The Influence of the pH and Salinity of Water in Breeding Sites on the Occurrence and Community Composition of Immature Mosquitoes in the Green Belt of the City of São Paulo, Brazil
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Mosquito Collections and Species Identification
2.3. Physicochemical Parameters of the Water
2.4. Data Analysis
3. Results
3.1. Immature Mosquito Species
3.2. Physicochemical Parameters
3.3. Analysis by Parameter
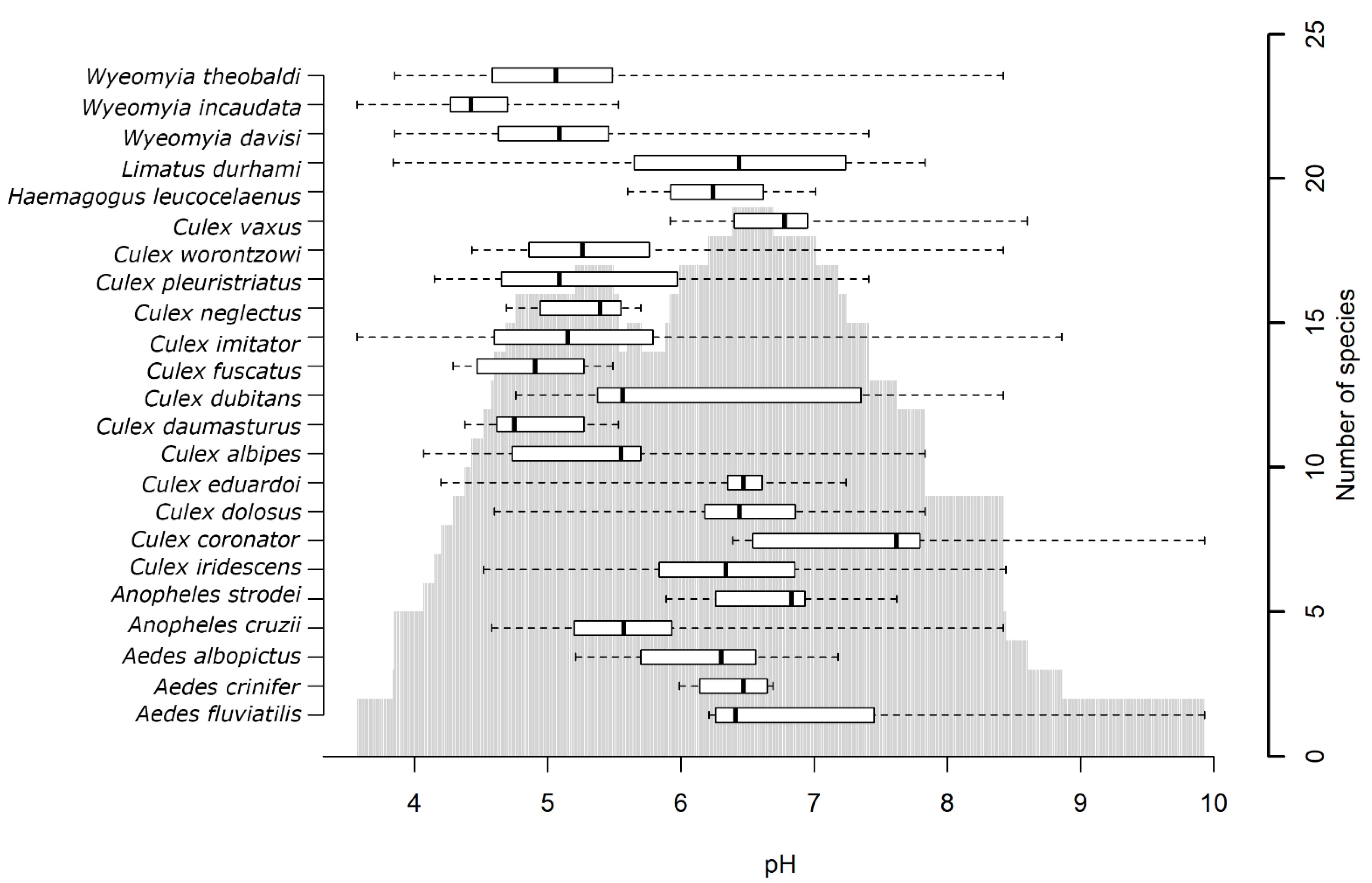
3.4. Analysis by Mosquito Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franklinos, L.H.V.; Jones, K.E.; Redding, D.W.; Abubakar, I. The effect of global change on mosquito-borne disease. Lancet Infect. Dis. 2019, 19, e302–e312. [Google Scholar] [CrossRef]
- Consoli, R.A.G.B.; Lourenço-de-Oliveira, R. Principais Mosquitos de Importância Sanitária no Brasil; Editora FIOCRUZ: Rio de Janeiro, Brazil, 1994; Volume 11, ISBN 8585676035. [Google Scholar]
- Wilke, A.B.B.; Chase, C.; Vasquez, C.; Carvajal, A.; Medina, J.; Petrie, W.D.; Beier, J.C. Urbanization creates diverse aquatic habitats for immature mosquitoes in urban areas. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Forattini, O.P. Culicidologia Médica; EDUSP, Ed.; Editora da universidade de São Paulo: São Paulo, Brazil, 2002; Volume 2, ISBN 85-314-0699-4. [Google Scholar]
- Chase, J.M.; Knight, T.M. Drought-induced mosquito outbreaks in wetlands. Ecol. Lett. 2003, 6, 1017–1024. [Google Scholar] [CrossRef]
- Nikookar, S.H.; Fazeli-Dinan, M.; Azari-Hamidian, S.; Mousavinasab, S.N.; Aarabi, M.; Ziapour, S.P.; Esfandyari, Y.; Enayati, A. Correlation between mosquito larval density and their habitat physicochemical characteristics in Mazandaran Province, northern Iran. PLoS Negl. Trop. Dis. 2017, 11, e0005835. [Google Scholar] [CrossRef]
- Yee, D.A.; Kneitel, J.M.; Juliano, S.A. Environmental Correlates of Abundances of Mosquito Species and Stages in Discarded Vehicle Tires. J. Med. Entomol. 2010, 47, 53–62. [Google Scholar] [CrossRef]
