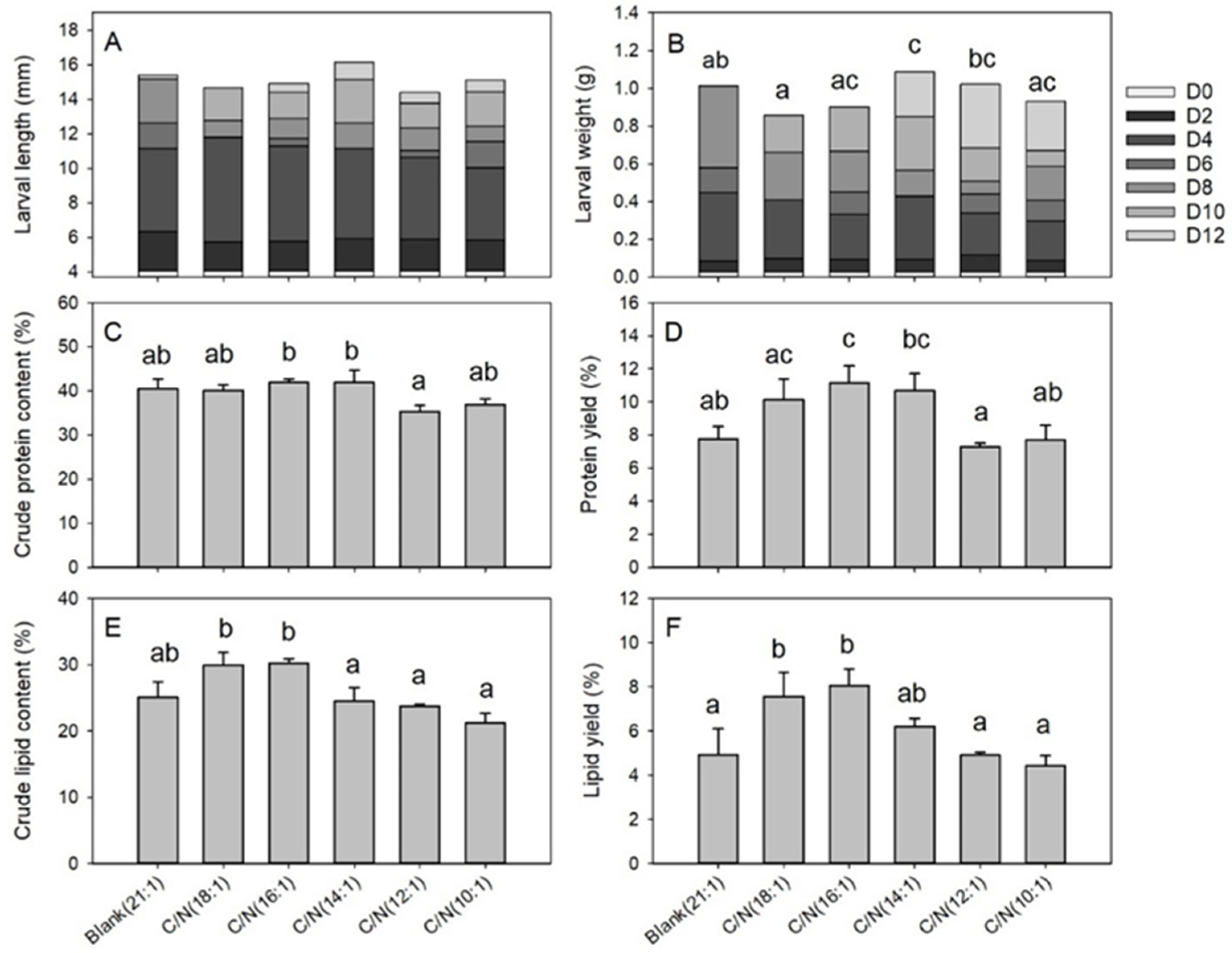
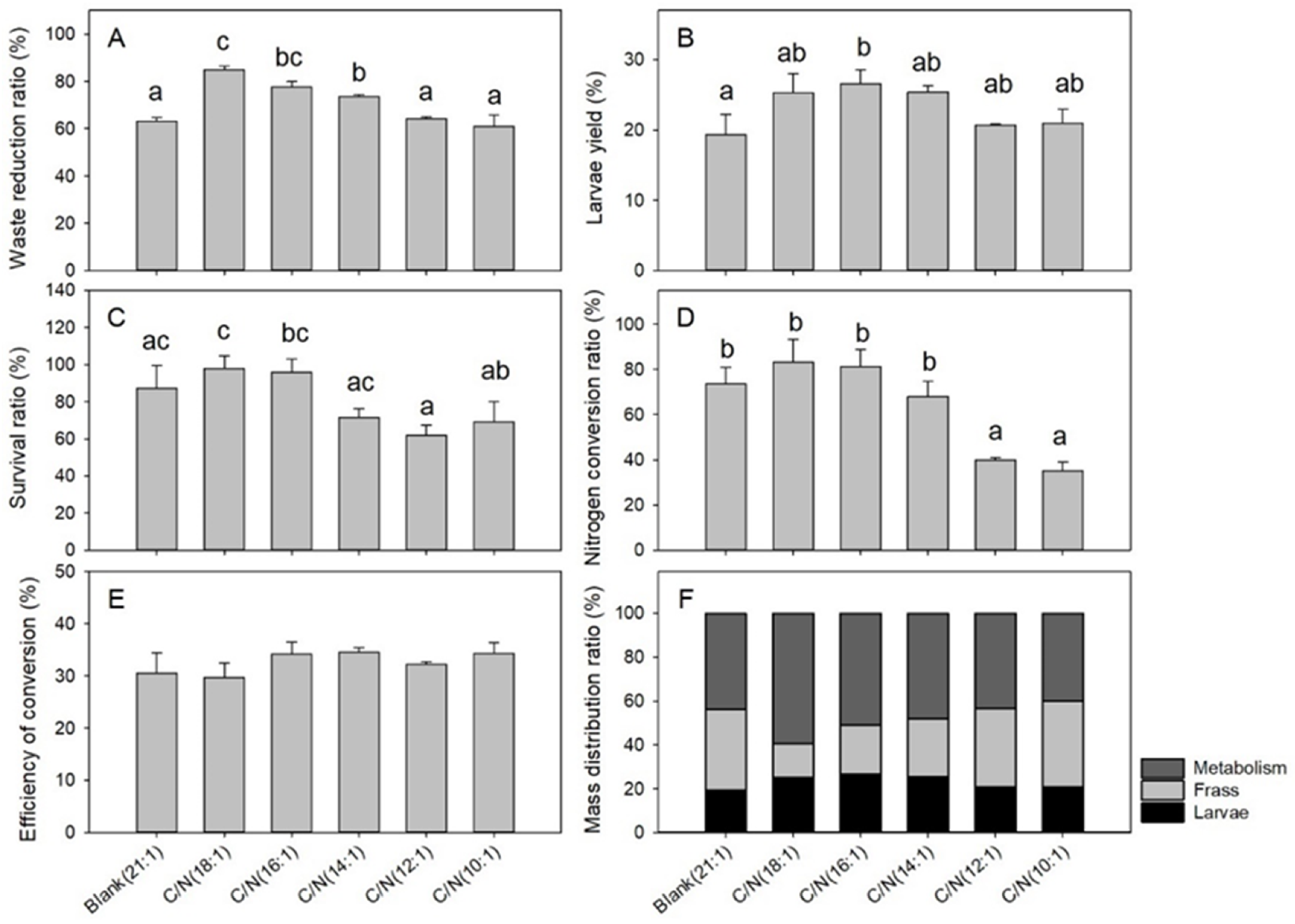
3.2. Effects of C/N on the Food Waste Treatment
3.3. Effects of C/N on Larval Enzyme Activity and Amino Acid Composition
4. Discussion
4.1. Effects of Nitrogen Source on the Bioconversion Process
4.2. Effects of C/N on the Bioconversion Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.; Yun, E.; Goo, T. Antimicrobial Activity of an Extract of Hermetia illucens Larvae Immunized with Lactobacillus casei against Salmonella Species. Insects 2020, 11, 704. [Google Scholar] [CrossRef]
- Fischer, H.; Romano, N.; Sinha, A.K. Conversion of Spent Coffee and Donuts by Black Soldier Fly (Hermetia illucens) Larvae into Potential Resources for Animal and Plant Farming. Insects 2021, 12, 332. [Google Scholar] [CrossRef]
- Cickova, H.; Newton, G.L.; Lacy, R.C.; Kozanek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Huis, A.V. Black soldier fly from pest to ‘crown jewel’ of the insects as feed industry: An historical perspective. J. Insects Food Feed. 2020, 6, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.B.; Tomberlin, J.K.; Cai, M.M.; Xiao, X.P.; Zheng, L.Y.; Yu, Z.N. Research and industrialisation of Hermetia illucens L. in China. J. Insects Food Feed. 2020, 6, 5–12. [Google Scholar] [CrossRef]
- Gladun, V.V. The first record of Hermetia illucens (Diptera, Stratiomyidae) from Russia. Nat. Conserv. Res. 2019, 4. [Google Scholar] [CrossRef]
- Marshall, S.A.; Woodley, N.E.; Hauser, M. The historical spread of the Black Soldier Fly, Hermetia illucens (L.) (Diptera, Stratiomyidae, Hermetiinae), and its establishment in Canada. J. Kans. Entomol. Soc. 2015, 146, 51–56. [Google Scholar]
