Microbial Control Agents for Fungus Gnats (Diptera: Sciaridae: Lycoriella) Affecting the Production of Oyster Mushrooms, Pleurotus spp.
Abstract
:Simple Summary
Abstract
1. Introduction
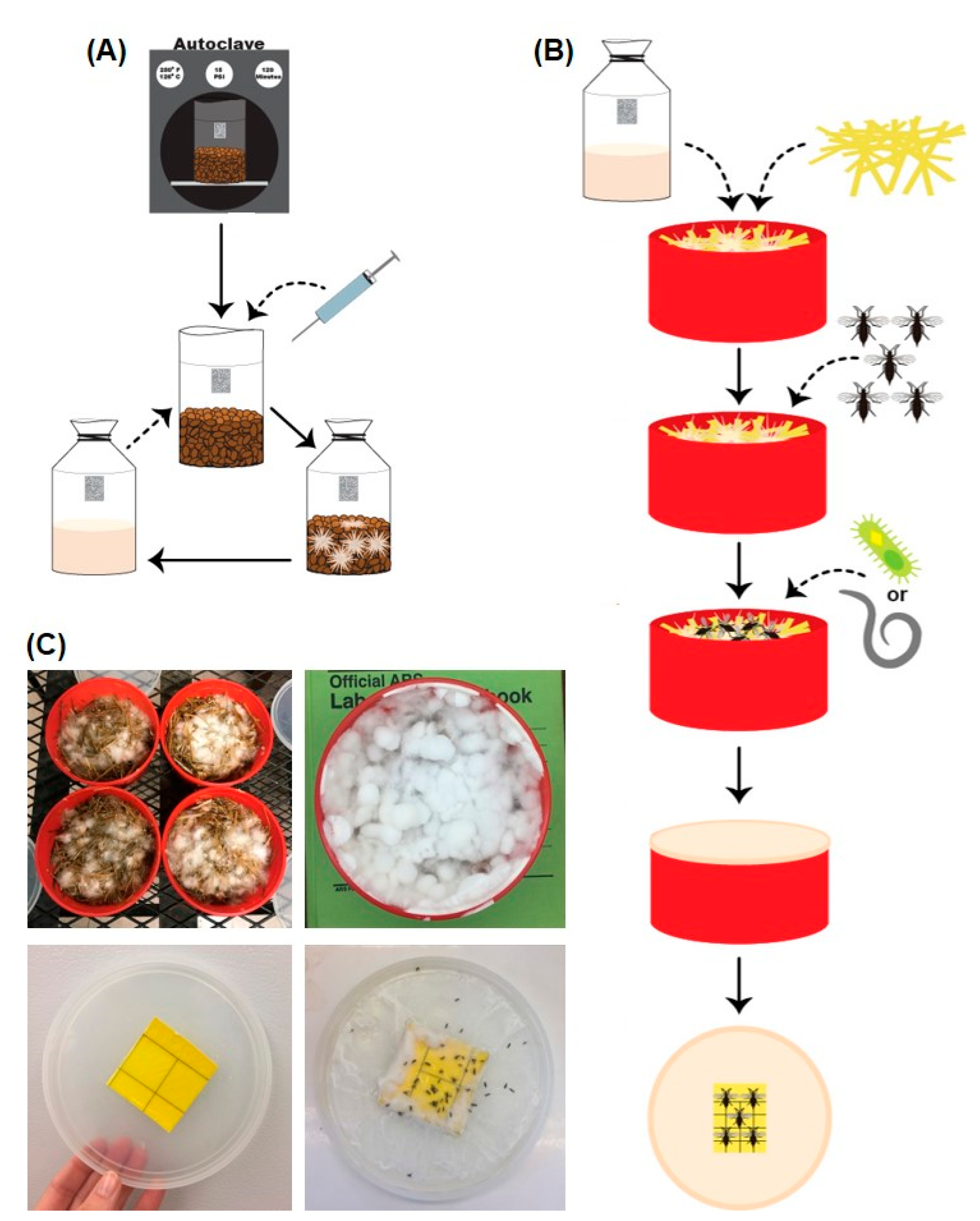
2. Materials and Methods
2.1. Oyster Mushroom Cultures
2.2. Container Bioassays
2.3. Straw Pasteurization
2.4. Bioassays with P. columbinus
2.5. Bioassays with P. ostreatus
2.6. Influence of Treatment on Mycelial Growth
2.7. Statistics
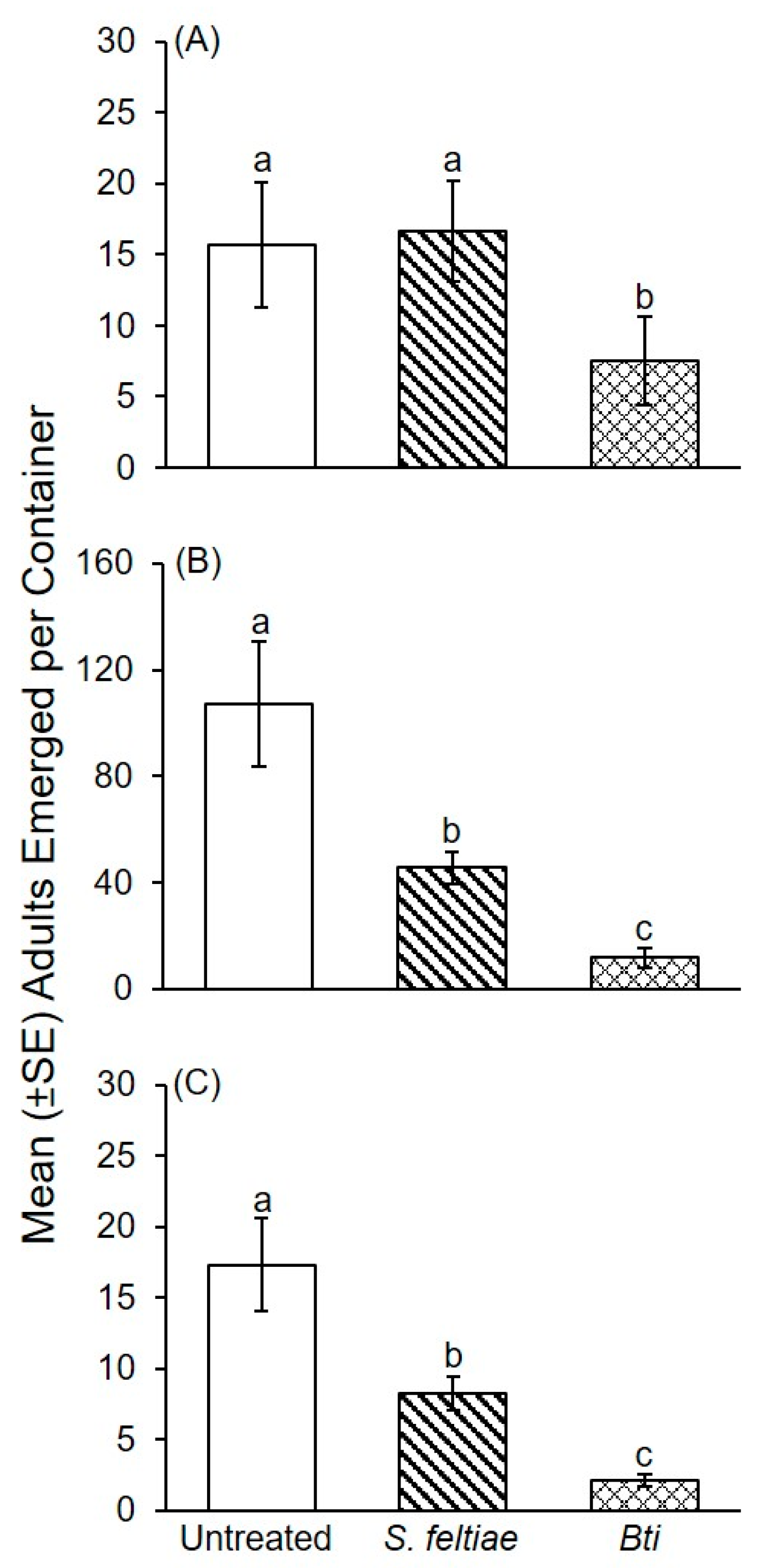
3. Results
3.1. Bioassays with P. columbinus
3.2. Bioassays with P. ostreatus
3.3. Influence of Treatment on Mycelial Growth
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enshasy, H.; Maftoun, P.; Johari, H.J.; Soltani, M.; Malik, R.; Othman, N. The edible mushroom Pleurotus spp.: I. Biodiversity and nutritional values. Int. J. Biotechnol. Wellness Ind. 2015, 4, 67–83. [Google Scholar] [CrossRef]
- Pavlik, M.; Pavlík, Š. Wood decomposition activity of oyster mushroom (Pleurotus ostreatus) isolate in situ. J. For. Sci. 2013, 59, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Piškur, B.; Bajc, M.; Robek, R.; Humar, M.; Sinjur, I.; Kadunc, A.; Oven, P.; Rep, G.; Petkovšek, S.A.S.; Kraigher, H.; et al. Influence of Pleurotus ostreatus inoculation on wood degradation and fungal colonization. Bioresour. Technol. 2011, 102, 10611–10617. [Google Scholar] [CrossRef] [PubMed]
- Gregori, A.; Švagelj, M.; Pohleven, J. Cultivation techniques and medicinal properties of Pleurotus spp. Food Technol. Biotechnol. 2007, 45, 238–249. [Google Scholar]
- Sánchez, C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl. Microbiol. Biotechnol. 2010, 85, 1321–1337. [Google Scholar] [CrossRef]
- National Agricultural Statistics Service (NASS). Mushrooms. National Agricultural Statistics Service, Agricultural Statistics Board, USDA. 2020. ISSN 1949-1530. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/r781wg03d/3j333r948/x920gm28k/mush0820.pdf (accessed on 1 September 2021).
