Insect Epigenetic Mechanisms Facing Anthropogenic-Derived Contamination, an Overview
Abstract
:Simple Summary
Abstract
1. Introduction
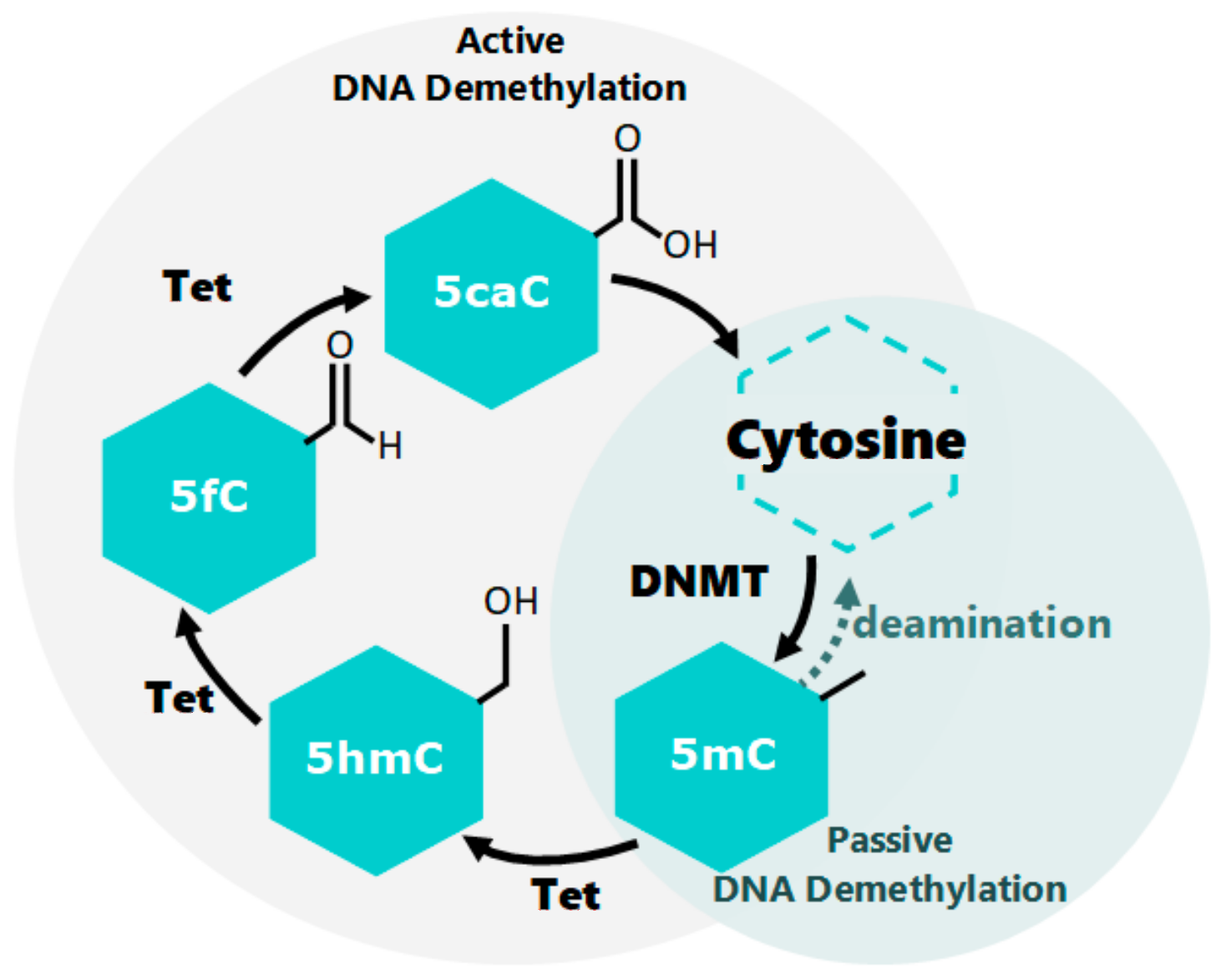
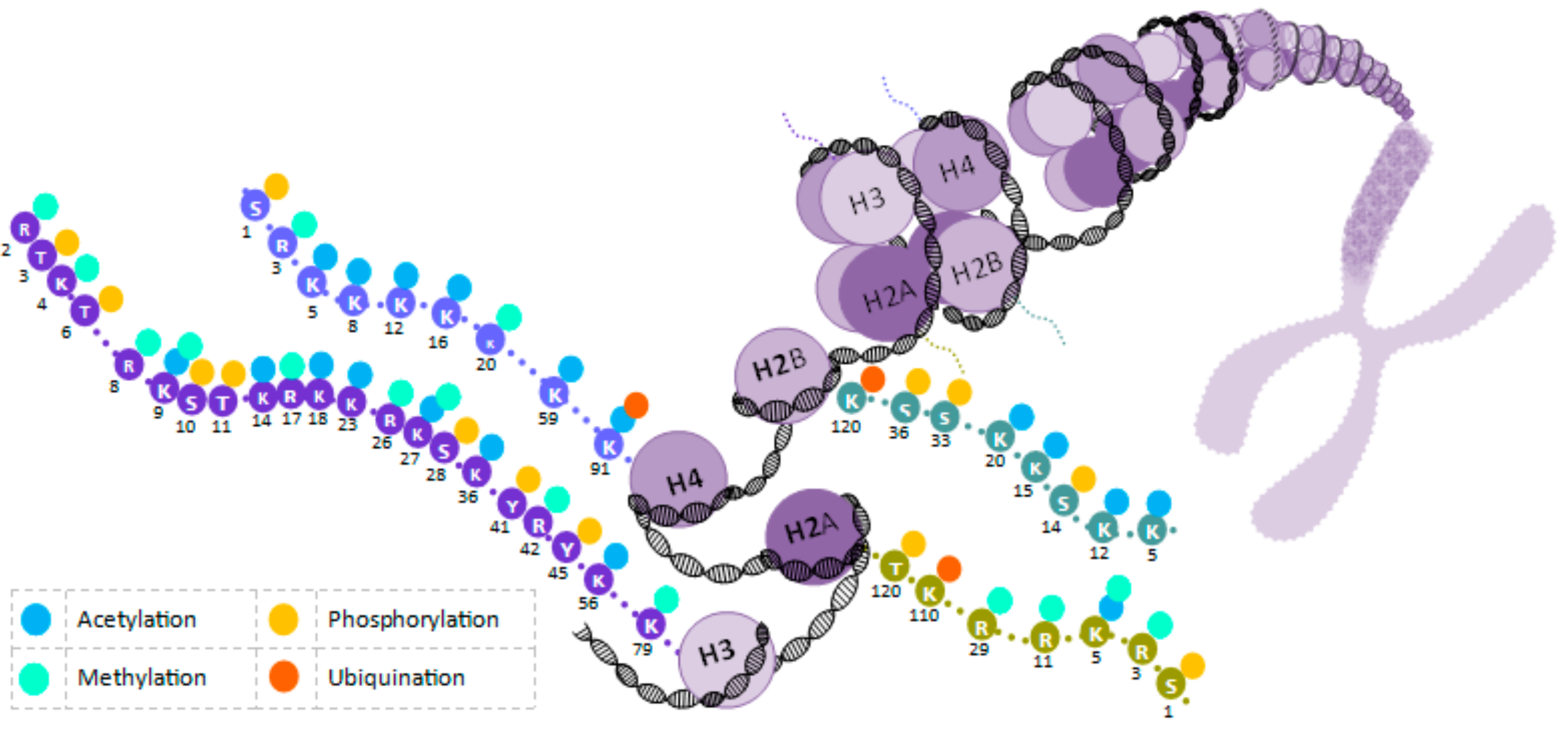
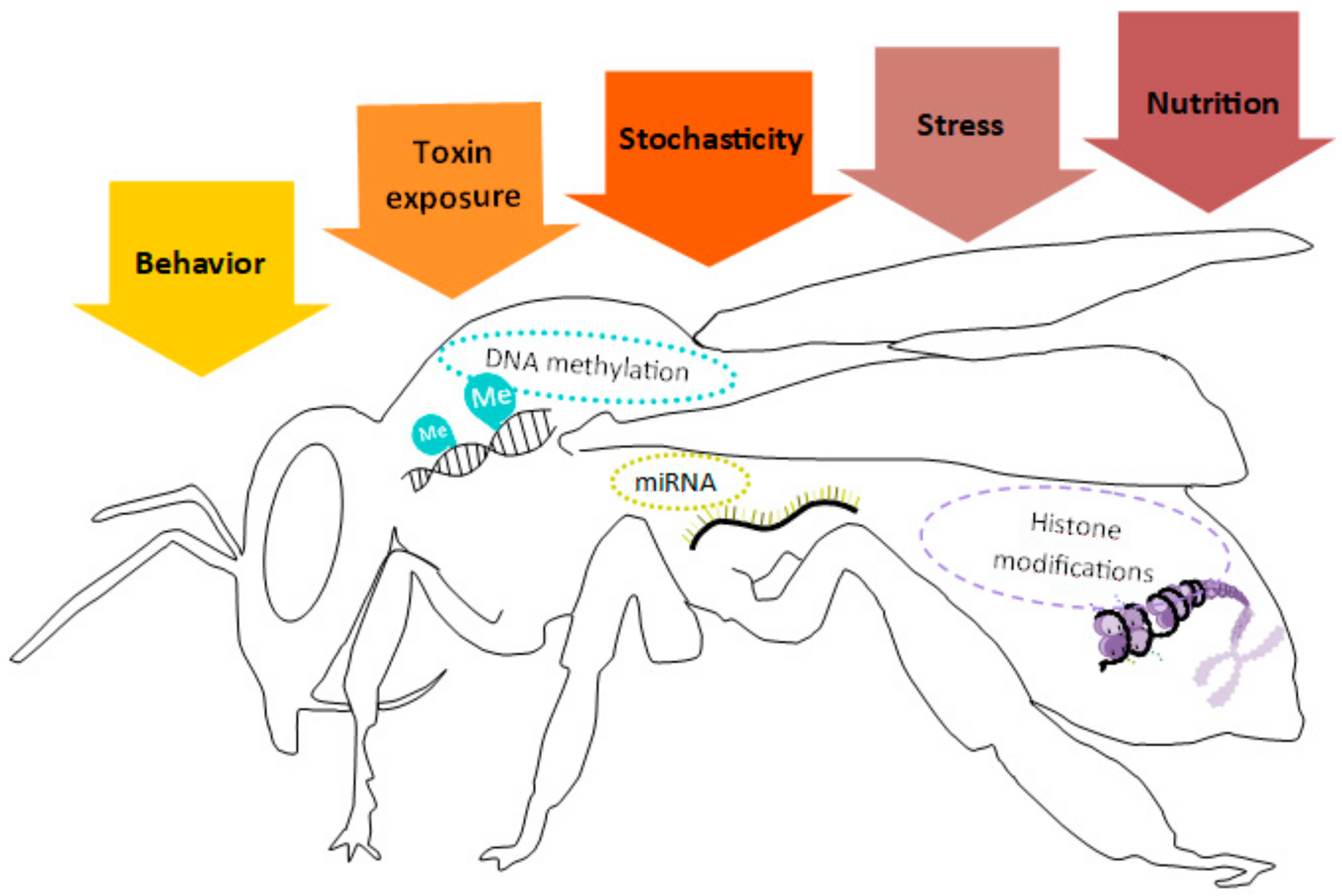
2. Epigenetic Molecular Mechanisms
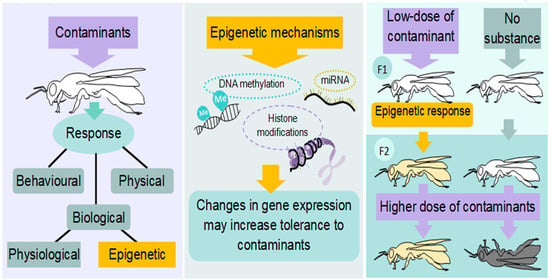
Insect Epigenetics and Contamination
3. Pesticide Chemical Behavior in the Environment
4. Pesticide Effects on Pest and Non-Target Organisms
5. Neonicotinoid Insecticides
Neonicotinoid Effects on Bees
6. The Link between Insect EMMs and Current Loss of Biodiversity
7. Conclusions
8. Further Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burggren, W. Epigenetic Inheritance and its Role in Evolutionary Biology: Re-evaluation and New Perspectives. Biology 2016, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Twyman, R.M.; Vilcinskas, A. Insects as Models to Study the Epigenetic Basis of Disease. Prog. Biophys. Mol. Biol. 2015, 118, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ware, G.W. Effects of Pesticides on Nontarget Organisms. In Residue Reviews; Springer: New York, NY, USA, 1980; pp. 173–201. [Google Scholar]
- Bird, A. Perceptions of Epigenetics. Nature 2007, 447, 396. [Google Scholar] [CrossRef]
- Jablonka, E.; Lamb, M.J. Evolution in Four Dimensions: Genetic, Epigenetic, Behavioral, and Symbolic Variation in the History of Life, 1st ed.; MIT Press: Cambridge, MA, USA, 2005; Volume 5. [Google Scholar]
- Jaenisch, R.; Bird, A. Epigenetic Regulation of Gene Expression: How the Genome Integrates Intrinsic and Environmental signals. Nat. Gen. 2003, 33, 245. [Google Scholar] [CrossRef]
- Glastad, K.M.; Hunt, B.G.; Goodisman, M.A. Epigenetics in Insects: Genome Regulation and the Generation of Phenotypic Diversity. Ann. Rev. Entomol. 2019, 64, 185–203. [Google Scholar] [CrossRef] [Green Version]
- Berens, A.J.; Hunt, J.H.; Toth, A.L. Nourishment Level Affects Caste-related Gene Expression in Polistes wasps. BMC Gen. 2015, 16, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Patalano, S.; Hore, T.A.; Reik, W.; Sumner, S. Shifting Behaviour: Epigenetic Reprogramming in Eusocial Insects. Curr. Opin. Cell Biol. 2012, 24, 367–373. [Google Scholar] [CrossRef]
- Kavi, L.A.; Kaufman, P.E.; Scott, J.G. Genetics and Mechanisms of Imidacloprid Resistance in House flies. Pest. Biochem. Physiol. 2014, 109, 64–69. [Google Scholar] [CrossRef]
- Oppold, A.; Kress, A.; Bussche, J.V.; Diogo, J.B.; Kuch, U.; Oehlmann, J.; Vandegehuchte, M.B.; Müller, R. Epigenetic Alterations and Decreasing Insecticide Sensitivity of the Asian Tiger Mosquito Aedes albopictus. EcoToxicol. Environ. Saf. 2015, 122, 45–53. [Google Scholar] [CrossRef]
- Feldhaar, H.; Otti, O. Pollutants and Their Interaction with Diseases of Social Hymenoptera. Insects 2020, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Ardura, A.; Clusa, L.; Zaiko, A.; Garcia-Vazquez, E.; Miralles, L. Stress Related Epigenetic Changes May Explain Opportunistic Success in Biological Invasions in Antipode mussels. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 Percent Decline Over 27 Years in Total Flying Insect Biomass in Protected Areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ Warning to Humanity on Insect Extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Van der Sluijs, J.P. Insect Decline, an Emerging Global Environmental Risk. Curr. Opin. Environ. Sustain. 2020, 46, 39–42. [Google Scholar] [CrossRef]
- Pearson, M.M. Phase Variation of the mrp Fimbrial Promoter. In Proteus mirabilis; Humana: New York, NY, USA, 2019; pp. 121–127. [Google Scholar]
- Frandi, A.; Collier, J. Multilayered Control of Chromosome Replication in Caulobacter crescentus. Biochem. Soc. Trans. 2019, 47, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinner, M.K.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; Ben Maamar, M.; McCarrey, J.R. Transgenerational Sperm DNA Methylation Epimutation Developmental Origins Following Ancestral Vinclozolin Exposure. Epigenetics 2019, 14, 721–739. [Google Scholar] [CrossRef] [Green Version]
- House, M.; Lukens, L. The Role of Germinally Inherited Epialleles in Plant Breeding: An Update. In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications; Springer: Cham, Switzerland, 2019; pp. 115–128. [Google Scholar]
- Tucci, V.; Isles, A.R.; Kelsey, G.; Ferguson-Smith, A.C.; Erice Imprinting Group. Genomic Imprinting and Physiological Processes in Mammals. Cell 2019, 176, 952–965. [Google Scholar] [CrossRef] [Green Version]
- Di Felice, F.; Egidi, A.; D’Alfonso, A.; Camilloni, G. Fob1p recruits DNA Topoisomerase I to Ribosomal Genes Locus and Contributes to its Transcriptional Silencing Maintenance. Int. J. Biochem. Cell Biol. 2019, 110, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Żylicz, J.J.; Bousard, A.; Žumer, K.; Dossin, F.; Mohammad, E.; da Rocha, S.T.; Schwalb, B.; Syx, L.; Dingli, F.; Loew, D.; et al. The Implication of Early Chromatin Changes in X Chromosome Inactivation. Cell 2019, 176, 182–197. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, A.I.; Dementyeva, E.V.; Zakharova, I.S.; Zakian, S.M. Diverse Developmental Strategies of X Chromosome Dosage Compensation in Eutherian Mammals. Int. J. Dev. Biol. 2019, 63, 223–233. [Google Scholar] [CrossRef]
- Zhong, P.; Li, J.; Luo, L.; Zhao, Z.; Tian, Z. TOP1α Regulates FLOWERING LOCUS C Expression by Coupling Histone Modification and Transcription Machinery. Development 2019, 146, 4. [Google Scholar] [CrossRef] [Green Version]
- Posner, R.; Toker, I.A.; Antonova, O.; Star, E.; Anava, S.; Azmon, E.; Hendricks, M.; Bracha, S.; Gingold, H.; Rechavi, O. Neuronal Small RNAs Control Behavior Transgenerationally. Cell 2019, 177, 1814–1826. [Google Scholar] [CrossRef] [Green Version]
- Dupont, C.; Kappeler, L.; Saget, S.; Grandjean, V.; Lévy, R. Role of miRNA in the Transmission of Metabolic Diseases Associated with Paternal Diet-induced Obesity. Front. Gen. 2019, 10, 337. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Raven, P.H. Vertebrates on the Brink s Indicators of Biological Annihilation and the Sixth Mass Extinction. Proc. Natl. Acad. Sci. USA 2020, 117, 13596–13602. [Google Scholar] [CrossRef]