- Dickson, L.B.; Jiolle, D.; Minard, G.; Moltini-Conclois, I.; Volant, S.; Ghozlane, A.; Bouchier, C.; Ayala, D.; Paupy, C.; Moro, C.V.; et al. Carryover effects of larval exposure to different environmental bacteria drive adult trait variation in a mosquito vector. Sci. Adv. 2017, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.M.; Flis, B.J.; Remold, S.K. Differences in the effects of salinity on larval growth and developmental programs of a freshwater and a euryhaline mosquito species (Insecta: Diptera, Culicidae). J. Exp. Biol. 2004, 207, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Ukubuiwe, A.C.; Ojianwuna, C.C.; Olayemi, I.K.; Arimoro, F.O.; Ukubuiwe, C.C. Quantifying the roles of water pH and hardness levels in development and biological fitness indices of Culex quinquefasciatus Say (Diptera: Culicidae). J. Basic Appl. Zool. 2020, 81. [Google Scholar] [CrossRef]
- Emidi, B.; Kisinza, W.N.; Mmbando, B.P.; Malima, R.; Mosha, F.W. Effect of physicochemical parameters on Anopheles and Culex mosquito larvae abundance in different breeding sites in a rural setting of Muheza, Tanzania. Parasites Vectors 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Sousa, A.R.; de Oliveira-Christe, R.; Camargo, A.A.; Scinachi, C.A.; Milani, G.M.; Urbinatti, P.R.; Natal, D.; Ceretti-Junior, W.; Marrelli, M.T. Influence of water’s physical and chemical parameters on mosquito (Diptera: Culicidae) assemblages in larval habitats in urban parks of São Paulo, Brazil. Acta Trop. 2020, 205, 105394. [Google Scholar] [CrossRef]
- Torreias, S.R.d.S.; Ferreira-Keppler, R.L.; Godoy, B.S.; Hamada, N. Mosquitoes (Diptera, Culicidae) inhabiting foliar tanks of Guzmania brasiliensis Ule (Bromeliaceae) in central Amazonia, Brazil. Rev. Bras. Entomol. 2010, 54, 618–623. [Google Scholar] [CrossRef]
- Williams, D.D. Environmental Constraints in Temporary Fresh Waters and Their Consequences for the Insect Fauna. J. N. Am. Benthol. Soc. 1996, 15, 634–650. [Google Scholar] [CrossRef]
- Muturi, E.J.; Mwangangi, J.; Shililu, J.; Muriu, S.; Jacob, B.; Kabiru, E.; Gu, W.; Mbogo, C.; Githure, J.; Novak, R. Mosquito species succession and physicochemical factors affecting their abundance in rice fields in Mwea, Kenya. J. Med. Entomol. 2007, 44, 336–344. [Google Scholar] [CrossRef][Green Version]