- Mohamed, M.F.; Refaei, E.F.S.; Abdalla, M.M.A.; Abdelgalil, S.H. Fruiting bodies yield of oyster mushroom (Pleurotus columbinus) as affected by different portions of compost in the substrate. Int. J. Recycl. Org. Waste Agric. 2016, 5, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.-F.; Qu, S.-X.; Lin, J.-S.; Li, H.-P.; Hou, L.-J.; Jiang, N.; Luo, X.; Ma, L. Identification of Cyt2Ba from a new strain of Bacillus thuringiensis and its toxicity in Bradysia difformis. Curr. Microbiol. 2020, 77, 2859–2866. [Google Scholar] [CrossRef]
- Gbolagade, J.; Ajayi, A.; Oku, I.; Wankasi, D. Nutritive value of common wild edible mushrooms from southern Nigeria. Glob. J. Biotechnol. Biochem. 2006, 1, 16–21. [Google Scholar]
- Dundar, A.; Acay, H.; Yildiz, A. Yield performances and nutritional contents of three oyster mushroom species cultivated on wheat stalk. Afr. J. Biotechnol. 2008, 7, 3497–3501. [Google Scholar]
- Lavi, I.; Levinson, D.; Peri, I.; Nimri, L.; Hadar, Y.; Schwartz, B. Orally administered glucans from the edible mushroom Pleurotus pulmonarius reduce acute inflammation in dextran sulfate sodium-induced experimental colitis. Br. J. Nutr. 2010, 103, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Oseni, T.O.; Dlamini, S.O.; Earnshaw, D.M.; Masarirambi, M.T. Effect of substrate pre-treatment methods on oyster mushroom (Pleurotus ostreatus) production. Int. J. Agric. Biol. 2012, 14, 251–255. [Google Scholar]
- Zadrazil, F. Changes in in vitro digestibility of wheat straw during fungal growth and after harvest of oyster mushrooms (Pleurotus spp.) on laboratory and industrial scale. J. Appl. Animal Res. 1997, 11, 37–48. [Google Scholar] [CrossRef]
- Shamshad, A. The development of integrated pest management for the control of mushroom sciarid flies, Lycoriella ingenua (Dufour) and Bradysia ocellaris (Comstock), in cultivated mushrooms. Pest Manag. Sci. 2010, 66, 1063–1074. [Google Scholar] [CrossRef]
- Erler, F.; Polat, E.; Demir, H.; Catal, M.; Tuna, G. Control of mushroom sciarid fly Lycoriella ingenua populations with insect growth regulators applied by soil drench. J. Econ. Entomol. 2011, 104, 839–844. [Google Scholar] [CrossRef]
- Keil, C.B. Field and laboratory evaluation of a Bacillus thuringiensis var. israelensis formulation for control of fly pests of mushrooms. J. Econ. Èntomol. 1991, 84, 1180–1188. [Google Scholar] [CrossRef] [Green Version]
- Nagy, A.; Manczinger, L.; Tombácz, D.; Hatvani, L.; Gyõrfi, J.; Antal, Z.; Sajben, E.; Vágvõllgyi, C.; Kredics, L. Biological control of oyster mushroom green mould disease by antagonistic Bacillus species. Biol. Control Fungal Bact. Plant Pathog. 2012, 78, 289–293. [Google Scholar]
- Mazin, M.; Harvey, R.; Andreadis, S.; Pecchia, J.; Cloonan, K.; Rajotte, E.G. Mushroom sciarid fly, Lycoriella ingenua (Diptera: Sciaridae) adults and larvae vector mushroom green mold (Trichoderma aggressivum ft. aggressivum) spores. Appl. Èntomol. Zoöl. 2019, 54, 369–376. [Google Scholar] [CrossRef]
- Coles, P.S.; Mazin, M.; Nogin, G. The association between mushroom sciarid flies, cultural techniques, and green mold disease incidence on commercial mushroom farms. J. Econ. Entomol. 2021, 114, 555–559. [Google Scholar] [CrossRef]
- White, P.F. Effects of bendiocarb and diflubenzuron on mushroom cropping. Ann. Appl. Biol. 1986, 108, 11–20. [Google Scholar] [CrossRef]