- Habel, J.C.; Samways, M.J.; Schmitt, T. Mitigating the Precipitous Decline of Terrestrial European Insects: Requirements for a New Strategy. Biodivers. Conserv. 2019, 28, 1343–1360. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological Annihilation Via the Ongoing Sixth Mass Extinction Signaled by Vertebrate Population Losses and Declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giersch, J.J.; Hotaling, S.; Kovach, R.P.; Jones, L.A.; Muhlfeld, C.C. Climate-Induced Glacier and Snow Loss Imperils Alpine Stream Insects. Glob. Chang. Biol. 2017, 23, 2577–2589. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s Sixth Mass Extinction Already Arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Kim, K.C. Biodiversity, Conservation and Inventory: Why Insects Matter. Biodivers. Conserv. 1993, 2, 191–214. [Google Scholar] [CrossRef]
- Hamilton, C.; Gemenne, F.; Bonneuil, C. Thinking the anthropocene. In The Anthropocene and The Global Environmental Crisis: Rethinking Modernity In A New Epoch, 1st ed.; Hamilton, C., Bonneuil, C., Gemenne, F., Eds.; Routledge: Abingdon, UK, 2015; pp. 1–13. [Google Scholar]
- Rusiecki, J.A.; Beane Freeman, L.E.; Bonner, M.R.; Alexander, M.; Chen, L.; Andreotti, G.; Barry, K.H.; Moore, L.E.; Byun, H.M.; Kamel, F.; et al. High Pesticide Exposure Events and DNA Methylation Among Pesticide Applicators in The Agricultural Health Study. Environ. Mol. Mutagenesis 2017, 58, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Morais, S.; Dias, E.; Pereira, M.L. Carbamates: Human exposure and health effects. In The Impact of Pesticides; Academy Publication: London, UK, 2012; pp. 21–38. [Google Scholar]
- Requena-Mullor, M.; Navarro-Mena, A.; Wei, R.; López-Guarnido, O.; Lozano-Paniagua, D.; Alarcon-Rodriguez, R. Evaluation of Gonadal Alterations in a Population Environmentally Exposed to a Mixture of Endocrine Active Pesticides. Int. J. Environ. Res. Public Health 2021, 18, 2355. [Google Scholar] [CrossRef]
- Ekroos, J.; Kleijn, D.; Batáry, P.; Albrecht, M.; Báldi, A.; Blüthgen, N.; Knop, E.; Kovács-Hostyánszki, A.; Smith, H.G. High Land-use Intensity in Grasslands Constrains Wild Bee Species Richness in Europe. Biol. Conserv. 2020, 241, 108255. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic Valuation of the Vulnerability of World Agriculture Confronted with Pollinator Decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Xider, K.M. Histopathological and Morphological Alterations in Salivary Gland of House Fly Induced by Oral Administration Thiamethoxam. Kurd. J. Appl. Res. 2018, 3, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Bebane, P.S.; Hunt, B.J.; Pegoraro, M.; Jones, A.C.; Marshall, H.; Rosato, E.; Mallon, E.B. The Effects of the Neonicotinoid Imidacloprid on Gene Expression and DNA Methylation in the Buff-Tailed Bumblebee Bombus terrestris. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190718. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Honey Constituents Up-Regulate Detoxification and Immunity Genes in The Western Honey Bee Apis mellifera. Proc. Natl. Acad. Sci. USA 2013, 110, 8842–8846. [Google Scholar] [CrossRef] [Green Version]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of Neonicotinoids and Fipronil on Non-Target Invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef] [Green Version]
- Brevik, K.; Lindström, L.; McKay, S.D.; Chen, Y.H. Transgenerational Effects of Insecticides—Implications for Rapid Pest Evolution in Agroecosystems. Curr. Opin. Insect Sci. 2018, 26, 34–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppold, A.M.; Müller, R. Epigenetics: A hidden target of insecticides. Adv. Insect Phys. 2017, 53, 313–324. [Google Scholar]
- Gressel, J. Evolving Understanding of The Evolution of Herbicide Resistance. Pest Man. Sci. 2009, 65, 1164–1173. [Google Scholar] [CrossRef]
- Chiron, F.; Chargé, R.; Julliard, R.; Jiguet, F.; Muratet, A. Pesticide Doses, Landscape Structure and their Relative Effects on Farmland Birds. Agric. Ecosyst. Environ. 2014, 185, 153–160. [Google Scholar] [CrossRef]
- Chensheng, L.U.; Warchol, K.M.; Callahan, R.A. In Situ Replication of Honey Bee Colony Collapse Disorder. Bull. Insect 2012, 65, 99–106. [Google Scholar]
- Burdge, G.C.; Lillycrop, K.A. Nutrition, Epigenetics, and Developmental Plasticity: Implications for Understanding Human Disease. Ann. Rev. Nutr. 2010, 30, 315–339. [Google Scholar] [CrossRef]
- Feil, R.; Fraga, M.F. Epigenetics and The Environment: Emerging Patterns and Implications. Nat. Rev. Gen. 2012, 13, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Laubach, Z.M.; Perng, W.; Dolinoy, D.C.; Faulk, C.D.; Holekamp, K.E.; Getty, T. Epigenetics and the Maintenance of Developmental Plasticity: Extending the Signalling Theory Framework. Biol. Rev. 2018, 93, 1323–1338. [Google Scholar] [CrossRef] [PubMed]
- Vidaki, A.; Daniel, B.; Court, D.S. Forensic DNA Methylation Profiling—Potential Opportunities and Challenges. Forensic Sci. Int. Gen. 2013, 7, 499–507. [Google Scholar] [CrossRef]
- Hunt, B.G.; Glastad, K.M.; Yi, S.V.; Goodisman, M.A. The Function of Intragenic DNA Methylation: Insights From Insect Epigenomes. Integr. Comp. Biol. 2013, 53, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Villagra, C.; Frías-Lasserre, D. Epigenetic Molecular Mechanisms in Insects. Neot. Entomol. 2020, 49, 615–642. [Google Scholar] [CrossRef]
- Kim, D.; Thairu, M.W.; Hansen, A.K. Novel Insights Into Insect-Microbe Interactions—Role of Epigenomics and Small Rnas. Front. Plant Sci. 2016, 7, 1164. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N 6-Methyladenosine in Nuclear RNA is a Major Substrate of the Obesity-Associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Dinger, M.E.; Mercer, T.R.; Mehler, M.F. RNA Regulation of Epigenetic Processes. Bioessays 2009, 31, 51–59. [Google Scholar] [CrossRef]
- Bushati, N.; Cohen, S.M. microRNA Functions. Ann. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef]
- Morales-Nebreda, L.; McLafferty, F.S.; Singer, B.D. DNA Methylation as a Transcriptional Regulator of the Immune System. Transl. Res. 2019, 204, 1–8. [Google Scholar] [CrossRef]
- Alavian-Ghavanini, A.; Rüegg, J. Understanding Epigenetic Effects of Endocrine Disrupting Chemicals: From Mechanisms to Novel Test Methods. Basic Clin. Pharmacol. Toxicol. 2018, 122, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Modified Histone Antibodies. Available online: https://www.cusabio.com/catalog-67-1.html (accessed on 12 July 2020).