- Clark, T.M.; Vieira, M.A.L.; Huegel, K.L.; Flury, D.; Carper, M. Strategies for regulation of hemolymph pH in acidic and alkaline water by the larval mosquito Aedes aegypti (L.) (Diptera; Culicidae). J. Exp. Biol. 2007, 210, 4359–4367. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.M.; Flis, B.J.; Remold, S.K. pH tolerances and regulatory abilities of freshwater and euryhaline Aedine mosquito larvae. J. Exp. Biol. 2004, 207, 2297–2304. [Google Scholar] [CrossRef]
- Donini, A.; Gaidhu, M.P.; Strasberg, D.R.; O’Donnell, M.J. Changing salinity induces alterations in hemolymph ion concentrations and Na+ and Cl- transport kinetics of the anal papillae in the larval mosquito, Aedes aegypti. J. Exp. Biol. 2007, 210, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Arduino, M.d.B.; Marques, G.R.d.A.M.; Serpa, L.L.N. Registro de larvas e pupas de Aedes aegypti e Aedes albopictus em recipientes com água salina em condições naturais. BEPA Bol. Epidemiol. Paul. 2010, 7, 22–28. [Google Scholar]
- Rozeboom, L.E.; Komp, W.H.W. A Review of the Species of Culex of the Subgenus Melanoconion (Diptera: Culicidae)1. Ann. Entomol. Soc. Am. 1950, 43, 75–114. [Google Scholar] [CrossRef]
- Lane, J. Neotropical Culicidae; Editora da Universidade de São Paulo: São Paulo, Brazil, 1953; Volume 1. [Google Scholar]
- Galindo, P.; Blanton, F.S.; Peyton, E.L. A Revision of the Uranotaenia of Panama with Notes on Other American Species of the Genus (Diptera: Culicidae)1. Ann. Entomol. Soc. Am. 1954, 47, 107–177. [Google Scholar] [CrossRef]
- Bram, R.A. Classification of Culex subgenus Culex in the new world (Diptera: Culicidae). Proc. Natl. Mus. 1967, 120, 1–120. [Google Scholar] [CrossRef]
- Corrêa, R.R.; Ramalho, G.R. Revision of the Genus Phoniomyia. Folia Clin. Biol. 1956, 25, 1–176. [Google Scholar]
- Sorensen, T.J. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. K. Dan. Vidensk. Selsk. 1948, 4, 3–48. [Google Scholar]
- Legendre, P.; Oksanen, J.; ter Braak, C.J.F. Testing the significance of canonical axes in redundancy analysis. Methods Ecol. Evol. 2011, 2, 269–277. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan community ecology package: Ordination methods, diversity analysis and other functions for community and vegetation ecologists. R Package Ver 2.2-0. 2017. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 5 September 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 5 September 2021).