- Melo, A.L.D.A.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2014, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, M.; Boisvert, J. Effects of Bacillus thuringiensis var. israelensis on target and nontarget organisms: A review of laboratory and field experiments. Biocontrol Sci. Technol. 2000, 10, 517–561. [Google Scholar] [CrossRef]
- Erler, F.; Polat, E.; Demir, H.; Cetin, H.; Erdemir, T. Evaluation of microbial products for the control of the mushroom phorid fly, Megaselia halterata (Wood). J. Èntomol. Sci. 2009, 44, 89–97. [Google Scholar] [CrossRef]
- Jess, S.; Schweizer, H. Biological control of Lycoriella ingenua (Diptera: Sciaridae) in commercial mushroom (Agaricus bisporus) cultivation: A comparison between Hypoaspis miles and Steinernema feltiae. Pest Manag. Sci. 2009, 65, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.K.; Gaugler, R. Entomopathogenic nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Grewal, P.; Georgis, R. Entomopathogenic nematodes. In Biopesticides: Use and Delivery; Hall, F.R., Menn, J.J., Eds.; Humana Press: Totowa, NJ, USA, 1999; Volume 5. [Google Scholar]
- Scheepmaker, J.W.A.; Geels, F.P.; Smits, P.H.; Van Griensven, L.J.L.D. Control of the mushroom pests Lycoriella auripila (Diptera: Sciaridae) and Megaselia halterata (Diptera: Phoridae) by Steinernema feltiae (Nematoda: Steinernematidae) in field experiments. Ann. Appl. Biol. 1997, 131, 359–368. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Geels, F.P.; Rutjens, A.J.; Smits, P.H.; Van Griensven, L.J.L.D. Comparison of the efficacy of entomopathogenic nematodes for the biological control of the mushroom pests Lycoriella auripila (Sciaridae) and Megaselia halterata (Phoridae). Biocontrol Sci. Technol. 1998, 8, 277–287. [Google Scholar] [CrossRef]
- Jess, S.; Kilpatrick, M. An integrated approach to the control of Lycoriella solani (Diptera: Sciaridae) during production of the cultivated mushroom (Agaricus bisporus). Pest Manag. Sci. 2000, 56, 477–485. [Google Scholar] [CrossRef]
- Navarro, M.J.; Gea, F.J. Entomopathogenic nematodes for the control of phorid and sciarid flies in mush-room crops. Pesqui. Agropecuária Bras. 2014, 49, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.-H.; Park, I.-K.; Choi, K.-S.; Shin, S.-C.; Ahn, Y.-J. Toxicity of medicinal plant extracts to Lycoriella ingenua (Diptera: Sciaridae) and Coboldia fuscipes (Diptera: Scatopsidae). J. Asia-Pac. Èntomol. 2008, 11, 221–223. [Google Scholar] [CrossRef]
- Inam-ul-Haq, M.; Khan, N.A.; Khan, M.A.; Khan, M.A.; Javed, N.; Binyamin, R.; Irshad, G. Use of medicinal plants in different composts for yield improvement of various strains of oyster mushroom. Pakistan J. Bot. 2010, 42, 3275–3283. [Google Scholar]
- Kwok, O.C.H.; Plattner, R.; Weisleder, D.; Wicklow, D.T. A nematicidal toxin from Pleurotus ostreatus NRRL 3526. J. Chem. Ecol. 1992, 18, 127–136. [Google Scholar] [CrossRef]
- Raymond, B.; Federici, B.A. In defence of Bacillus thuringiensis, the safest and most successful microbial insecticide available to humanity—A response to EFSA. FEMS Microbiol. Ecol. 2017, 93, fix084. [Google Scholar] [CrossRef] [Green Version]
- Richardson, P.N. Susceptibility of mushroom pests to the insect-parasitic nematodes Steinernema feltiae and Heterorhabditis heliothidis. Ann. Appl. Biol. 1987, 111, 433–438. [Google Scholar] [CrossRef]