- Koch, M.W.; Metz, L.M.; Kovalchuk, O. Epigenetics and Mirnas in the Diagnosis and Treatment of Multiple Sclerosis. Trends Mol. Med. 2013, 19, 23–30. [Google Scholar] [CrossRef]
- Bernstein, B.E.; Meissner, A.; Lander, E.S. The Mammalian Epigenome. Cell 2007, 128, 669–681. [Google Scholar] [CrossRef] [Green Version]
- Faulk, C.; Dolinoy, D.C. Timing is Everything: The When and How of Environmentally Induced Changes in the Epigenome of Animals. Epigenetics 2011, 6, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Burggren, W.W. Epigenetics in Insects: Mechanisms, Phenotypes and Ecological and Evolutionary Implications. Adv. Insect Phys. 2017, 53, 1–30. [Google Scholar]
- Kozeretska, I.A.; Serga, S.V.; Koliada, A.K.; Vaiserman, A.M. Epigenetic Regulation of Longevity in Insects. Adv. Insect Phys. 2017, 53, 87–114. [Google Scholar]
- Youngson, N.A.; Whitelaw, E. Transgenerational Epigenetic Effects. Ann. Rev. Gen. Hum. Gen. 2008, 9, 233–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikhodeyev, O.N. The Mechanisms of Epigenetic Inheritance: How Diverse Are They? Biol. Rev. 2018, 93, 1987–2005. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Wang, Y.; Ma, L.; Zhang, W.; Xu, B. Genome-Wide Differential DNA Methylation in Reproductive, Morphological, and Visual System Differences Between Queen Bee and Worker Bee (Apis mellifera). Front. Gen. 2020, 11, 770. [Google Scholar] [CrossRef]
- Gulati, P.; Kohli, S.; Narang, A.; Brahmachari, V. A Comparative Analysis of Histone Methyltransferases and Demethylases in Insect Genome: A Meta-Analysis. bioRxiv 2019, 598946. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, K.; Dubovskiy, I.; Grizanova, E.; Lehmann, R.; Vilcinskas, A. Epigenetic Mechanisms Mediate the Experimental Evolution of Resistance Against Parasitic Fungi in the Greater Wax Moth Galleria mellonella. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ney, G.; Schul, J. Epigenetic and Genetic Variation Between Two Behaviorally Isolated Species of Neoconocephalus (Orthoptera: Tettigonioidea). J. Orthoptera Res. 2019, 28, 11. [Google Scholar] [CrossRef]
- Lo, N.; Simpson, S.J.; Sword, G.A. Epigenetics and Developmental Plasticity in Orthopteroid Insects. Curr. Opin. Insect Sci. 2018, 25, 25–34. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Clark, J.; Diakoff, S.J.; Denlinger, D.L. Transcriptional Evidence for Small RNA Regulation of Pupal Diapause in the Flesh Fly, Sarcophaga bullata. Insect Biochem. Mol. Biol. 2013, 43, 982–989. [Google Scholar] [CrossRef]
- Khosla, S.; Mendiratta, G.; Brahmachari, V. Genomic Imprinting in the Mealybugs. Cytogen. Gen. Res. 2006, 113, 41–52. [Google Scholar] [CrossRef]
- Morandin, C.; Brendel, V.P.; Sundström, L.; Helanterä, H.; Mikheyev, A.S. Changes in Gene DNA Methylation and Expression Networks Accompany Caste Specialization and Age-Related Physiological Changes in a Social Insect. Mol. Ecol. 2019, 28, 1975–1993. [Google Scholar] [CrossRef]
- Simola, D.F.; Graham, R.J.; Brady, C.M.; Enzmann, B.L.; Desplan, C.; Ray, A.; Zwiebel, L.J.; Bonasio, R.; Reinberg, D.; Liebig, J.; et al. Epigenetic (Re) Programming of Caste-Specific Behavior in the Ant Camponotus floridanus. Science 2016, 351, 6268. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, S.V.; Hunt, B.G.; Rehan, S.M. The Effect of Maternal Care on Gene Expression and DNA Methylation in a Subsocial Bee. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Richard, G.; Le Trionnaire, G.; Danchin, E.; Sentis, A. Epigenetics and Insect Polyphenism: Mechanisms and Climate Change Impacts. Curr. Opin. Insect Sci. 2019, 35, 138–145. [Google Scholar] [CrossRef]
- Chatterjee, N.; Gim, J.; Choi, J. Epigenetic Profiling to Environmental Stressors in Model and Non-Model Organisms: Ecotoxicology Perspective. Environ. Health Toxicol. 2018, 33, 3. [Google Scholar] [CrossRef] [PubMed]
- Brevik, K.; Bueno, E.M.; McKay, S.; Schoville, S.D.; Chen, Y.H. Insecticide Exposure Affects Intergenerational Patterns of DNA Methylation in the Colorado Potato Beetle, Leptinotarsa decemlineata. Evol. Appl. 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Seebacher, F.; Krause, J. Epigenetics of Social Behaviour. Trends Ecol. Evol. 2019, 34, 818–830. [Google Scholar] [CrossRef]
- Bantz, A.; Camon, J.; Froger, J.A.; Goven, D.; Raymond, V. Exposure to Sublethal Doses of Insecticide and their Effects on Insects at Cellular and Physiological Levels. Curr. Opin. Insect Sci. 2018, 30, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Jeanrenaud, A.C.; Brooke, B.D.; Oliver, S. Larval Metal Pollutant Exposure Alters the Epigenetic Architecture of the Major Malaria Vector Anopheles arabiensis (Diptera: Culicidae). Res. Sq. 2020. [Google Scholar] [CrossRef]
- Planelló, R.; Martínez-Guitarte, J.L.; Morcillo, G. The Endocrine Disruptor Bisphenol a Increases the Expression of HSP70 and Ecdysone Receptor Genes in the Aquatic Larvae of Chironomus riparius. Chemosphere 2008, 7, 1870–1876. [Google Scholar] [CrossRef]
- Roelofs, D.; Janssens, T.K.; Timmermans, M.J.; Nota, B.; Marien, J.; Bochdanovits, Z.; Ylstra, B.; Van Straalen, N.M. Adaptive Differences in Gene Expression Associated with Heavy Metal Tolerance in the Soil Arthropod Orchesella cincta. Mol. Ecol. 2009, 18, 3227–3239. [Google Scholar] [CrossRef]
- Gintenreiter, S.; Ortel, J.; Nopp, H.J. Effects of Different Dietary Levels of Cadmium, Lead, Copper, and Zinc on the Vitality of the Forest Pest Insect Lymantria dispar L. (Lymantriidae, Lepid). Arch. Environ. Contam. Toxicol. 1993, 25, 62–66. [Google Scholar] [CrossRef]
- Dai, T.M.; Lü, Z.C.; Liu, W.X.; Wan, F.H.; Hong, X.Y. The Homology Gene Btdnmt1 is Essential for Temperature Tolerance in Invasive Bemisia Tabaci Mediterranean Cryptic Species. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rayms-Keller, A.; McGaw, M.; Oray, C.; Carlson, J.O.; Beaty, B.J. Molecular Cloning and Characterization of a Metal Responsive Aedes Aegypti Intestinal Mucin Cdna. Insect Mol. Biol. 2000, 9, 419–426. [Google Scholar] [CrossRef]
- Raes, H.; Braeckman, B.P.; Criel, G.R.; Rzeznik, U.; Vanfleteren, J.R. Copper Induces Apoptosis in Aedes C6/36 Cells. J. Exp. Zool. 2000, 286, 1–2. [Google Scholar] [CrossRef]
- Carlisle, D.M.; Clements, W.H. Growth and Secondary Production of Aquatic Insects Along a Gradient of Zn Contamination in Rocky Mountain Streams. J. N. Am. Benth. Soc. 2003, 22, 582–597. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Du, Y.; Xue, H.; Wu, Y.; Zhou, B. Aluminum Induces Neurodegeneration and its Toxicity Arises from Increased Iron Accumulation and Reactive Oxygen Species (ROS) Production. Neurobiol. Aging 2012, 33, 199-e1. [Google Scholar] [CrossRef]
- Hitchcock, S.W. Field and Laboratory Studies of DDT And Aquatic Insects; Connecticut Agricultural Experiment Station: New Haven, CT, USA, 1965. [Google Scholar]
- Moye, W.C.; Luckmann, W.H. Fluctuations in Populations of Certain Aquatic Insects Following Application of Aldrin Granules to Sugar Creek, Iroquois County, Illinois. J. Econ. Entomol. 1964, 57, 318–322. [Google Scholar] [CrossRef]
- Gupta, A.P.; Sutherland, D.J. Effects of Sublethal Doses of Chlordane on the Hemocytes and Midgut Epithelium of Periplaneta americana. Ann. Entomol. Soc. Am. 1968, 61, 910–918. [Google Scholar] [CrossRef]
- Wafford, K.A.; Sattelle, D.B.; Gant, D.B.; Eldefrawi, A.T.; Eldefrawi, M.E. Noncompetitive Inhibition of GABA Receptors in Insect and Vertebrate CNS By Endrin and Lindane. Pest. Biochem. Physiol. 1989, 33, 213–219. [Google Scholar] [CrossRef]
- Lummis, S.C.; Buckingham, S.D.; Rauh, J.J.; Sattelle, D.B. Blocking Actions of Heptachlor at an Insect Central Nervous System GABA Receptor. Proc. R. Soc. Lond. B 1990, 240, 97–106. [Google Scholar]
- Maund, S.J.; Peither, A.; Taylor, E.J.; Jüttner, I.; Beyerle-Pfnür, R.; Lay, J.P.; Pascoe, D. Toxicity of Lindane to Freshwater Insect Larvae in Compartments of an Experimental Pond. EcoToxicol. Environ. Saf. 1992, 23, 76–88. [Google Scholar] [CrossRef]
- Matsumura, F.; Hogendijk, C.J. Insect Resistance to Insecticides, Enzymatic Degradation of Parathion in Organophosphate-Susceptible and-Resistant Houseflies. J. Agric. Food Chem. 1964, 12, 447–453. [Google Scholar] [CrossRef]
- Krueger, H.R.; O’brien, R.D. Relationship Between Metabolism and Differential Toxicity of Malathion in Insects and Mice. J. Econ. Entomol. 1959, 52, 1063–1067. [Google Scholar] [CrossRef]
- Magbanua, F.S.; Townsend, C.R.; Hageman, K.J.; Piggott, J.J.; Matthaei, C.D. Individual and Combined Effects of Fine Sediment and Glyphosate Herbicide on Invertebrate Drift and Insect Emergence: A Stream Mesocosm ExperimEntomol. Fresh. Sci. 2016, 35, 139–151. [Google Scholar] [CrossRef]
- Hamm, R.L.; Kaufman, P.E.; Reasor, C.A.; Rutz, D.A.; Scott, J.G. Resistance to cyfluthrin and tetrachlorvinphos in the lesser mealworm, Alphitobius diaperinus, collected from the eastern United States. Pest Man. Sci. 2006, 62, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Knorr, M.; Spencer, A.G.; Jespersen, J.B. Selection and Reversion of Azamethiphos-Resistance in a Field Population of the Housefly Musca domestica (Diptera: Muscidae), and the Underlying Biochemical Mechanisms. J. Econ. Entomol. 2000, 93, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Alston, D.G.; Tepedino, V.J.; Bradley, B.A.; Toler, T.R.; Griswold, T.L.; Messinger, S.M. Effects of the Insecticide Phosmet on Solitary Bee Foraging and Nesting in Orchards of Capitol Reef National Park, Utah. Environ. Entomol. 2007, 36, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Chernaki-Leffer, A.M.; Sosa-Gómez, D.R.; Almeida, L.M.; Lopes, I.D. Susceptibility of Alphitobius diaperinus (Panzer) (Coleoptera, Tenebrionidae) to Cypermethrin, Dichlorvos and Triflumuron in Southern Brazil. Rev. Bras. Entomol. 2011, 55, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Jales, J.T.; de Moura Barbosa, T.; Dos Santos, L.C.; Rachetti, V.D.; Gama, R.A. Carrion Decomposition and Assemblage of Necrophagous Dipterans Associated with Terbufos (Organophosphate) Intoxicated Rat Carcasses. Act. Trop. 2020, 212, 105652. [Google Scholar] [CrossRef]
