Characterization of Thermal and Time Exposure to Improve Artificial Diet for Western Corn Rootworm Larvae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Egg Treatment
2.2. Experimental Approach
3. Diet Preparation
3.1. Insect Artificial Diet Bioassays
3.2. Data Processing and Statistical Analyses
4. Results
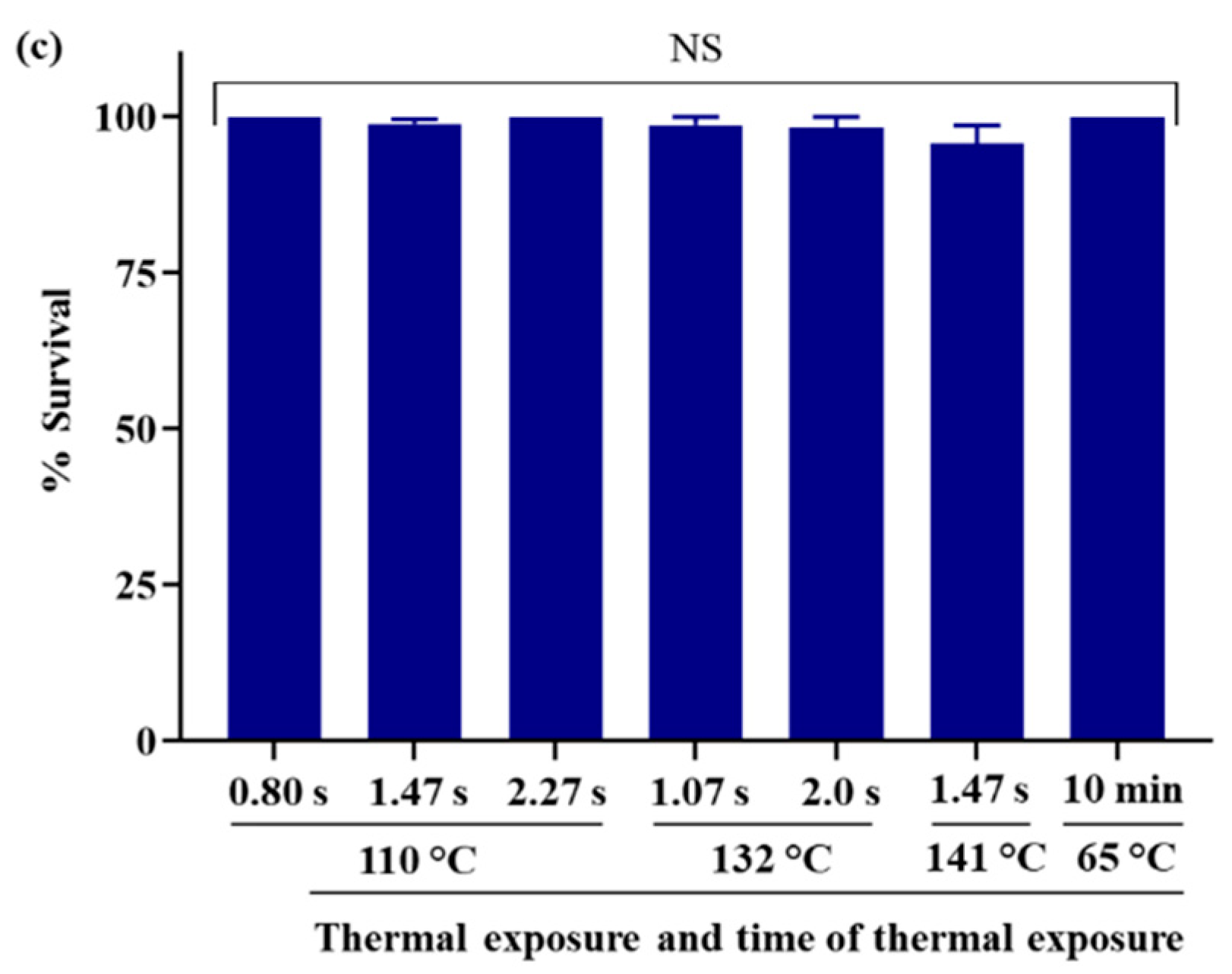
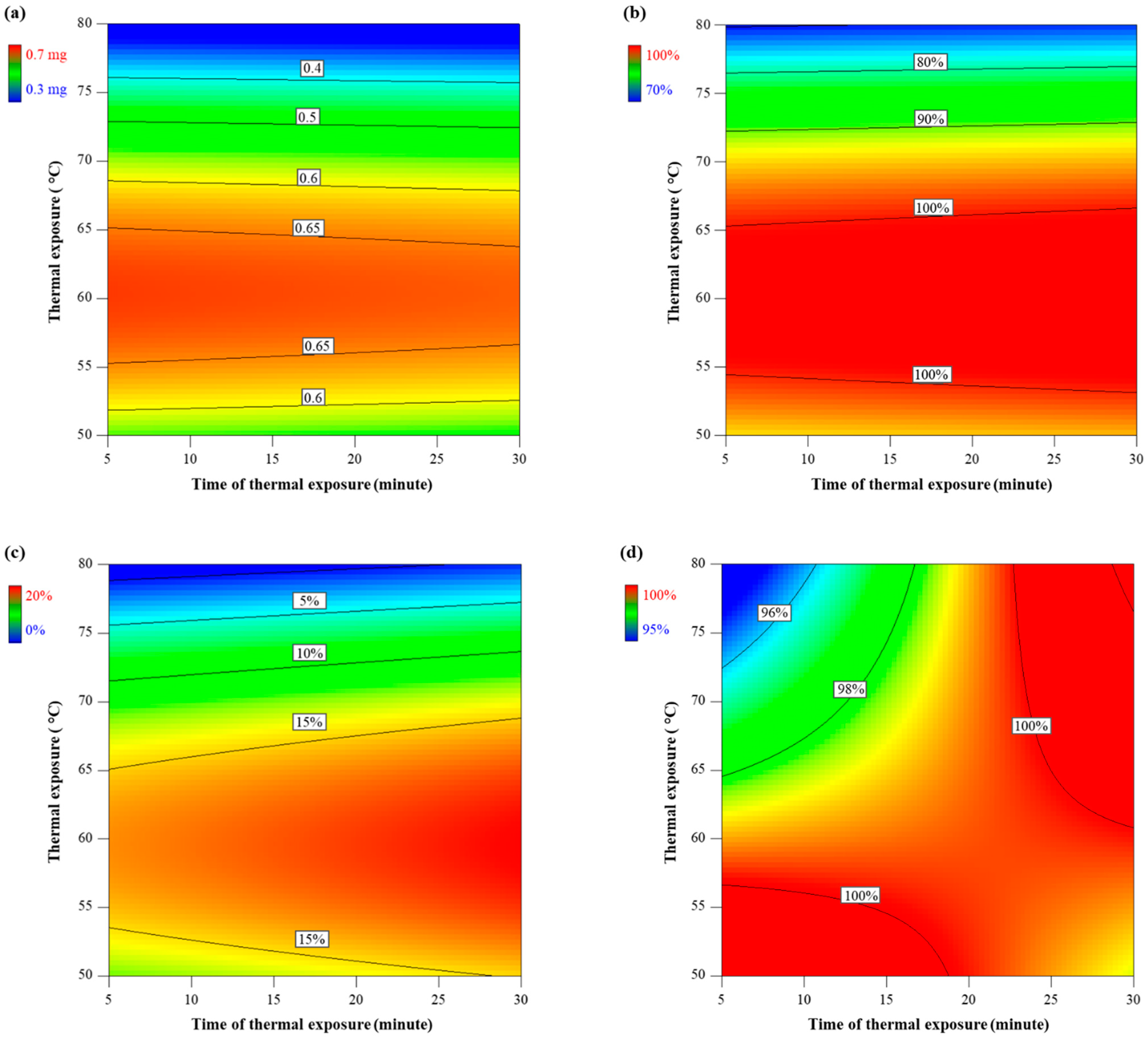
4.1. Diet Quality with High Thermal Exposure and Short Time of Thermal Exposure
4.2. Diet Quality with Mild Thermal Exposure and Extended Time of Thermal Exposure
4.3. Contamination
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veres, A.; Wyckhuys, K.A.; Kiss, J.; Tóth, F.; Burgio, G.; Pons, X.; Avilla, C.; Vidal, S.; Razinger, J.; Bazok, R. An update of the Worldwide Integrated Assessment (WIA) on systemic pesticides. Part 4: Alternatives in major cropping systems. Environ. Sci. Pollut. Res. 2020, 27, 29867–29899. [Google Scholar] [CrossRef]
- Wechsler, S.; Smith, D. Has resistance taken root in US corn fields? Demand for insect control. Am. J. Agr. Econ. 2018, 100, 1136–1150. [Google Scholar] [CrossRef]
- Branson, T.F.; Krysan, J.L. Feeding and oviposition behavior and life cycle strategies of Diabrotica: An evolutionary view with implications for pest management. Environ. Entomol. 1981, 10, 826–831. [Google Scholar] [CrossRef]
- Moeser, J.; Vidal, S. Nutritional resources used by the invasive maize pest Diabrotica virgifera virgifera in its new south-east-European distribution range. Entomol. Exp. Appl. 2005, 114, 55–63. [Google Scholar] [CrossRef]
- Culy, M.D.; Edwards, C.R.; Cornelius, J.R. Effect of silk feeding by western corn rootworm (Coleoptera: Chrysomelidae) on yield and quality of inbred corn in seed corn production fields. J. Econ. Entomol. 1992, 85, 2440–2446. [Google Scholar] [CrossRef]