- Gopalakrishnan, R.; Das, M.; Baruah, I.; Veer, V.; Dutta, P. Physicochemical characteristics of habitats in relation to the density of container-breeding mosquitoes in Asom, India. J. Vector Borne Dis. 2013, 50, 215–219. [Google Scholar]
- Yap, H.H.; Lee, C.Y.; Chong, N.L.; Foo, A.E.; Lim, M.P. Oviposition site preference of Aedes albopictus in the laboratory. J. Am. Mosq. Control. Assoc. 1995, 11, 128–132. [Google Scholar] [PubMed]
- Kengne, P.; Charmantier, G.; Blondeau-Bidet, E.; Costantini, C.; Ayala, D. Tolerance of disease-vector mosquitoes to brackish water and their osmoregulatory ability. Ecosphere 2019, 10. [Google Scholar] [CrossRef]
- Bradley, T.J. Saline-water Insects: Ecology, Physiology and Evolution. In Aquatic Insects: Challenges to Populations; Institute of Evolutionary Biology: Edinburgh, UK, 2008; ISBN 978-1-84593-396-8. [Google Scholar]
- Patrick, M.L.; Bradley, T.J. Regulation of compatible solute accumulation in larvae of the mosquito Culex tarsalis: Osmolarity versus salinity. J. Exp. Biol. 2000, 203, 831–839. [Google Scholar] [CrossRef]
- de Brito, M.; Paulo Forattini, O. Productivity of Aedes albopictus’ breeding containers in Paraíba Valley, Brazil. Rev. Saude Publica 2004, 38, 209–215. [Google Scholar] [CrossRef]
- Ceretti-junior, W.; Christe, R.D.O.; Rizzo, M.; Strobel, C.; Otavio, M.; Junior, D.M.; Helena, M.; Homem, S.; Fernandes, A.; Medeiros-sousa, A.R.; et al. Species Composition and Ecological Aspects of Immature Mosquitoes (Diptera: Culicidae) in Bromeliads in Urban Parks in the City of São. J. Arthropod-Borne Dis. 2016, 10, 102–112. [Google Scholar] [PubMed]
- Marques, G.R.A.M.; Forattini, O.P. Aedes albopictus em bromélias de solo em Ilhabela, litoral do Estado de São Paulo. Rev. Saude Publica 2005, 39, 548–552. [Google Scholar] [CrossRef][Green Version]
- Müller, G.A.; Marcondes, C.B. Bromeliad-associated mosquitoes from Atlantic forest in Santa Catarina Island, southern Brazil (Diptera, Culicidae), with new records for the State of Santa Catarina. Iheringia. Série Zool. 2006, 96, 315–319. [Google Scholar] [CrossRef]
- Cardoso, C.A.A.; Lourenço-De-Oliveira, R.; Codeço, C.T.; Motta, M.A. Mosquitoes in Bromeliads at Ground Level of the Brazilian Atlantic Forest: The Relationship between Mosquito Fauna, Water Volume, and Plant Type. Ann. Entomol. Soc. Am. 2015, 108, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.R.A.M.; Forattini, O.P. Culicídeos em bromélias: Diversidade de fauna segundo influência antrópica, litoral de São Paulo. Rev. Saude Publica 2008, 42, 979–985. [Google Scholar] [CrossRef]
- Wilke, A.B.B.; Vasquez, C.; Carvajal, A.; Medina, J.; Chase, C.; Cardenas, G.; Mutebi, J.P.; Petrie, W.D.; Beier, J.C. Proliferation of Aedes aegypti in urban environments mediated by the availability of key aquatic habitats. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Simões, R.F.; Wilke, A.B.B.; Chagas, C.R.F.; Menezes, R.M.T.d.; Suesdek, L.; Multini, L.C.; Silva, F.S.; Grech, M.G.; Marrelli, M.T.; Kirchgatter, K. Wing Geometric Morphometrics as a Tool for the Identification of Culex Subgenus Mosquitoes of Culex (Diptera: Culicidae). Insects 2020, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Demari-Silva, B.; Multini, L.C.; Suesdek, L.; Oliveira, T.M.P.; Sallum, M.A.M.; Marrelli, M.T. Wing morphometry and genetic variability between Culex coronator and Culex usquatus (Diptera: Culicidae), two sibling species of the coronator group. J. Med. Entomol. 2017, 54. [Google Scholar] [CrossRef] [PubMed]