- Eldefrawi, M.E.; Hoskins, W.M. Relation of the Rate of Penetration and Metabolism to the Toxicity of Sevin to Three Insect Species. J. Econ. Entomol. 1961, 54, 401–405. [Google Scholar] [CrossRef]
- Davis, J.W.; Cowan, C.B., Jr. Field Evaluation of Three Formulations of Aldicarb for Control of Cotton Insects. J. Econ. Entomol. 1972, 65, 231–232. [Google Scholar] [CrossRef]
- Spurr, H.W., Jr.; Sousa, A.A. Potential Interactions of Aldicarb and its Metabolites on Nontarget Organisms in the EnvironmEntomol. J. Environ. Qual. 1974, 3, 130–133. [Google Scholar] [CrossRef]
- Ball, H.J.; Su, P.P. Effect of Sublethal Dosages of Carbofuran and Carbaryl on Fecundity and Longevity of the Female Western Corn Rootworm. J. Econ. Entomol. 1979, 72, 873–876. [Google Scholar] [CrossRef]
- Gammon, D.W. Neural Effects of Allethrin on the Free Walking Cockroach Periplaneta americana: An Investigation Using Defined Doses At 15 and 32 C. Pest. Sci. 1978, 9, 79–91. [Google Scholar] [CrossRef]
- Dai, P.L.; Wang, Q.; Sun, J.H.; Liu, F.; Wang, X.; Wu, Y.Y.; Zhou, T. Effects of Sublethal Concentrations of Bifenthrin and Deltamethrin on Fecundity, Growth, and Development of the Honeybee Apis mellifera ligustica. Environ. Toxicol. Chem. 2010, 29, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Nadda, G.; Saxena, P.N.; Srivastava, G. Effects of Sublethal Doses of Beta-Cyfluthrin on Mutant Drosophila melanogaster (Diptera: Drosophilidae). Appl. Entomol. Zool. 2005, 40, 265–271. [Google Scholar] [CrossRef]
- Saha, S.; Kaviraj, A. Acute Toxicity of Synthetic Pyrethroid Cypermethrin to Some Freshwater Organisms. Bull. Environ. Contam. Toxicol. 2008, 80, 49–52. [Google Scholar] [CrossRef]
- Schleier, J.J.; Peterson, R.K. Toxicity and Risk of Permethrin and Naled to Non-Target Insects after Adult Mosquito ManagemEntomol. Ecotoxicology 2010, 19, 1140–1146. [Google Scholar] [CrossRef]
- Oberhauser, K.S.; Manweiler, S.A.; Lelich, R.; Blank, M.; Batalden, R.V.; De Anda, A. Impacts of Ultra-Low Volume Resmethrin Applications on Non-Target Insects. J. Am. Mosqu. Contr. Assoc. 2009, 25, 83–93. [Google Scholar] [CrossRef]
- Britch, S.C.; Linthicum, K.J.; Kline, D.L.; Aldridge, R.L.; Golden, F.V.; Wittie, J.; Henke, J.; Hung, K.; Gutierrez, A.; Snelling, M.; et al. Transfluthrin Spatial Repellent on US Military Materials Reduces Culex tarsalis Incursion in a Desert. Environ. Entomol. J. Am. Mosqu. Contr. Assoc. 2020, 36, 37–42. [Google Scholar] [CrossRef]
- Abdel-Haleem, D.R.; Genidy, N.A.; Fahmy, A.R.; Azm, A.E.; Fatma, S.M.; Ismail, N.S. Comparative Modelling, Toxicological and Biochemical Studies of Imidacloprid and Thiamethoxam Insecticides on the house fly, Musca domestica L. (Diptera: Muscidae). Egyp. Acad. J. Biol. Sci. A Entomol. 2018, 11, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Paleolog, J.; Wilde, J.; Siuda, M.; Bąk, B.; Wójcik, Ł.; Strachecka, A. Imidacloprid Markedly Affects Hemolymph Proteolysis, Biomarkers, DNA Global Methylation, and the Cuticle Proteolytic Layer in Western Honeybees. Apidologie 2020, 51, 620–630. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.T.; Tang, C.K.; Wu, C.P.; Wu, P.C.; Yang, E.C.; Tai, C.C.; Wu, Y.L. Histone Deacetylase Inhibitor Treatment Restores Memory-Related Gene Expression and Learning Ability in Neonicotinoid-Treated Apis mellifera. Insect Mol. Biol. 2018, 27, 512–521. [Google Scholar] [CrossRef]
- El Hassani, A.K.; Dacher, M.; Gary, V.; Lambin, M.; Gauthier, M.; Armengaud, C. Effects of Sublethal Doses of Acetamiprid and Thiamethoxam on the Behavior of the Honeybee (Apis mellifera). Arch. Environ. Contam. Toxicol. 2008, 54, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Maloney, E.M.; Liber, K.; Headley, J.V.; Peru, K.M.; Morrissey, C.A. Neonicotinoid Insecticide Mixtures: Evaluation of Laboratory-Based Toxicity Predictions Under Semi-Controlled Field Conditions. Environ. Pollut. 2018, 243, 1727–1739. [Google Scholar] [CrossRef]
- Liang, P.; Tian, Y.A.; Biondi, A.; Desneux, N.; Gao, X.W. Short-Term and Transgenerational Effects of the Neonicotinoid Nitenpyram on Susceptibility to Insecticides in Two Whitefly Species. Ecotoxicology 2012, 21, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Beketov, M.A.; Liess, M. Acute and Delayed Effects of the Neonicotinoid Insecticide Thiacloprid on Seven Freshwater Arthropods. Environ. Toxicol. Chem. 2008, 27, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, K.; Harada, T.; Adachi, Y.; Mori, M.; Ihara, M.; Hayasaka, D. Comparative Ecotoxicity of Imidacloprid and Dinotefuran to Aquatic Insects In Rice Mesocosms. EcoToxicol. Environ. Saf. 2017, 138, 122–129. [Google Scholar] [CrossRef]
- Martínez-Paz, P.; Morales, M.; Martínez-Guitarte, J.L.; Morcillo, G. Genotoxic Effects of Environmental Endocrine Disruptors on the Aquatic Insect Chironomus riparius Evaluated Using the Comet Assay. Mut. Res. Gen. Toxicol. Environ. Mutagenesis 2013, 758, 41–47. [Google Scholar] [CrossRef]
- Morales, M.; Martínez-Paz, P.; Martín, R.; Planelló, R.; Urien, J.; Martínez-Guitarte, J.L.; Morcillo, G. Transcriptional Changes Induced by In Vivo Exposure to Pentachlorophenol (PCP) in Chironomus riparius (Diptera) Aquatic Larvae. Aqu. Toxicol. 2014, 157, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Planelló, R.; Herrero, O.; Martínez-Guitarte, J.L.; Morcillo, G. Comparative Effects of Butyl Benzyl Phthalate (BBP) and Di (2-Ethylhexyl) Phthalate (DEHP) on the Aquatic Larvae of Chironomus riparius Based on Gene Expression Assays Related to the Endocrine System, the Stress Response and Ribosomes. Aqu. Toxicol. 2011, 105, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kwak, I.S. Characterization of Heat Shock Protein 40 and 90 in Chironomus Riparius Larvae: Effects of Di (2-ethylhexyl) Phthalate Exposure on Gene Expressions and Mouthpart Deformities. Chemosphere 2008, 74, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Bovier, T.F.; Rossi, S.; Mita, D.G.; Digilio, F.A. Effects of the Synthetic Estrogen 17-A-Ethinylestradiol on Drosophila melanogaster: Dose and Gender Dependence. EcoToxicol. Environ. Saf. 2018, 162, 625–632. [Google Scholar] [CrossRef]
- Unni, B.G.; Peck, K.E.; Pytel, M.M.; Dahm, K.H.; Bhaskaran, G.; Singh, H.R.; Kakoty, Y.; Devi, B.; Wann, S.B. Dimethyl Sulphoxide Inhibits In Vitro Synthesis of Juvenile Hormone I and II and Stimulates Juvenile Hormone III by corpora allata of Insects. Curr. Sci. 2009, 96, 1114–1119. [Google Scholar]
- Al-Jaibachi, R.; Cuthbert, R.N.; Callaghan, A. Up and Away: Ontogenic Transference as a Pathway for Aerial Dispersal of Microplastics. Biol. Lett. 2018, 14, 20180479. [Google Scholar] [CrossRef] [Green Version]
- Akindele, E.O.; Ehlers, S.M.; Koop, J.H. Freshwater Insects of Different Feeding Guilds Ingest Microplastics in Two Gulf of Guinea Tributaries in Nigeria. Environ. Sci. Pollut. Res. 2020, 27, 33373–33379. [Google Scholar] [CrossRef]
- Desneux, N.; Rafalimanana, H.; Kaiser, L. Dose–Response Relationship in Lethal and Behavioural Effects of Different Insecticides on the Parasitic Wasp Aphidius ervi. Chemosphere 2004, 54, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Nieh, J.C. Lethal and Sublethal Synergistic Effects of a New Systemic Pesticide, flupyradifurone (Sivanto®), on Honeybees. Proc. R. Soc. B 2019, 286, 20190433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, K.F. Sublethal Effects of Neurotoxic Insecticides on Insect Behavior. Ann. Rev. Entomol. 1988, 33, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Colin, T.; Meikle, W.G.; Wu, X.; Barron, A.B. Traces of a Neonicotinoid Induce Precocious Foraging and Reduce Foraging Performance in Honey Bees. Environ. Sci. Technol. 2019, 53, 8252–8261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, J.; Preetha, G. Pesticide Toxicity To Arthropod Predators: Exposure, Toxicity And Risk Assessment Methodologies. In Pesticide Toxicity to Non-Target Organisms; Stanley, J., Preetha, G., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–98. [Google Scholar]
- Müller, T.; Römer, C.I.; Müller, C. Parental Sublethal Insecticide Exposure Prolongs Mating Response and Decreases Reproductive Output in Offspring. J. Appl. Ecol. 2019, 56, 1528–1537. [Google Scholar] [CrossRef]
- Collotta, M.; Bertazzi, P.A.; Bollati, V. Epigenetics and Pesticides. Toxicology 2013, 307, 35–41. [Google Scholar] [CrossRef]
- Hardstone, M.C.; Scott, J.G. Is Apis mellifera More Sensitive to Insecticides Than Other Insects? Pest Man. Sci. 2010, 66, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Picó, Y.; Alvarez-Ruiz, R.; Alfarhan, A.H.; El-Sheikh, M.A.; Alshahrani, H.O.; Barceló, D. Pharmaceuticals, Pesticides, Personal Care Products and Microplastics Contamination Assessment of Al-Hassa Irrigation Network (Saudi Arabia) and Its Shallow Lakes. Sci. Total Environ. 2020, 701, 135021. [Google Scholar] [CrossRef]