- Ball, H.J.; Weekman, G.T. Differential resistance of corn rootworms to insecticides in Nehraska and adjoining States. J. Econ. Entomol. 1963, 56, 553–555. [Google Scholar] [CrossRef]
- Meinke, L.J.; Siegfried, B.D.; Wright, R.J.; Chandler, L.D. Adult susceptibility of Nebraska western corn rootworm (Coleoptera: Chrysomelidae) populations to selected insecticides. J. Econ. Entomol. 1998, 91, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.E.; Wang, H.; Zukoff, S.N.; Meinke, L.J.; French, B.W.; Siegfried, B.D. Evidence of field-evolved resistance to Bifenthrin in western corn rootworm (Diabrotica virgifera virgifera LeConte) populations in Western Nebraska and Kansas. PLoS ONE 2015, 10, e0142299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.E.; Souza, D.; Zukoff, S.N.; Meinke, L.J.; Siegfried, B.D. Cross-resistance and synergism bioassays suggest multiple mechanisms of pyrethroid resistance in western corn rootworm populations. PLoS ONE 2017, 12, e0179311. [Google Scholar] [CrossRef] [Green Version]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Keweshan, R.S.; Dunbar, M.W. Field-evolved resistance to Bt maize by western corn rootworm. PLoS ONE 2011, 6, e22629. [Google Scholar] [CrossRef] [Green Version]
- Gassmann, A.J.; Shrestha, R.B.; Jakka, S.R.; Dunbar, M.W.; Clifton, E.H.; Paolino, A.R.; Ingber, D.A.; French, B.W.; Masloski, K.E.; Dounda, J.W. Evidence of resistance to Cry34/35Ab1 corn by western corn rootworm (Coleoptera: Chrysomelidae): Root injury in the field and larval survival in plant-based bioassays. J. Econ. Entomol. 2016, 109, 1872–1880. [Google Scholar] [CrossRef]