- Üstüner, T.; Hasbenlí, A.; Rozkošný, R. The first record of Hermetia illucens (Linnaeus, 1758) (Diptera, Stratiomyidae) from the Near East. Stud. Dipterol. 2003, 10, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Holmes, L.A.; Vanlaerhoven, S.L.; Tomberlin, J.K. Lower temperature threshold of black soldier fly (Diptera: Stratiomyidae) development. J. Insects Food Feed. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Harnden, L.M.; Tomberlin, J.K. Effects of temperature and diet on black soldier fly, Hermetia illucens (L.) (Diptera: Stratiomyidae), development. Forensic Sci. Int. 2016, 266, 109–116. [Google Scholar] [CrossRef]
- Cammack, J.; Tomberlin, J. The Impact of Diet Protein and Carbohydrate on Select Life-History Traits of The Black Soldier Fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.; Tanga, C.M. Biochar and gypsum amendment of agro-industrial waste for enhanced black soldier fly larval biomass and quality frass fertilizer. PLoS ONE 2020, 15, e238154. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)—Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Lopes, I.G.; Lalander, C.; Vidotti, R.M.; Vinnerås, B. Using Hermetia illucens larvae to process biowaste from aquaculture production. J. Clean. Prod. 2020, 251, 119753. [Google Scholar] [CrossRef]
- Pang, W.; Hou, D.; Nowar, E.E.; Chen, H.; Wang, S. The influence on carbon, nitrogen recycling, and greenhouse gas emissions under different C/N ratios by black soldier fly. Environ. Sci. Pollut. Res. 2020, 27, 42767–42777. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Hao, C.; Garza, E.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrügg, C.; Mathys, A. Decomposition of biowaste macronutrients, microbes, and chemicals in black soldier fly larval treatment: A review. Waste Manag. 2018, 82, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Vinnerås, B.; Sveriges, L. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Surendra, K.C.; Tomberlin, J.K.; van Huis, A.; Cammack, J.A.; Heckmann, L.L.; Khanal, S.K. Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Manag. 2020, 117, 58–80. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; Broekhoven, V.S.; Huis, V.A.; Loon, V.J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food By-Products. PLoS ONE 2015, 10, e144601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, K.U.; Cai, M.; Xiao, X.; Zheng, L.; Hui, W.; Soomro, A.A.; Zhou, Y.; Wu, L.; Yu, Z.; Zhang, J. Cellulose decomposition and larval biomass production from the co-digestion of dairy manure and chicken manure by mini-livestock (Hermetia illucens L.). J. Environ. Manag. 2017, 196, 458–465. [Google Scholar] [CrossRef]
- Fischer, H.; Romano, N. Fruit, vegetable, and starch mixtures on the nutritional quality of black soldier fly (Hermetia illucens) larvae and resulting frass. J. Insects Food Feed. 2021, 7, 319–327. [Google Scholar] [CrossRef]
- Green, T.R.; Popa, R. Enhanced Ammonia Content in Compost Leachate Processed by Black Soldier Fly Larvae. Appl. Biochem. Biotechnol. 2012, 166, 1381–1387. [Google Scholar] [CrossRef]
- Grossule, V.; Lavagnolo, M.C. The treatment of leachate using Black Soldier Fly (BSF) larvae: Adaptability and resource recovery testing. J. Environ. Manag. 2020, 253, 109707. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; Rehman, K.U.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef] [Green Version]
- Erickson, M.C.; Islam, M.; Sheppard, C.; Liao, J.; Doyle, M.P. Reduction of Escherichia coli O157:H7 and Salmonella enterica Serovar Enteritidis in Chicken Manure by Larvae of the Black Soldier Fly. J. Food Prot. 2004, 67, 685. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, W.; Xu, J.; Xue, H.; Stanford, K.; McAllister, T.A.; Xu, W.; Martinez-Abarca, F. Evaluation of compost, vegetable and foozzd waste as amendments to improve the composting of NaOH/NaClO-contaminated poultry manure. PLoS ONE 2018, 13, e205112. [Google Scholar] [CrossRef] [Green Version]