- Caceres-Jensen, L.; Rodríguez-Becerra, J.; Sierra-Rosales, P.; Escudey, M.; Valdebenito, J.; Neira-Albornoz, A.; Dominguez-Vera, V.; Villagra, C.A. Electrochemical Method to Study the Environmental Behavior of Glyphosate on Volcanic Soils: Proposal of Adsorption-Desorption and Transport Mechanisms. J. Hazard. Mater. 2019, 379, 120746. [Google Scholar] [CrossRef]
- Caceres-Jensen, L.C.; Rodríguez-Becerra, J.; Escudey, M. Impact of Physical/Chemical Properties of Volcanic Ash-Derived Soils on Mechanisms Involved During Sorption of Ionisable and Non-Ionisable Herbicides. In Advanced Sorption Process Applications; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Augustyniak, M.; Płachetka-Bożek, A.; Kafel, A.; Babczyńska, A.; Tarnawska, M.; Janiak, A.; Loba, A.; Dziewięcka, M.; Karpeta-Kaczmarek, J.; Zawisza-Raszka, A. Phenotypic Plasticity, Epigenetic or Genetic Modifications in Relation to the Duration of Cd-Exposure Within a Microevolution Time Range in the Beet Armyworm. PLoS ONE 2016, 11, e0167371. [Google Scholar]
- Jadiya, P.; Nazir, A. Environmental Toxicants as Extrinsic Epigenetic Factors for Parkinsonism: Studies Employing Transgenic C. Elegans Model. CNS Neuro. Disor-Drug Targ. 2012, 11, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Colosio, C.; Rubino, F.M.; Moretto, A. Pesticides. In International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Academic Press: New York, NY, USA, 2016; pp. 454–462. [Google Scholar]
- Mužinić, V.; Želježić, D. Non-Target Toxicity of Novel Insecticides. Arch. Indust. Hyg. Toxicol. 2018, 69, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Darvas, B.; Polgar, L.A. Novel-Type Insecticides: Specificity and Effects on Non-Target Organisms. In Insecticides with Novel Modes of Action; Ishaaya, I., Horowitz, A.R., Eds.; Springer: Berlin, Germany, 1998; pp. 188–259. [Google Scholar]
- Smith, C.J.; Perfetti, T.A. A Comparison of The Persistence, Toxicity, and Exposure to High-Volume Natural Plant-Derived and Synthetic Pesticides. Toxicol. Res. Appl. 2020, 4, 2397847320940561. [Google Scholar] [CrossRef]
- Zahm, S.H.; Ward, M.H. Pesticides and Childhood Cancer. Environ. Health Persp. 1998, 106, 893–908. [Google Scholar]
- Howe, C.M.; Berrill, M.; Pauli, B.D.; Helbing, C.C.; Werry, K.; Veldhoen, N. Toxicity of Glyphosate-Based Pesticides to Four North American Frog Species. Environ. Toxicol. Chem. 2004, 23, 1928–1938. [Google Scholar] [CrossRef]
- Isenring, R. Pesticides Reduce Biodiversity. Pest. News 2010, 88, 4–7. [Google Scholar]
- Lerro, C.C.; Freeman, L.E.; DellaValle, C.T.; Andreotti, G.; Hofmann, J.N.; Koutros, S.; Parks, C.G.; Shrestha, S.; Alavanja, M.C.; Blair, A.; et al. Pesticide Exposure and Incident Thyroid Cancer Among Male Pesticide Applicators in Agricultural Health Study. Environ. Int. 2021, 146, 106187. [Google Scholar] [CrossRef]
- Seide, V.E.; Bernardes, R.C.; Pereira, E.J.; Lima, M.A. Glyphosate is Lethal and Cry Toxins Alter the Development of the Stingless Bee Melipona quadrifasciata. Environ. Pollut. 2018, 243, 1854–1860. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Tasei, J.N. Impact of Agrochemicals on Non-Apis Bees. In Honey Bees: Estimating the Environmental Impact of Chemicals; Taylor & Francis Group: Abingdon, UK, 2002; pp. 101–131. [Google Scholar]
- Hoshi, N. Adverse Effects of Pesticides on Regional Biodiversity and Their Mechanisms. In Risks and Regulation of New Technologies; Matsuda, T., Wolff, J., Tanagawa, T., Eds.; Springer: Singapore, 2021; pp. 235–247. [Google Scholar]
- Gill, R.J.; Raine, N.E. Chronic Impairment of Bumblebee Natural Foraging Behaviour Induced by Sublethal Pesticide Exposure. Funct. Ecol. 2014, 28, 1459–1471. [Google Scholar] [CrossRef]
- Atwood, D.; Paisley-Jones, C. Pesticides Industry Sales and Usage 2008–2012 Market Estimates; US EPA: Washington, DC, USA, 2017. [Google Scholar]
- Caceres-Jensen, L.; Neira-Albornoz, A.; Escudey, M. Herbicides Mechanisms Involved in the Sorption Kinetic Of Ionisable And Non Ionisable Herbicides: Impact of Physical/Chemical Properties Of Soils And Experimental Conditions. In Kinetic Modeling for Environmental Systems; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Junqueira, L.V.; Mendes, K.F.; Sousa, R.N.; Almeida, C.D.; Alonso, F.G.; Tornisielo, V.L. Sorption-Desorption Isotherms and Biodegradation of Glyphosate in Two Tropical Soils Aged with Eucalyptus Biochar. Arch. Agric. Soil Sci. 2020, 66, 1651–1667. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.H.; Guo, Q.N.; Ma, L.Y.; Yang, H. Physiochemical Assessment of Environmental Behaviors of Herbicide Atrazine in Soils Associated With its Degradation and Bioavailability to Weeds. Chemosphere 2021, 262, 127830. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kania, J.; Kmiecik, E.; Malina, G.; Wątor, K. Fate of Selected Neonicotinoid Insecticides in Soil–Water Systems: Current State of The Art and Knowledge Gaps. Chemosphere 2020, 255, 126981. [Google Scholar] [CrossRef]
- Cáceres-Jensen, L.; Gan, J.; Baez, M.; Fuentes, R.; Escudey, M. Adsorption of Glyphosate on Variable-Charge, Volcanic Ash–Derived Soils. J. Environ. Qual. 2009, 38, 1449–1457. [Google Scholar] [CrossRef]
- Annabi, E.; Ben Salem, I.; Abid-Essefi, S. Acetamiprid, a Neonicotinoid Insecticide, Induced Cytotoxicity and Genotoxicity in PC12 Cells. Toxicol. Mech. Methods 2019, 29, 580–586. [Google Scholar] [CrossRef]
- Bansal, O.P. Sorption, Degradation and Movement of Three Carbamate Pesticides in Soils. Ind. J. Agric. Sci. 2011, 81, 578–581. [Google Scholar]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Impact of Pesticides on Soil Microbial Diversity, Enzymes, and Biochemical Reactions. Adv. Agric. 2009, 102, 159–200. [Google Scholar]
- Mattina, M.J.; White, J.; Eitzer, B.; Iannucci-Berger, W. Cycling of Weathered Chlordane Residues in the Environment: Compositional and Chiral Profiles in Contiguous Soil, Vegetation, And Air Compartments. Environ. Toxicol. Chem. 2002, 21, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Folmar, L.C.; Sanders, H.O.; Julin, A.M. Toxicity of the Herbicide Glyphosate and Several of its Formulations to Fish and Aquatic Invertebrates. Arch. Environ. Contam. Toxicol. 1979, 8, 269–278. [Google Scholar] [CrossRef]
- Aparicio, V.C.; De Gerónimo, E.; Marino, D.; Primost, J.; Carriquiriborde, P.; Costa, J.L. Environmental Fate of Glyphosate and Aminomethylphosphonic Acid in Surface Waters And Soil Of Agricultural Basins. Chemosphere 2013, 93, 1866–1873. [Google Scholar] [CrossRef]
- Gill, J.P.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate Toxicity for Animals. Environ. Chem. Lett. 2018, 16, 401–426. [Google Scholar] [CrossRef]
- Caceres-Jensen, L.; Rodriguez-Becerra, J.; Escudey, M.; Joo-Nagata, J.; Villagra, C.A.; Dominguez-Vera, V.; Neira-Albornoz, A.; Cornejo-Huentemilla, M. Nicosulfuron Sorption Kinetics and Sorption/Desorption on Volcanic Ash-Derived Soils: Proposal of Sorption and Transport Mechanisms. J. Hazard. Mater. 2020, 385, 121576. [Google Scholar] [CrossRef] [PubMed]
- Murano, H.; Suzuki, K.; Kayada, S.; Saito, M.; Yuge, N.; Arishiro, T.; Watanabe, A.; Isoi, T. Influence of Humic Substances and Iron and Aluminum Ions on the Sorption of Acetamiprid to an Arable Soil. Sci. Total Environ. 2018, 615, 1478–1484. [Google Scholar] [CrossRef]
- Fernandez-Bayo, J.D.; Nogales, R.; Romero, E. Evaluation of the Sorption Process for Imidacloprid And Diuron In Eight Agricultural Soils from Southern Europe Using Various Kinetic Models. J. Agric. Food Chem. 2008, 56, 5266–5272. [Google Scholar] [CrossRef]
- Eggleton, P. The State of the World’s Insects. Ann. Rev. Environ. Res. 2020, 26, 45. [Google Scholar] [CrossRef]
- Studzińska, M.B.; Sallé, G.; Roczeń-Karczmarz, M.; Szczepaniak, K.; Demkowska-Kutrzepa, M.; Tomczuk, K. A Survey of Ivermectin Resistance in Parascaris Species Infected Foals in South-Eastern Poland. Act. Vet. Scand. 2020, 62, 1–5. [Google Scholar]
- Motaung, T.E. Chloronicotinyl Insecticide Imidacloprid: Agricultural Relevance, Pitfalls and Emerging Opportunities. Crop Prot. 2020, 131, 105097. [Google Scholar] [CrossRef]
- Saaristo, M.; Brodin, T.; Balshine, S.; Bertram, M.G.; Brooks, B.W.; Ehlman, S.M.; McCallum, E.S.; Sih, A.; Sundin, J.; Wong, B.B.; et al. Direct and Indirect Effects of Chemical Contaminants on the Behaviour, Ecology and Evolution of Wildlife. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181297. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Foppen, R.P.; van Turnhout, C.A.; de Kroon, H.; Jongejans, E. Declines in Insectivorous Birds are Associated With High Neonicotinoid Concentrations. Nature 2014, 511, 341–343. [Google Scholar] [CrossRef]
- Yusmalinar, S.; Anggtaeni, T.; Khusnan, K.; Wibowo, I.; Putra, R.E.; Ahmad, I. Reproductive Ability Enhancement of Housefly (Musca domestica Linn) (Diptera: Muscidae) Through Hormesis by Application of Sublethal Doses of Imidacloprid and Permethrin. J. Entomol. 2017, 14, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Tang, J. Utilization of Biodiversity in Agriculture: Today and Tomorrow. Chin. J. Ecol. Agric. 2013, 21, 54–60. [Google Scholar]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop Production in the USA is Frequently Limited by a Lack of Pollinators. Proc. R. Soc. B. Biol. Sci. 2020, 287, 20200922. [Google Scholar] [CrossRef] [PubMed]