- Zukoff, S.N.; Zukoff, A.L.; Geisert, R.W.; Hibbard, B.E. Western corn rootworm (Coleoptera: Chrysomelidae) larval movement in eCry3.1Ab+mCry3A seed blend scenarios. J. Econ. Entomol. 2016, 109, 1834–1845. [Google Scholar] [CrossRef]
- Ludwick, D.C.; Meihls, L.N.; Ostlie, K.R.; Potter, B.D.; French, L.; Hibbard, B.E. Minnesota field population of western corn rootworm (Coleoptera: Chrysomelidae) shows incomplete resistance to Cry34Ab1/Cry35Ab1 and Cry3Bb1. J. Appl. Entomol. 2017, 141, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Calles-Torrez, V.; Knodel, J.J.; Boetel, M.A.; French, B.W.; Fuller, B.W.; Ransom, J.K. Field-evolved resistance of northern and western corn rootworm (Coleoptera: Chrysomelidae) populations to corn hybrids expressing single and pyramided Cry3Bb1 and Cry34/35Ab1 Bt proteins in North Dakota. J. Econ. Entomol. 2019, 112, 1875–1886. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Shrestha, R.B.; Kropf, A.L.; St Clair, C.R.; Brenizer, B.D. Field-evolved resistance by western corn rootworm to Cry34/35Ab1 and other Bacillus thuringiensis traits in transgenic maize. Pest Manag. Sci. 2020, 76, 268–276. [Google Scholar] [CrossRef]
- Levine, E.; Spencer, J.L.; Isard, S.A.; Onstad, D.W.; Gray, M.E. Adaptation of the western corn rootworm to crop rotation: Evolution of a new strain in response to a management practice. Am. Entomol. 2002, 48, 94–117. [Google Scholar] [CrossRef] [Green Version]
- Gray, M.E.; Sappington, T.W.; Miller, N.J.; Moeser, J.; Bohn, M.O. Adaptation and invasiveness of western corn rootworm: Intensifying research on a worsening pest. Ann. Rev. Entomol. 2009, 54, 303–321. [Google Scholar] [CrossRef] [Green Version]
- EPA. Memo from EPA on Corn Rootworm Resistance Management; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2016.