- Janssen, R.H.; Vincken, J.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, Q.; Zhang, J.; Yu, Z. Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 2012, 41, 75–79. [Google Scholar] [CrossRef]
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag. 2020, 102, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Wei, D.; Wei, D.; Yuan, G.; Wang, J. The effect of dietary restriction on longevity, fecundity, and antioxidant responses in the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). J. Insect Physiol. 2013, 59, 1008–1016. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 14 April 2021).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Barragan-Fonseca, K.B.; Dicke, M.; Loon, V.J.J.A. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Smet, S.D. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]


| Groups | N Source | N Element | N Source | Final Carbon and Nitrogen Properties | ||
|---|---|---|---|---|---|---|
| Supplied 1 | Amount | Amount | after Nitrogen Source Supplement | |||
| g/100 g FW | g/100 g FW | C (%) | N (%) | C/N | ||
| Trial 1 | ||||||
| Blank | None | 0 | 0 | 47.0 | 2.26 | 21:1 |
| NH4CL | NH4CL | 1 | 3.82 | 47.0 | 3.26 | 14:1 |
| NaNO3 | NaNO3 | 1 | 6.06 | 47.0 | 3.26 | 14:1 |
| Urea | Urea | 1 | 2.14 | 47.4 | 3.26 | 15:1 |
| Uric acid | Uric acid | 1 | 3.00 | 48.1 | 3.26 | 15:1 |
| Gly | Gly | 1 | 5.35 | 48.7 | 3.26 | 15:1 |
| Glu | L-Glu | 1 | 10.53 | 51.3 | 3.26 | 16:1 |
| Glu/Asp | L-Glu/L-Asp (1:1) | 1 | 10.00 | 50.9 | 3.26 | 16:1 |
| Soybean flour | Soybean flour | 1 | 16.72 | 55.1 | 3.26 | 17:1 |
| Fish meal | Fish meal | 1 | 9.52 | 50.8 | 3.26 | 16:1 |
| Trial 2 | ||||||
| Blank (21:1) | None | 0 | 0 | 47.0 | 2.26 | 21:1 |
| C/N(18:1) | Urea | 0.36 | 0.76 | 47.2 | 2.62 | 18:1 |
| C/N(16:1) | Urea | 0.68 | 1.46 | 47.3 | 2.94 | 16:1 |