- Multini, L.C.; de Souza, A.L.d.S.; Marrelli, M.T.; Wilke, A.B.B. The influence of anthropogenic habitat fragmentation on the genetic structure and diversity of the malaria vector Anopheles cruzii (Diptera: Culicidae). Sci. Rep. 2020, 10, 18018. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.R.; Santos, R.L.; Forattini, O.P. Aedes albopictus in bromeliads of anthropic environment in São Paulo State, Brazil. Rev. Saude Publica 2001, 35, 243–248. [Google Scholar] [CrossRef][Green Version]
- Lambrechts, L.; Scott, T.W.; Gubler, D.J. Consequences of the Expanding Global Distribution of Aedes albopictus for Dengue Virus Transmission. PLoS Negl. Trop. Dis. 2010, 4, e646. [Google Scholar] [CrossRef]
- Multini, L.C.; da Silva de Souza, A.L.; Marrelli, M.T.; Wilke, A.B.B. Population structuring of the invasive mosquito Aedes albopictus (Diptera: Culicidae) on a microgeographic scale. PLoS ONE 2019, 14, e0220773. [Google Scholar] [CrossRef]
- Smartt, C.T.; Stenn, T.M.S.; Chen, T.Y.; Teixeira, M.G.; Queiroz, E.P.; Souza Dos Santos, L.; Queiroz, G.A.N.; Ribeiro Souza, K.; Kalabric Silva, L.; Shin, D.; et al. Evidence of zika virus RNA fragments in Aedes albopictus (Diptera: Culicidae) field-collected eggs from Camaçari, Bahia, Brazil. J. Med. Entomol. 2017, 54, 1085–1087. [Google Scholar] [CrossRef]
- Amraoui, F.; Pain, A.; Piorkowski, G.; Vazeille, M.; Couto-Lima, D.; de Lamballerie, X.; Lourenço-de-Oliveira, R.; Failloux, A.B. Experimental Adaptation of the Yellow Fever Virus to the Mosquito Aedes albopictus and Potential risk of urban epidemics in Brazil, South America. Sci. Rep. 2018, 8, 4–11. [Google Scholar] [CrossRef]
- Pagès, F.; Peyrefitte, C.N.; Mve, M.T.; Jarjaval, F.; Brisse, S.; Iteman, I.; Gravier, P.; Nkoghe, D.; Grandadam, M. Aedes albopictus Mosquito: The Main Vector of the 2007 Chikungunya Outbreak in Gabon. PLoS ONE 2009, 4, e4691. [Google Scholar] [CrossRef]
- Benelli, G.; Wilke, A.B.B.; Beier, J.C. Aedes albopictus (Asian Tiger Mosquito). Trends Parasitol. 2020, 36, 942–943. [Google Scholar] [CrossRef]
- Camargo-Neves, V.L.F.d.; Poletto, D.W.; Rodas, L.A.C.; Pachioli, M.L.; Cardoso, R.P.; Scandar, S.A.S.; Sampaio, S.M.P.; Koyanagui, P.H.; Botti, M.V.; Mucci, L.F.; et al. Entomological investigation of a sylvatic yellow fever area in São Paulo State, Brazil. Cad. Saude Publica 2005, 21, 1278–1286. [Google Scholar] [CrossRef]
- de Abreu, F.V.S.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Santos, A.A.C.d.; de Miranda, R.M.; Bonelly, I.d.S.; Neves, M.S.A.S.; Bersot, M.I.; Santos, T.P.d.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef]