- Meihls, L.N.; Higdon, M.L.; Siegfried, B.D.; Miller, N.J.; Sappington, T.W.; Ellersieck, M.R.; Spencer, T.A.; Hibbard, B.E. Increased survival of western corn rootworm on transgenic corn within three generations of on-plant greenhouse selection. Proc. Natl. Acad. Sci. USA 2008, 105, 19177–19182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowatzki, T.M.; Lefko, S.A.; Binning, R.R.; Thompson, S.D.; Spencer, T.; Siegfried, B. Validation of a novel resistance monitoring technique for corn rootworm (Coleoptera: Chrysomelidae) and event DAS-59122-7 maize. J. Appl. Entomol. 2008, 132, 177–188. [Google Scholar] [CrossRef]
- Zukoff, S.N.; Ostlie, K.R.; Potter, B.; Meihls, L.N.; Zukoff, A.L.; French, L.; Ellersieck, M.R.; Wade French, B.; Hibbard, B.E. Multiple assays indicate varying levels of cross resistance in Cry3Bb1-selected field populations of the western corn rootworm to mCry3A, eCry3.1Ab, and Cry34/35Ab1. J. Econ. Entomol. 2016, 109, 1387–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrone, P.G.; Ferri, F.D.; Mosley, T.R.; Meinke, L.J. Improvements in laboratory rearing of the southern corn rootworm, Diabrotica undecimpuncta howardi Barber (Coleoptera: Chrysomelidae), on an artificial diet and corn. J. Econ. Entomol. 1985, 78, 290–293. [Google Scholar] [CrossRef]
- Sutter, G.R.; Krysan, J.L.; Guss, P.L. Rearing the southern corn rootworm on artificial diet. J. Econ. Entomol. 1971, 64, 65–67. [Google Scholar] [CrossRef]
- Pleau, M.J.; Huesing, J.E.; Head, G.P.; Feir, D.J. Development of an artificial diet for the western corn rootworm. Entomol. Exp. Appl. 2002, 105, 1–11. [Google Scholar] [CrossRef]
- Huynh, M.P.; Meihls, L.N.; Hibbard, B.E.; Lapointe, S.L.; Niedz, R.P.; Ludwick, D.C.; Coudron, T.A. Diet improvement for western corn rootworm (Coleoptera: Chrysomelidae) larvae. PLoS ONE 2017, 12, e0187997. [Google Scholar] [CrossRef] [Green Version]
- Ludwick, D.C.; Meihls, L.N.; Huynh, M.P.; Pereira, A.E.; French, B.W.; Coudron, T.A.; Hibbard, B.E. A new artificial diet for western corn rootworm larvae is compatible with and detects resistance to all current Bt toxins. Sci. Rep. 2018, 8, 5379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, M.P.; Hibbard, B.E.; Vella, M.; Lapointe, S.L.; Niedz, R.P.; Shelby, K.S.; Coudron, T.A. Development of an improved and accessible diet for western corn rootworm larvae using response surface modeling. Sci. Rep. 2019, 9, 16009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meihls, L.N.; Huynh, M.P.; Ludwick, D.C.; Coudron, T.A.; French, B.W.; Shelby, K.S.; Hitchon, A.J.; Schaafsma, A.W.; Pereira, A.E.; Hibbard, B.E. Comparison of six artificial diets for support of western corn rootworm bioassays and rearing. J. Econ. Entomol. 2018, 111, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Paramasiva, I.; Sharma, H.C.; Krishnayya, P.V. Antibiotics influence the toxicity of the delta endotoxins of Bacillus thuringiensis towards the cotton bollworm, Helicoverpa armigera. BMC Microbiol. 2014, 14, 200. [Google Scholar] [CrossRef] [Green Version]
- Visweshwar, R.; Sharma, H.; Akbar, S.; Sreeramulu, K. Elimination of gut microbes with antibiotics confers resistance to Bacillus thuringiensis toxin proteins in Helicoverpa armigera (Hubner). Appl. Biochem. Biotechnol. 2015, 177, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-C.; Spencer, J.L.; Curzi, M.J.; Zavala, J.A.; Seufferheld, M.J. Gut bacteria facilitate adaptation to crop rotation in the western corn rootworm. Proc. Natl. Acad. Sci. USA 2013, 110, 11917–11922. [Google Scholar] [CrossRef] [Green Version]