| C/N(14:1) | Urea | 1.10 | 2.36 | 47.5 | 3.36 | 14:1 |
| C/N(12:1) | Urea | 1.66 | 3.55 | 47.7 | 3.92 | 12:1 |
| C/N(10:1) | Urea | 2.44 | 5.23 | 48.0 | 4.70 | 10:1 |
| Groups | POD (U/g) | SOD (U/g) | CAT (U/g) | GSH-px (U/g) |
|---|---|---|---|---|
| Blank (21:1) | 0.056 ± 0.079 | 0.149 ± 0.082 ab | 0.071 ± 0.034 | 7.40 ± 0.78 |
| C/N (18:1) | 0.00 ± 0.00 | 0.249 ± 0.081 b | 0.094 ± 0.026 | 8.46 ± 1.77 |
| C/N (16:1) | 0.049 ± 0.048 | 0.213 ± 0.133 ab | 0.035 ± 0.034 | 9.91 ± 4.56 |
| C/N (14:1) | 0.00 ± 0.00 | 0.058 ± 0.009 ab | 0.039 ± 0.027 | 7.12 ± 2.03 |
| C/N (12:1) | 0.170 ± 0.121 | 0.00 ± 0.00 a | 0.037 ± 0.018 | 4.90 ± 0.62 |
| C/N (10:1) | 0.00 ± 0.00 | 0.00 ± 0.00 a | 0.042 ± 0.008 | 5.94 ± 1.39 |
| F = 2.266, p = 0.114 | F = 4.561, p = 0.015 | F = 1.710, p = 0.207 | F = 0.816, p = 0.561 |
| Amino Acids | Blank (21:1) | C/N (18:1) | C/N (16:1) | C/N (14:1) | C/N (12:1) | C/N (10:1) | Overall |
|---|---|---|---|---|---|---|---|
| Cys | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.9 ± 0.1 |
| Met | 3.3 ± 0.2 | 3.4 ± 0.1 | 3.2 ± 0.1 | 3.3 ± 0.2 | 3.2 ± 0.2 | 3.1 ± 0.1 | 3.2 ± 0.2 |
| Asp | 8.5 ± 0.4 | 9.2 ± 1.0 | 9.1 ± 0.8 | 8.8 ± 0.3 | 9.4 ± 0.8 | 9.5 ± 0.6 | 9.1 ± 0.8 |
| Thr | 4.4 ± 0.2 | 4.5 ± 0.3 | 4.5 ± 0.2 | 4.3 ± 0.2 | 4.5 ± 0.2 | 4.5 ± 0.2 | 4.4 ± 0.2 |
| Ser | 5.0 ± 0.2 | 5.0 ± 0.3 | 5.1 ± 0.3 | 5.0 ± 0.2 | 4.9 ± 0.2 | 5.0 ± 0.2 | 5.0 ± 0.3 |
| Glu | 10.6 ± 0.5 | 11.2 ± 0.7 | 11.6 ± 0.3 | 11.7 ± 1.2 | 10.8 ± 0.6 | 12.3 ± 0.5 | 11.4 ± 0.9 |
| Gly | 6.2 ± 0.3 | 6.0 ± 0.2 | 6.2 ± 0.2 | 6.1 ± 0.3 | 5.9 ± 0.3 | 5.8 ± 0.2 | 6.0 ± 0.3 |
| Ala | 11.1 ± 0.3 | 10.2 ± 0.5 | 9.9 ± 0.6 | 10.0 ± 0.8 | 10.0 ± 0.2 | 9.1 ± 0.3 | 10.0 ± 0.8 |
| Val | 7.0 ± 0.5 | 6.9 ± 0.5 | 7.0 ± 0.3 | 6.7 ± 0.2 | 6.8 ± 0.5 | 6.4 ± 0.4 | 6.8 ± 0.4 |
| Ile | 4.9 ± 0.3 | 4.8 ± 0.3 | 4.8 ± 0.2 | 4.8 ± 0.2 | 4.9 ± 0.3 | 4.8 ± 0.3 | 4.8 ± 0.3 |