- Grewal, P.S.; Richardson, P.N.; Collins, G.; Edmondson, R.N. Comparative effects of Steinernema feltiae (Nematoda: Steinernematidae) and insecticides on yield and cropping of the mushroom Agaricus bisporus. Ann. Appl. Biol. 1992, 121, 511–520. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Geels, F.P.; Smits, P.H.; Van Griensven, L.J.L.D. Influence of Steinernema feltiae and diflubenzuron on yield and economics of the cultivated mushroom Agaricus bisporus in Dutch mushroom culture. Biocontrol Sci. Technol. 1998, 8, 269–275. [Google Scholar] [CrossRef]
- Thorn, R.G.; Barron, G.L. Carnivorous mushrooms. Science 1984, 224, 76–78. [Google Scholar] [CrossRef] [Green Version]
- Barron, G.L.; Thorn, R.G. Destruction of nematodes by species of Pleurotus. Can. J. Bot. 1987, 65, 774–778. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Thorn, R.G. Nematode-trapping in Pleurotus tuberregium. Mycologia 1994, 86, 696–699. [Google Scholar] [CrossRef]
- Pineda-Alegría, J.A.; Sánchez-Vázquez, J.E.; González-Cortazar, M.; Zamilpa, A.; López-Arellano, M.E.; Cue-vas-Padilla, E.J.; Mendoza-de-Gives, P.; Aguilar-Marcelino, L. The edible mushroom Pleurotus djamor produces metabolites with lethal activity against the parasitic nematode Haemonchus contortus. J. Med. Food 2017, 20, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Marlin, M.; Wolf, A.; Alomran, M.; Carta, L.; Newcombe, G. Nematophagous Pleurotus species consume some nematode species but are themselves consumed by others. Forests 2019, 10, 404. [Google Scholar] [CrossRef] [Green Version]
- Panevska, A.; Hodnik, V.; Skočaj, M.; Novak, M.; Modic, Š.; Pavlic, I.; Podržaj, S.; Zarić, M.; Resnik, N.; Maček, P.; et al. Pore-forming protein complexes from Pleurotus mushrooms kill western corn rootworm and Colorado potato beetle through targeting membrane ceramide phosphoethanolamine. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panevska, A.; Skočaj, M.; Modic, Š.; Razinger, J.; Sepčić, K. Aegerolysins from the fungal genus Pleurotus—Bioinsecticidal proteins with multiple potential applications. J. Invertebr. Pathol. 2020, 107474. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, V.M.; Sward, G.F.H.; Ranger, C.M.; Reding, M.E.; Canas, L. Microbial Control Agents for Fungus Gnats (Diptera: Sciaridae: Lycoriella) Affecting the Production of Oyster Mushrooms, Pleurotus spp. Insects 2021, 12, 786. https://doi.org/10.3390/insects12090786
Anderson VM, Sward GFH, Ranger CM, Reding ME, Canas L. Microbial Control Agents for Fungus Gnats (Diptera: Sciaridae: Lycoriella) Affecting the Production of Oyster Mushrooms, Pleurotus spp. Insects. 2021; 12(9):786. https://doi.org/10.3390/insects12090786
Chicago/Turabian StyleAnderson, Valerie M., Grace F. H. Sward, Christopher M. Ranger, Michael E. Reding, and Luis Canas. 2021. "Microbial Control Agents for Fungus Gnats (Diptera: Sciaridae: Lycoriella) Affecting the Production of Oyster Mushrooms, Pleurotus spp." Insects 12, no. 9: 786. https://doi.org/10.3390/insects12090786
APA StyleAnderson, V. M., Sward, G. F. H., Ranger, C. M., Reding, M. E., & Canas, L. (2021). Microbial Control Agents for Fungus Gnats (Diptera: Sciaridae: Lycoriella) Affecting the Production of Oyster Mushrooms, Pleurotus spp. Insects, 12(9), 786. https://doi.org/10.3390/insects12090786