- Paddock, K.J.; Pereira, A.E.; Finke, D.L.; Ericsson, A.C.; Hibbard, B.E.; Shelby, K.S. Host resistance to Bacillus thuringiensis is linked to altered bacterial community within a specialist insect herbivore. Mol. Ecol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.P.; Hibbard, B.E.; Lapointe, S.L.; Niedz, R.P.; French, B.W.; Pereira, A.E.; Finke, D.L.; Shelby, K.S.; Coudron, T.A. Multidimensional approach to formulating a specialized diet for northern corn rootworm larvae. Sci. Rep. 2019, 9, 3709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.C. Insect Diets: Science and Technology, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Huynh, M.P.; Bernklau, E.J.; Coudron, T.A.; Shelby, K.S.; Bjostad, L.B.; Hibbard, B.E. Characterization of corn root factors to improve artificial diet for western corn rootworm (Coleoptera: Chrysomelidae) larvae. J. Insect Sci. 2019, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.P.; Kovaleva, E.; Campbell, J.H.; Anderson, P.E.; Brown, S.G.; Davis, D.C.; Valdes, J.J.; Welch, R.W.; Bentley, W.E.; van Beek, N.A. Production of a recombinant antibody fragment in whole insect larvae. Mol. Biotechnol. 2007, 36, 44–51. [Google Scholar] [CrossRef]
- Allen, D.M. Mean square error of prediction as a criterion for selecting variables. Technometrics 1971, 13, 469–475. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons: New York, NY, USA, 2016. [Google Scholar]
- Anderson, M.J.; Whitcomb, P.J. Using graphical diagnostics to deal with bad data. Qual. Eng. 2007, 19, 111–118. [Google Scholar] [CrossRef]
- Damodaran, S. Amino acids, peptides and proteins. In Fennema’s Food Chemistry; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008; Volume 4, pp. 425–439. [Google Scholar]
- Vanderzant, E. Improvements in the rearing diet for Chrysopa carnea and the amino acid requirements for growth. J. Econ. Entomol. 1973, 66, 336–338. [Google Scholar] [CrossRef]
- Vanderzant, E. An artificial diet for larvae and adults of Chrysopa carnea, an insect predator of crop pests. J. Econ. Entomol. 1969, 62, 256–257. [Google Scholar] [CrossRef]
- Griffin, J.; Lindig, O.; McLaughlin, R. Flash sterilizers: Sterilizing artificial diets for insects. J. Econ. Entomol. 1974, 67, 689. [Google Scholar] [CrossRef]
- Oyediran, I.O.; Hibbard, B.E.; Clark, T.L. Prairie grasses as hosts of the western corn rootworm (Coleoptera: Chrysomelidae). Environ. Entomol. 2004, 33, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Siegfried, B.D.; Vaughn, T.T.; Spencer, T. Baseline susceptibility of western corn rootworm (Coleoptera: Crysomelidae) to Cry3Bb1 Bacillus thuringiensis toxin. J. Econ. Entomol. 2005, 98, 1320–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.E.; Huynh, M.P.; Sethi, A.; Miles, A.L.; Wade French, B.; Ellersieck, M.R.; Coudron, T.A.; Shelby, K.S.; Hibbard, B.E. Baseline susceptibility of a laboratory strain of northern corn rootworm, Diabrotica barberi (Coleoptera: Chrysomelidae) to Bacillus thuringiensis traits in seedling, single plant, and diet-toxicity assays. J. Econ. Entomol. 2020, 113, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.E.; Huynh, M.P.; Carlson, A.R.; Haase, A.; Kennedy, R.M.; Shelby, K.S.; Coudron, T.A.; Hibbard, B.E. Assessing the single and combined toxicity of the bioinsecticide Spear® and Cry3Bb1 protein against susceptible and resistant western corn rootworm larvae (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2021, in press. [Google Scholar] [CrossRef]