| Leu | 7.5 ± 0.2 | 7.5 ± 0.5 | 7.5 ± 0.3 | 7.5 ± 0.1 | 7.9 ± 0.4 | 7.8 ± 0.1 | 7.6 ± 0.3 |
| Tyr | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.0 | 0.6 ± 0.1 | 0.5 ± 0.0 | 0.6 ± 0.1 |
| Phe | 4.5 ± 0.5 | 4.7 ± 0.4 | 4.6 ± 0.1 | 4.4 ± 0.2 | 4.7 ± 0.5 | 4.6 ± 0.3 | 4.6 ± 0.4 |
| Lys | 5.9 ± 0.2 | 5.9 ± 0.1 | 6.1 ± 0.2 | 6.0 ± 0.2 | 6.3 ± 0.1 | 6.4 ± 0.2 | 6.1 ± 0.3 |
| His | 3.5 ± 0.2 | 3.6 ± 0.2 | 3.5 ± 0.1 | 3.5 ± 0.1 | 3.3 ± 0.2 | 3.1 ± 0.1 | 3.4 ± 0.2 |
| Arg | 5.2 ± 0.1 | 4.9 ± 0.5 | 4.8 ± 0.4 | 5.2 ± 0.0 | 5.0 ± 0.1 | 5.3 ± 0.1 | 5.1 ± 0.3 |
| Pro | 7.3 ± 0.1 | 7.1 ± 0.3 | 7.0 ± 1.1 | 7.5 ± 0.4 | 7.2 ± 0.1 | 7.5 ± 0.4 | 7.3 ± 0.5 |
| Trp | 1.3 ± 0.4 | 1.6 ± 0.4 | 1.7 ± 0.1 | 1.6 ± 0.4 | 1.9 ± 0.1 | 1.5 ± 0.0 | 1.6 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhang, S.; Sun, S.; Wu, M.; Bao, Y.; Tong, H.; Ren, M.; Jin, N.; Xu, J.; Zhou, H.; et al. Effects of Different Nitrogen Sources and Ratios to Carbon on Larval Development and Bioconversion Efficiency in Food Waste Treatment by Black Soldier Fly Larvae (Hermetia illucens). Insects 2021, 12, 507. https://doi.org/10.3390/insects12060507
Lu Y, Zhang S, Sun S, Wu M, Bao Y, Tong H, Ren M, Jin N, Xu J, Zhou H, et al. Effects of Different Nitrogen Sources and Ratios to Carbon on Larval Development and Bioconversion Efficiency in Food Waste Treatment by Black Soldier Fly Larvae (Hermetia illucens). Insects. 2021; 12(6):507. https://doi.org/10.3390/insects12060507
Chicago/Turabian StyleLu, Yan, Shouyu Zhang, Shibo Sun, Minghuo Wu, Yongming Bao, Huiyan Tong, Miaomiao Ren, Ning Jin, Jianqiang Xu, Hao Zhou, and et al. 2021. "Effects of Different Nitrogen Sources and Ratios to Carbon on Larval Development and Bioconversion Efficiency in Food Waste Treatment by Black Soldier Fly Larvae (Hermetia illucens)" Insects 12, no. 6: 507. https://doi.org/10.3390/insects12060507