- Melathopoulos, A.P.; Cutler, G.C.; Tyedmers, P. Where is the Value in Valuing Pollination Ecosystem Services to Agriculture? Ecol. Econ. 2015, 109, 59–70. [Google Scholar] [CrossRef]
- Kremen, C.; Bugg, R.L.; Nicola, N.; Smith, S.A.; Thorp, R.W.; Williams, N.M. Native Bees, Native Plants and Crop Pollination in California. Fremontia 2002, 30, 41–49. [Google Scholar]
- Elbert, A.; Haas, M.; Springer, B.; Thielert, W.; Nauen, R. Applied Aspects of Neonicotinoid Uses in Crop Protection. Pest Man. Sci. 2008, 64, 1099–1105. [Google Scholar] [CrossRef]
- Lu, C.; Chang, C.H.; Palmer, C.; Zhao, M.; Zhang, Q. Neonicotinoid Residues in Fruits and Vegetables: An Integrated Dietary Exposure Assessment Approach. Environ. Sci. Technol. 2018, 52, 3175–3184. [Google Scholar] [CrossRef]
- Basley, K.; Davenport, B.; Vogiatzis, K.; Goulson, D. Effects of Chronic Exposure to Thiamethoxam on Larvae of the Hoverfly Eristalis tenax (Diptera, Syrphidae). PeerJ 2018, 6, e4258. [Google Scholar] [CrossRef] [Green Version]
- Georgieva, M.; Bonchev, G.; Zehirov, G.; Vasileva, V. Neonicotinoid Insecticides Exert Diverse Cytotoxic and Genotoxic Effects on Cultivated Sunflower. Environ. Sci. Pollut. G 2021, 1–15. [Google Scholar] [CrossRef]
- Craddock, H.A.; Huang, D.; Turner, P.C.; Quirós-Alcalá, L.; Payne-Sturges, D.C. Trends in Neonicotinoid Pesticide Residues in Food and Water in the United States, 1999–2015. Environ. Health. 2019, 18, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farahat, N.M.; Zyaan, O.H.; Khaled, A.S.; Hussein, M.A. Toxic and Biochemical Effects of Imidacloprid and Tannic Acid on the Culex pipiens Larvae (Diptera: Culicidae). Int. J. Mosqu. Res. 2018, 5, 111–115. [Google Scholar]
- Byholm, P.; Mäkeläinen, S.; Santangeli, A.; Goulson, D. First evidence of neonicotinoid residues in a long-distance migratory raptor, the European honey buzzard (Pernis apivorus). Sci. Total Environ. 2018, 639, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Piiroinen, S.; Goulson, D. Chronic Neonicotinoid Pesticide Exposure and Parasite Stress Differentially Affects Learning in Honeybees and Bumblebees. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mörtl, M.; Vehovszky, Á.; Klátyik, S.; Takács, E.; Győri, J.; Székács, A. Neonicotinoids: Spreading, Translocation and Aquatic Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordoni, L.; Gabbianelli, R. Nutrigenomics of Food Pesticides. In Principles of Nutrigenetics and Nutrigenomics; Caterina, R.D., Martinez, J.A., Kohlmeier, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 513–518. [Google Scholar]
- Parkinson, R.H.; Zhang, S.; Gray, J.R. Neonicotinoid and Sulfoximine Pesticides Differentially Impair Insect Escape Behavior and Motion Detection. Proc. Nat. Acad. Sci. USA 2020, 117, 5510–5515. [Google Scholar] [CrossRef] [Green Version]
- Hoshi, N.; Hirano, T.; Omotehara, T.; Tokumoto, J.; Umemura, Y.; Mantani, Y.; Tanida, T.; Warita, K.; Tabuchi, Y.; Yokoyama, T.; et al. Insight into the Mechanism of Reproductive Dysfunction Caused by Neonicotinoid Pesticides. Biol. Pharm. Bull. 2014, 37, 1439–1443. [Google Scholar] [CrossRef] [Green Version]
- Simon-Delso, N.; Amaralrogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic Insecticides (Neonicotinoids And Fipronil): Trends, Uses, Mode of Action and Metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Millot, F.; Decors, A.; Mastain, O.; Quintaine, T.; Berny, P.; Vey, D.; Lasseur, R.; Bro, E. Field Evidence of Bird Poisonings by Imidaclo-Prid-Treated Seeds: A Review of Incidents Reported by the French SAGIR Network from 1995 to 2014. Environ. Sci. Pollut. Res. 2017, 24, 5469–5485. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Ito, T.; Otsuka, S.; Nansai, H.; Abe, K.; Nakao, Y.; Ohgane, J.; Yoneda, M.; Sone, H. Epigenetic Effects of Insecticides on Early Differentiation of Mouse Embryonic Stem Cells. Toxicol. Vitr. 2021, 75, 105174. [Google Scholar] [CrossRef] [PubMed]
- Arican, Y.E.; Karaman, E.F.; Özden, S. The Subcronic Effects of Acetamipride on the Global DNA Methylation Levels in Sprague-Dawley Rat Brain and Liver. Istanb. J. Pharm. 2019, 49, 167–172. [Google Scholar] [CrossRef]
- Bradford, B.R.; Whidden, E.; Gervasio, E.D.; Checchi, P.M.; Raley-Susman, K.M. Neonicotinoid-Containing Insecticide Disruption Of Growth, Locomotion, and Fertility in Caenorhabditis elegans. PLoS ONE 2020, 15, e0238637. [Google Scholar] [CrossRef]
- Vehovszky, Á.; Farkas, A.; Ács, A.; Stoliar, O.; Székács, A.; Mörtl, M.; Győri, J. Neonicotinoid Insecticides Inhibit Cholinergic Neuro-Transmission in a Molluscan (Lymnaea stagnalis) Nervous System. Aquat. Toxicol. 2015, 167, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson-Robert, O.; Labrie, G.; Chagnon, M.; Fournier, V. Planting of Neonicotinoid-Coated Corn Raises Honey Bee Mortality and Sets Back Colony DevelopmEntomol. PeerJ 2017, 5, e3670. [Google Scholar] [CrossRef] [Green Version]
- Henríquez-Piskulich, P.; Schapheer, C.; Vereecken, N.; Villagra, C. Agroecological Strategies to Safeguard Insect Pollinators in Biodiversity Hotspots: Chile as a Case Study. Sustainability 2021, 13, 6728. [Google Scholar] [CrossRef]
- Stanley, D.A.; Garratt, M.P.; Wickens, J.B.; Wickens, V.J.; Potts, S.G.; Raine, N.E. Neonicotinoid Pesticide Exposure Impairs Crop Pollination Services Provided By Bumblebees. Nature 2015, 528, 548–550. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Q.; Zhang, L.; Gao, X. Characterization of Imidacloprid Resistance in the Housefly Musca domestica (Diptera: Muscidae). Pest. Biochem. Phys. 2012, 102, 109–114. [Google Scholar] [CrossRef]
- Cutler, G.C. Insects, Insecticides and Hormesis: Evidence and Considerations for Study. Dose-Response 2013, 11, 154–177. [Google Scholar] [CrossRef]
- Botías, C.; David, A.; Hill, E.M.; Goulson, D. Contamination of Wild Plants Near Neonicotinoid Seed-Treated Crops, and Implications for Non-Target Insects. Sci. Total Environ. 2016, 566, 269–278. [Google Scholar] [CrossRef]
- Forister, M.L.; Cousens, B.; Harrison, J.G.; Anderson, K.; Thorne, J.H.; Waetjen, D.; Nice, C.C.; De Parsia, M.; Hladik, M.L.; Meese, R.; et al. Increasing Neonicotinoid Use and the Declining Butterfly Fauna of Lowland California. Biol. Lett. 2016, 12, 20160475. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, A.A.; Newman, A.E.M.; Raine, N.E.; Mitchell, G.W.; Norris, D.R. Effects of Early-Life Exposure to Sublethal Levels of a Common Neonicotinoid Insecticide on the Orientation and Migration of Monarch Butterflies (Danaus plexippus). J. Exp. Biol. 2020, 224, jeb230870. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.; Green, J.; Shuker, D.M.; Whitehorn, P.R. Exposure to the Neonicotinoid Imidacloprid Disrupts Sex Allocation Cue Use During Superparasitism in the Parasitoid Wasp Nasonia vitripennis. Ecol. Entomol. 2016, 41, 693–697. [Google Scholar] [CrossRef] [Green Version]
- Parra-Morales, L.B.; Alzogaray, R.; del Brio, J.; Cichón, L.; Garrido, S.A.; D’Hervé, F.; Montagna, M. Acetamiprid-Induced Response of Biotransformation and Antioxidant Parameters in the Codling Moth Cydia pomonella (Lepidoptera: Tortricidae). Int. J. Pest Manag. 2019, 67, 1–10. [Google Scholar] [CrossRef]
- Vaiserman, A.M. Hormesis and Epigenetics: Is There a Link? Ageing Res. Rev. 2011, 10, 413–421. [Google Scholar] [CrossRef]
- Lawrence, T.J.; Culbert, E.M.; Felsot, A.S.; Hebert, V.R.; Sheppard, W.S. Survey and Risk Assessment of Apis mellifera (Hymenoptera: Apidae) Exposure to Neonicotinoid Pesticides in Urban, Rural, and Agricultural Settings. J. Econ. Entomol. 2016, 109, 520–528. [Google Scholar] [CrossRef]
- Hu, Y.T.; Wu, T.C.; Yang, E.C.; Wu, P.C.; Lin, P.T.; Wu, Y.L. Regulation of Genes Related to Immune Signaling and Detoxification In Apis mellifera by an Inhibitor of Histone Deacetylation. Sci. Rep. 2017, 7, 1–4. [Google Scholar]
- Stoughton, S.J.; Liber, K.; Culp, J.; Cessna, A. Acute and Chronic Toxicity of Imidacloprid to the Aquatic Invertebrates Chironomus Tentans and Hyalella azteca Under Constant-and Pulse-Exposure Conditions. Arch. Environ. Contam. Toxicol. 2008, 54, 662–673. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Bishop, B.A.; Grafius, E.J. Inheritance and Synergism of Resistance to Imidacloprid in the Colorado Potato Beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2000, 93, 1508–1514. [Google Scholar] [CrossRef]
- Rundlöf, M.; Andersson, G.K.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed Coating With a Neonicotinoid Insecticide Negatively Affects Wild Bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef]
- Peng, Y.C.; Yang, E.C. Sublethal Dosage of Imidacloprid Reduces the Microglomerular Density of Honey Bee Mushroom Bodies. Sci. Rep. 2016, 6, 19298. [Google Scholar] [CrossRef] [Green Version]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid Clothianidin Adversely Affects Insect Immunity and Promotes Replication of a Viral Pathogen in Honey Bees. Proc. Nat. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biergans, S.D.; Claudianos, C.; Reinhard, J.; Galizia, C.G. DNA Methylation Mediates Neural Processing After Odor Learning in the Honeybee. Sci. Rep. 2017, 7, 43635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, O.P.; Poonia, R. Qualitative Decline of Pollinator Spectrum in Sunflower Agro Ecosystem. Ind. J. Ecol. 2018, 45, 592–597. [Google Scholar]