- Tillman, P.G.; McKibben, G.; Malone, S.; Harsh, D. Form-Fill-Seal Machine for Mass Rearing Noctuid Species; Mississippi Agricultural and Forestry Experiment Station: Mississippi State, MS, USA, 1997. [Google Scholar]
- Paddock, K.J.; Robert, C.A.; Erb, M.; Hibbard, B.E. Western Corn Rootworm, Plant and Microbe Interactions: A Review and Prospects for New Management Tools. Insects 2021, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, C.; Ivashuta, S.; Wiggins, E.; Flagel, L.; Moar, W.; Pleau, M.; Miller, K.; Zhang, Y.; Ramaseshadri, P.; Jiang, C. Development and characterization of the first dsRNA-resistant insect population from western corn rootworm, Diabrotica virgifera virgifera LeConte. PLoS ONE 2018, 13, e01970. [Google Scholar] [CrossRef] [Green Version]



| Treatment | High Temperature | Mild Temperature | ||
|---|---|---|---|---|
| Temperature (°C) | Time Exposure (Second) | Temperature (°C) | Time Exposure (Minute) | |
| 1 | 141 | 1.47 | 80 | 17 |
| 2 | 132 | 1.07 | 76 | 26 |
| 3 | 132 | 2.00 | 76 | 10 |
| 4 | 110 | 0.80 | 65 | 30 |
| 5 | 110 | 1.47 | 65 | 17 |
| 6 | 110 | 1.47 | 65 | 17 |
| 7 | 110 | 1.47 | 65 | 17 |
| 8 | 110 | 2.27 | 65 | 5 |
| 9 | 88 | 1.07 | 55 | 26 |
| 10 | 88 | 2.00 | 55 | 9 |
| 11 | 80 | 1.47 | 50 | 17 |
| 12 (control) | 65 | 600 | 65 | 10 |
| Weight p-Values | Regression Coefficients | % Molt to 2nd Instar p-Values | Regression Coefficients | % Molt to 3rd Instar p-Values | Regression Coefficients | Survival p-Values | Regression Coefficients | |
|---|---|---|---|---|---|---|---|---|
| Model | 0.0015 | - | 0.0410 | - | 0.0100 | - | 0.0531 | - |
| A | 0.8538 | −0.0005 | 0.8940 | 0.0005 | 0.5604 | 0.0098 | 0.0582 | −0.0088 |
| B | 0.0012 | 0.1321 | 0.0224 | 0.0969 | 0.0051 | 0.0511 | 0.1563 | −0.0033 |
| AB | - | - | - | - | - | - | 0.0596 | 0.0002 |
| B2 | 0.0025 | −0.0011 | 0.0478 | −0.0008 | 0.0204 | −0.0004 | - | - |
| Lack of fit | 0.1953 | 0.0005 | 0.9195 | 0.5496 | ||||
| Model type | Quadratic (reduced) | Quadratic (reduced) | Quadratic (reduced) | Two-factor interaction | ||||
| R2 | 0.8745 | 0.6710 | 0.7838 | 0.6443 | ||||
| R2adj | 0.8208 | 0.5300 | 0.6911 | 0.4919 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, M.P.; Pereira, A.E.; Geisert, R.W.; Vella, M.G.; Coudron, T.A.; Shelby, K.S.; Hibbard, B.E. Characterization of Thermal and Time Exposure to Improve Artificial Diet for Western Corn Rootworm Larvae. Insects 2021, 12, 783. https://doi.org/10.3390/insects12090783
Huynh MP, Pereira AE, Geisert RW, Vella MG, Coudron TA, Shelby KS, Hibbard BE. Characterization of Thermal and Time Exposure to Improve Artificial Diet for Western Corn Rootworm Larvae. Insects. 2021; 12(9):783. https://doi.org/10.3390/insects12090783
Chicago/Turabian StyleHuynh, Man P., Adriano E. Pereira, Ryan W. Geisert, Michael G. Vella, Thomas A. Coudron, Kent S. Shelby, and Bruce E. Hibbard. 2021. "Characterization of Thermal and Time Exposure to Improve Artificial Diet for Western Corn Rootworm Larvae" Insects 12, no. 9: 783. https://doi.org/10.3390/insects12090783
APA StyleHuynh, M. P., Pereira, A. E., Geisert, R. W., Vella, M. G., Coudron, T. A., Shelby, K. S., & Hibbard, B. E. (2021). Characterization of Thermal and Time Exposure to Improve Artificial Diet for Western Corn Rootworm Larvae. Insects, 12(9), 783. https://doi.org/10.3390/insects12090783