- Eisenhauer, N.; Bonn, A.; Guerra, C.A. Recognizing the Quiet Extinction of Invertebrates. Nat. Commun. 2019, 10, 1–3. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S., III; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. Planetary Boundaries: Exploring the Safe Operating Space for Humanity. Ecol. Soc. 2009, 14, 32. [Google Scholar] [CrossRef]
- Summerhayes, C.P.; Zalasiewicz, J. Global Warming and the Anthropocene. Geol. Today 2018, 34, 194–200. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Grimm, N.B. Nature-Based Approaches to Managing Climate Change Impacts in Cities. Phil. Tran. R. Soc. B 2020, 375, 20190124. [Google Scholar] [CrossRef] [Green Version]
- Nolan, R.H.; Boer, M.M.; Collins, L.; Resco de Dios, V.; Clarke, H.; Jenkins, M.; Kenny, B.; Bradstock, R.A. Causes and Consequences of Eastern Australia’s 2019–20 Season of Mega-Fires. Glob. Chang. Biol. 2020, 26, 1039–1041. [Google Scholar] [CrossRef] [Green Version]
- Edie, S.M.; Huang, S.; Collins, K.S.; Roy, K.; Jablonski, D. Loss of Biodiversity Dimensions Through Shifting Climates and Ancient Mass Extinctions. Integ. Comp. Biol. 2018, 58, 1179–1190. [Google Scholar] [CrossRef]
- Abraham, J.P.; Baringer, M.; Bindoff, N.L.; Boyer, T.; Cheng, L.J.; Church, J.A.; Conroy, J.L.; Domingues, C.M.; Fasullo, J.T.; Gilson, J.; et al. A Review of Global Ocean Temperature Observations: Implications for Ocean Heat Content Estimates and Climate Change. Rev. Geophys. 2013, 51, 450–483. [Google Scholar] [CrossRef]
- Cáceres, L.; Fuentes, R.; Escudey, M.; Fuentes, E.; Báez, M.E. Metsulfuron-Methyl Sorption/Desorption Behavior on Volcanic Ash-Derived Soils. Effect of Phosphate And Ph. J. Agric. Food Chem. 2010, 58, 6864–6869. [Google Scholar] [CrossRef] [PubMed]
- Mantyka-pringle, C.S.; Martin, T.G.; Rhodes, J.R. Interactions Between Climate and Habitat Loss Effects on Biodiversity: A Systematic Review and Meta-Analysis. Glob. Chang. Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.S. The Biodiversity Crisis: A Multifaceted Review. Curr. Sci. 2002, 82, 638–647. [Google Scholar]
- Luiza-Andrade, A.; Brasil, L.S.; Benone, N.L.; Shimano, Y.; Farias, A.P.; Montag, L.F.; Dolédec, S.; Juen, L. Influence of Oil Palm Monoculture on the Taxonomic and Functional Composition of Aquatic Insect Communities in Eastern Brazilian Amazonia. Ecol. Indic. 2017, 82, 478–483. [Google Scholar] [CrossRef]
- Guenat, S.; Kunin, W.E.; Dougill, A.J.; Dallimer, M. Effects of Urbanisation and Management Practices on Pollinators in Tropical Africa. J. Appl. Ecol. 2019, 56, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Elmqvist, T.; Zipperer, W.; Güneralp, B. Urbanization, Habitat Loss, Biodiversity Decline: Solution Pathways to Break the Cycle. In Routledge Handbook of Urbanization and Global Environmental Change; Seta, K., Solecki, W.D., Griffith, C.A., Eds.; Routledge: London, UK, 2016. [Google Scholar]
- Marcantonio, M.; Rocchini, D.; Geri, F.; Bacaro, G.; Amici, V. Biodiversity, Roads, & Landscape Fragmentation: Two Mediterranean Cases. Appl. Geogr. 2013, 42, 63–72. [Google Scholar]
- Goosem, M. Fragmentation Impacts Caused by Roads Through Rainforests. Curr. Sci. 2007, 93, 1587–1595. [Google Scholar]
- Geneletti, D. Biodiversity Impact Assessment of Roads: An Approach Based on Ecosystem Rarity. Environ. Impact Assess. Rev. 2003, 23, 343–365. [Google Scholar] [CrossRef]
- Butt, N.; Beyer, H.L.; Bennett, J.R.; Biggs, D.; Maggini, R.; Mills, M.; Renwick, A.R.; Seabrook, L.M.; Possingham, H.P. Biodiversity Risks From Fossil Fuel Extraction. Science 2013, 342, 425–426. [Google Scholar] [CrossRef] [Green Version]
- Virah-Sawmy, M.; Ebeling, J.; Taplin, R. Mining and Biodiversity Offsets: A Transparent and Science-Based Approach to Measure “No-Net-Loss”. J. Environ. Manag. 2014, 143, 61–70. [Google Scholar] [CrossRef]
- Maiti, S.K.; Chowdhury, A. Effects of Anthropogenic Pollution on Mangrove Biodiversity: A Review. J. Environ. Prot. 2013, 4, 1428–1434. [Google Scholar] [CrossRef] [Green Version]
- McNeely, J.A. The Sinking Ark: Pollution and the Worldwide Loss of Biodiversity. Biodivers. Conserv. 1992, 1, 2–18. [Google Scholar] [CrossRef]
- Baur, B.; Cremene, C.; Groza, G.; Rakosy, L.; Schileyko, A.A.; Baur, A.; Stoll, P.; Erhardt, A. Effects of Abandonment of Subalpine Hay Meadows on plant and Invertebrate Diversity in Transylvania, Romania. Biol. Conserv. 2006, 132, 261–273. [Google Scholar] [CrossRef]
- MacDonald, D.; Crabtree, J.R.; Wiesinger, G.; Dax, T.; Stamou, N.; Fleury, P.; Lazpita, J.G.; Gibon, A. Agricultural Abandonment in Mountain Areas of Europe: Environmental Consequences and Policy Response. J. Environ. Man. 2000, 59, 47–69. [Google Scholar] [CrossRef]
- Cristi de Barros, E.C.; Ventura, H.V.; Gontijo, P.C.; Pereira, R.R.; Picanço, M.C. Ecotoxicological Study of Insecticide Effects on Arthropods in Common Bean. J. Insect Sci. 2015, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Jaouannet, M.; Dempsey, D.M.; Imani, J.; Coustau, C.; Kogel, K.H. RNA-Based Technologies for Insect Control in Plant Production. Biotech. Adv. 2020, 39, 107463. [Google Scholar] [CrossRef] [PubMed]
- Rada, S.; Schweiger, O.; Harpke, A.; Kühn, E.; Kuras, T.; Settele, J.; Musche, M. Protected Areas do not Mitigate Biodiversity Declines: A Case Study on Butterflies. Div. Distr. 2019, 25, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Rostant, W.G.; Wedell, N.; Hosken, D.J. Transposable Elements and Insecticide Resistance. Adv. Gen. 2012, 78, 169–201. [Google Scholar]
- Gutzat, R.; Scheid, O.M. Epigenetic responses to stress: Triple defense? Curr. Opin. Plant Biol. 2012, 15, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-Specific Effects of Neonicotinoid Pesticides on Honey Bees and Wild Bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, R.F.; Lester, P.J.; Miller, A.S.; Ryan, K.G. A Neurotoxic Pesticide Changes the Outcome of Aggressive Interactions Between Native and Invasive Ants. Proc. R. Soc. B Biol. Sci. 2013, 280, 20132157. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, W.F.; Smagghe, G.; Guedes, R.N. Pesticides and Reduced-R isk Insecticides, Native Bees and Pantropical Stingless Bees: Pitfalls and Perspectives. Pest Man. Sci. 2015, 71, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Ben Maamar, M.; Sadler-Riggleman, I.; Beck, D.; Nilsson, E.; McBirney, M.; Klukovich, R.; Xie, Y.; Tang, C.; Yan, W. Alterations in Sperm DNA Methylation, Non-Coding RNA and Histone Retention Associate With DDT-Induced Epigenetic Transgenerational Inheritance of Disease. Epig. Chrom. 2018, 11, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Vargas, A.O.; Botelho, J.F.; Mpodozis, J. The Evolutionary Consequences of Epigenesis and Neutral Change: A Conceptual Approach at the Organismal Level. J. Exp. Zool. Part B Mol. Dev. Evol. 2020. [Google Scholar] [CrossRef]
- Stavert, J.R.; Pattemore, D.E.; Gaskett, A.C.; Beggs, J.R.; Bartomeus, I. Exotic Species Enhance Response Diversity to Land-Use Change But Modify Functional Composition. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170788. [Google Scholar] [CrossRef] [PubMed]
- Wilting, H.C.; Schipper, A.M.; Bakkenes, M.; Meijer, J.R.; Huijbregts, M.A. Quantifying Biodiversity Losses Due to Human Consumption: A Global-Scale Footprint Analysis. Environ. Sci. Technol. 2017, 51, 3298–3306. [Google Scholar]
- Biesmeijer, J.C.; Roberts, S.P.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.J.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and The Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Potts, S.; Packer, L. Pollinator Diversity and Crop Pollination Services are at Risk. Trends Ecol. Evol. 2005, 20, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Pettis, J.S.; Lichtenberg, E.M.; Andree, M.; Stitzinger, J.; Rose, R. Crop Pollination Exposes Honey Bees to Pesticides Which Alters Their Susceptibility to the Gut Pathogen Nosema ceranae. PLoS ONE 2013, 8, e70182. [Google Scholar]
- Pievani, T. The Sixth Mass Extinction: Anthropocene and the Human Impact on Biodiversity. Rend. Lincei 2014, 25, 85–93. [Google Scholar] [CrossRef]
- Schapheer, C.; Pellens, R.; Scherson, R. Arthropod-Microbiota Integration: Its Importance for Ecosystem Conservation. Front. Microbiol. 2021, 12, 1–18. [Google Scholar] [CrossRef]

| Epigenetic Inheritance Mechanism | Heritable Effect | Reference |
|---|---|---|
| C5-cytosine methylation/demethylation | Phase variation | Pearson (2019) [17] |
| Inheritance of methylated Cori * | Frandi & Collier (2019) [18] | |
| Epimutations | Skinner et al. (2019) [19] | |
| Paramutations | House & Lukens (2019) [20] | |
| Genomic imprinting | Tucci et al. (2019) [21] | |
| Transcriptional silencing | Di Felice et al. (2019) [22] | |
| X chromosome inactivation | Żylicz et al. (2019) [23] | |
| Histone modifications | Dosage compensation | Shevchenko et al. (2019) [24] |
| Vernalization | Zhong et al. (2019) [25] | |
| Post-transcriptional silencing through RNA interference | Transgenerational inheritance of neural processes | Posner et al. (2019) [26] |
| Heritable effects of starvation | Dupont et al. (2019) [27] |
| Chemical Group/ Functional Category | Elements or Compounds | Affected Species | Order: Family | Effects | EMMs Studied | References |
|---|---|---|---|---|---|---|
| Heavy Metals | Cu2+ | Aedes aegypti; Anopheles arabiensis | Diptera: Culicidae | Cell metabolisms, egg hatching, apoptosis; decrease in RNA methylation; DNAm methylation | DNA M., RNA-b M | Rayms-Keller et al., 2000 [89]; Raes et al., 2000 [90]; Jeanrenaud, Brooke & Oliver, 2020 [84] |
| Zn | Rhithrogena robusta | Ephemeroptera: Heptageniidae | Reduced individual growth rate | NO | Carlisle & Clements, 2003 [91] | |
| Pb | Lymantria dispar; Anopheles arabiensis | Lepidoptera: Erebidae; Diptera: Culicidae | Decrease in growth and reduction in hatching success; increased in RNA methylation patterns; DNA methylation | DNA M., RNA-b M. | Gintenreiter, Ortel & Nopp, 1993 [87]; Jeanrenaud, Brooke & Oliver, 2020 [84] | |
| Cd | Orchesella cincta; Anopheles arabiensis | Collembola: Entomobryidae; Diptera: Culicidae | Transcriptome stress response; increase in 5-hmC methylation | DNA M. | Roelofs et al., 2009 [86]; Jeanrenaud, Brooke & Oliver, 2020 [84] | |
| Heavy Metals | Al | Drosophila melanogaster | Diptera: Drosophilidae | Reduction in median life span; climbing ability and cognitive capacity | NO | Wu et al., 2012 [92] |
| Chlorinated hydrocarbons | DDT | Ephemerella subvaria, Ephemerella auravillii | Ephemeroptera: Ephemerellidae | Impaired subimago emergence | NO | Hitchcock, 1965 [93] |
| Aldrin | Cheumatopsyche analis | Trichoptera: Hydropsychidae | Hormetic response | NO | Moye & Luckmann, 1964 [94] | |
| Chlordane | Periplaneta americana | Blattodea: Blattidae | Increase in total hemocyte count; excessive vacuolization of epithelial cells in the midgut lumen | NO | Gupta & Sutherland, 1968 [95] | |
| Endrin | Periplaneta americana | Blattodea: Blattidae | Dose-dependent blocking of GABA receptors | NO | Wafford et al., 1989 [96] | |
| Chlorinated hydrocarbons | Heptachlor | Periplaneta americana | Blattodea: Blattidae | Dose-dependent blocking of GABA receptors | NO | Lummis et al., 1990 [97] |
| Lindane | Periplaneta americana; Chironomus ripariu | Blattodea: Blattidae; Diptera: Chironimidae | Dose-dependent blocking of GABA receptors; reduction in imago emergence | NO | Wafford et al., 1989 [96]; Maund et al., 1992 [98] | |
| Organophosphates | Parathion | Musca domestica | Diptera: Muscidae | Toxin degradation | NO | Matsumura & Hogendijk, 1964 [99] |
| Malathion | Periplaneta americana; Musca domestica | Blattodea: Blattidae; Diptera: Muscidae | Toxin metabolization | NO | Krueger & O’Brien, 1959 [100] | |
| Glyphosate | Deleatidium spp. | Ephemeroptera: Leptophlebiidae | Reduction in imago emergence | NO | Magbanua et al., 2016 [101] | |
| Organophosphates | Diazinon | Musca domestica | Diptera: Muscidae | Toxin degradation | NO | Matsumura & Hogendijk, 1964 [99] |
| Tetrachlorvinphos | Alphitobius diaperinus | Coleoptera: Tenebrionidae | Resistance to pesticide | NO | Hamm et al., 2006 [102] | |
| Azamethiphos | Musca domestica | Diptera: Muscidae | Resistance to pesticide | NO | Kristensen et al., 2000 [103] | |
| Phosmet | Megachile rotundata | Hymenoptera: Megachilidae | Reduced nesting and progeny production | NO | Alston et al., 2007 [104] | |
| Diclorvos | Alphitobius diaperinus | Coleoptera: Tenebrionidae | Resistance to pesticide | NO | Chernaki-Leffer et al., 2011 [105] | |
| Terbufos | Alphitobius diaperinus | Diptera: Sarcophagidae | Avoidance of pesticide | NO | Jales et al., 2020 [106] | |
| Carbamates | Sevin | Musca domestica | Diptera: Muscidae | Metabolization of pesticide | NO | Eldefrawi & Hoskims, 1964 [107] |
| Aldicarb | Pseudatomoscelis seriatus; Musca domestica | Hemiptera: Miridae; Diptera: Muscidae | Death | NO | Davis & Cowan, 1972 [108]; Spurr & Sousa, 1974 [109] | |
| Carbofuran | Diabrotica virgifera | Coleoptera: Chrysomelidae | Increase in oviposition; increase in longevity | NO | Ball & Su, 1979 [110] | |
| Carbaryl | Diabrotica virgifera | Coleoptera: Chrysomelidae | Increase in oviposition | NO | Ball & Su, 1979 [110] | |
| Pyrethroids | Allethrin | Periplaneta americana | Blattodea: Blattidae | Temperature dependent disruption of the nervous system | NO | Gammon, 1978 [111] |
| Bifenthrin | Apis mellifera ligustica | Hymenoptera: Apidae | Reduction in oviposition; reduction in cap rate; reduction in emergence rate; success rate of development | NO | Dai et al., 2010 [112] | |
| β Cyfluthrin | Drosophila melanogaster (Sepia mutant) | Diptera: Drosophilidae | Reduction imago emergence; prolongation of total developmental period | NO | Nadda, Saxena & Srivastava, 2005 [113] | |
| Cypermethrin | Alphitobius diaperinus | Coleoptera: Tenebrionidae | Resistance to pesticide | NO | Chernaki-Leffer et al., 2011 [105] | |
| Cyphenothrin | Ranatra filiformis | Hemiptera: Nepidae | Hyperactivity, death | NO | Saha & Kaviraj, 2007 [114] | |
| Pyrethroids | Deltamethrin | Apis mellifera ligustica | Hymenoptera: Apidae | Reduction in oviposition; lower hatch rate; reduction in cap rate; success rate of development | NO | Dai et al., 2010 [112] |
| Permethrin | Acheta domesticus | Orthoptera: Gryllidae | Death | NO | Schleier & Peterson, 2010 [115] | |
| Resmethrin | Danaus plexippus | Lepidoptera: Nymphalidae | Reduced adult size, death | NO | Oberhauser et al., 2009 [116] | |
| Transfluthrin | Culex tarsalis | Diptera: Culicidae | Avoidance of pesticide | NO | Britch et al., 2020 [117] | |
| Neonicotinoids (neuroinsecticides) | Thiamethoxam | Musca domestica | Diptera: Muscidae | Acetylcholine receptors hyperexcitation; ATPase activity | NO | Abdel-Haleem et al., 2018 [118] |
| Neonicotinoids (neuroinsecticides) | Imidacloprid | Apis mellifera | Hymenoptera: Apidae | Acetylcholine receptors hyperexcitation; Malpighian tubule deformation; changes in global DNA methylation | DNA M. | Paleolog et al., 2020 [119]; Hu et al., 2018 [120]; Brevik et al., 2020 [81] |
| Acetamiprid | Apis mellifera | Hymenoptera: Apidae | Reduction in sucrose sensitivity; increased locomotive activity | NO | El-hassani et al., 2007 [121] | |
| Clothianidin | Chironomus dilutus | Diptera: Chironomidae | Reduction of emergence | NO | Maloney et al., 2018 [122] | |
| Nitenpyram | Bemisia tabaci B biotype | Hemiptera: Aleyrodidae | Resistance to pesticide | NO | Liang et al., 2012 [123] | |
| Thiacloprid | Culex pipiens | Diptera: Culicidae | Preimaginal development duration | NO | Beketov & Liess, 2008 [124] | |
| Dinotefuran | Chironominae spp. | Diptera: Chironomidae | Population hormetic response | NO | Kobashi et al., 2017 [125] | |
| Endocrine disruptors (ED) | Bisphenol A | Chironomus riparius | Diptera: Chironomidae | Increase in mRNA for ecdysone receptor and increase in the expression of HSP70 | RNA-b M. | Planelló, Martínez-Guitarte & Morcillo, 2008 [85] |
| Tributyltin | Chironomus riparius | Diptera: Chironomidae | DNA breakage | NO | Martínez-Paz et al., 2013 [126] | |
| Pentachlorophenol | Chironomus riparius | Diptera: Chironomidae | Upregulation of Hsp70 gene transcription; downregulation of the Hsp27 transcription | NO | Morales et al., 2014 [127] | |
| Nonylphenol | Chironomus riparius | Diptera: Chironomidae | DNA breakage | NO | Martínez-Paz et al., 2013 [126] | |
| Triclosan | Chironomus riparius | Diptera: Chironomidae | DNA breakage | NO | Martínez-Paz et al., 2013 [126] | |
| Benzyl butyl phthalate | Chironomus riparius | Diptera: Chironomidae | Overexpression of the EcR gene | NO | Planelló et al., 2011 [128] | |
| Endocrine disruptors (ED) | DEHP/Di(2-ethylhexyl)phthalate | Chironomus riparius | Diptera: Chironomidae | Mouthparts deformities | NO | Park & Kwak, 2008 [129] |
| Ethinylestradiol | Drosophila melanogaster | Diptera: Drosophilidae | Reduction in lifespan, decrease in fertility | NO | Bovier et al., 2018 [130] | |
| Genistein/5,7-dihydroxy-3-(4-hydro- xyphenyl)chromen-4-one) | Aedes albopictus; Anopheles arabiensis | Diptera: Culicidae | DNA methylation; reduction in egg hatching | DNA M. | Oppold et al., 2015 [11]; Jeanrenaud, Brooke & Oliver, 2020 [84] | |
| Vinclozolin (Fungicide) | Aedes albopictus; Anopheles arabiensis | Diptera: Culicidae | DNA methylation; reduction in egg hatching | DNA M. | Oppold et al., 2015 [11]; Jeanrenaud, Brooke & Oliver, 2020 [84] | |
| DMSO/dimethyl sulphoxide | Antheraea assamensis | Lepidoptera: Saturniidae | Alterations in hormonal balance; alterations in silk production | NO | Unni et al., 2009 [131] | |
| Micro plastics | Polystyrene | Culex pipiens | Diptera: Culicidae | Accumulation in Malpighian tubules | NO | Al-Jaibachi, Cuthbert & Callaghan, 2018 [132] |
| Polypropylene | Lestes viridis | Odonata: Lestidae | Accumulation in body | NO | Akindele, Ehlers & Koop, 2020 [133] | |
| Acrylonitrile butadiene styrene (ABS) | Siphlonurus sp. | Ephemeroptera: Siphlonuridae | Accumulation in body | NO | Akindele, Ehlers & Koop, 2020 [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares-Castro, G.; Cáceres-Jensen, L.; Guerrero-Bosagna, C.; Villagra, C. Insect Epigenetic Mechanisms Facing Anthropogenic-Derived Contamination, an Overview. Insects 2021, 12, 780. https://doi.org/10.3390/insects12090780
Olivares-Castro G, Cáceres-Jensen L, Guerrero-Bosagna C, Villagra C. Insect Epigenetic Mechanisms Facing Anthropogenic-Derived Contamination, an Overview. Insects. 2021; 12(9):780. https://doi.org/10.3390/insects12090780
Chicago/Turabian StyleOlivares-Castro, Gabriela, Lizethly Cáceres-Jensen, Carlos Guerrero-Bosagna, and Cristian Villagra. 2021. "Insect Epigenetic Mechanisms Facing Anthropogenic-Derived Contamination, an Overview" Insects 12, no. 9: 780. https://doi.org/10.3390/insects12090780
APA StyleOlivares-Castro, G., Cáceres-Jensen, L., Guerrero-Bosagna, C., & Villagra, C. (2021). Insect Epigenetic Mechanisms Facing Anthropogenic-Derived Contamination, an Overview. Insects, 12(9), 780. https://doi.org/10.3390/insects12090780