- Hendy, A.; Hernandez-Acosta, E.; Valério, D.; Mendonça, C.; Costa, E.R.; Júnior, J.T.A.; Assunção, F.P.; Scarpassa, V.M.; Gordo, M.; Fé, N.F.; et al. The vertical stratification of potential bridge vectors of mosquito-borne viruses in a central Amazonian forest bordering Manaus, Brazil. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Montagner, F.R.G.; Silva, O.S.; Jahnke, S.M. Mosquito species occurrence in association with landscape composition in green urban areas. Braz. J. Biol. 2018, 78, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Rezende, H.R.; Virgens, T.M.d.; Liberato, M.A.; Valente, F.I.; Fernandes, A.; Urbinatti, P.R. Aspectos ecológicos de culicídeos imaturos em larvitrampas de floresta e ambiente antrópico adjacente no Municipio de Linhares, Espírito Santo, Brasil. Epidemiol. E Serviç. Saúde 2011, 20, 385–391. [Google Scholar] [CrossRef]
- Wilk-da-Silva, R.; Mucci, L.F.; Ceretti-Junior, W.; Duarte, A.M.R.d.C.; Marrelli, M.T.; Medeiros-Sousa, A.R. Influence of landscape composition and configuration on the richness and abundance of potential sylvatic yellow fever vectors in a remnant of Atlantic Forest in the city of São Paulo, Brazil. Acta Trop. 2020, 204, 105385. [Google Scholar] [CrossRef] [PubMed]
- Multini, L.C.; Marrelli, M.T.; Beier, J.C.; Wilke, A.B.B. Increasing Complexity Threatens the Elimination of Extra-Amazonian Malaria in Brazil. Trends Parasitol. 2019, 35, 1–4. [Google Scholar] [CrossRef]
- Medeiros-Sousa, A.R.; de Oliveira Christe, R.; de Castro Duarte, A.M.R.; Mucci, L.F.; Ceretti-Junior, W.; Marrelli, M.T. Effects of anthropogenic landscape changes on the abundance and acrodendrophily of Anopheles (Kerteszia) cruzii, the main vector of malaria parasites in the Atlantic Forest in Brazil. Malar. J. 2019, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.M.R.; Pereira, D.M.; de Paula, M.B.; Fernandes, A.; Urbinatti, P.R.; Ribeiro, A.F.; Mello, M.H.S.; Matos, M.O.; Mucci, L.F.; Fernandes, L.N.; et al. Natural infection in anopheline species and its implications for autochthonous malaria in the Atlantic forest in Brazil. Parasit. Vectors 2013, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Ceretti-Junior, W.; Oliveira-Christe, R.; Wilk-da-Silva, R.; Mucci, L.F.; Duarte, A.M.R.d.C.; Fernandes, A.; Barrio-Nuevo, K.M.; Carvalho, M.P.; Marrelli, M.T.; Medeiros-Sousa, A.R. Diversity analysis and an updated list of mosquitoes (Diptera: Culicidae) found in Cantareira State Park, São Paulo, Brazil. Acta Trop. 2020, 212, 105669. [Google Scholar] [CrossRef]
- Deane, L.M.; Ferreira Neto, J.a.; Lima, M.M. The vertical dispersión of Anopheles (Kerteszia) cruzii in a forest in southern Brazil suggests that human cases of malaria of simian origin might be expected. Mem. Inst. Oswaldo Cruz 1984, 79, 461–463. [Google Scholar] [CrossRef] [PubMed]


| Location | Collection Site | Geographical Coordinates |
|---|---|---|
| PEC | PEC-1 | 23°24′37.44” S 46°37′12.30” W |
| PEC-2 | 23°26′51.90” S 46°38′5.46” W | |
| PEC-3 | 23°27′13.62” S 46°38′9.70” W | |
| APA | APA-4 | 23°56′22.68” S 46°41′39.54” W |
| APA-5 | 23°54′37.60” S 46°42′7.69” W | |
| APA-6 | 23°54′23.71” S 46°42′29.15” W | |
| APA-7 | 23°53′8.90” S 46°44′29.39” W |
| Statistical Analysis | |||||
|---|---|---|---|---|---|
| CAP | Total inertia | Explained | Not explained | p | |
| 101.05 | 33.08 | 67.97 | 0.001 | ||
| CCA | Total inertia | Explained | Not explained | p | |
| 12.45 | 0.47 | 11.98 | 0.001 | ||
| Partial CCA-excluding the effect of “type of aquatic habitat” | Total inertia | Conditional | Explained | Not explained | p |
| 12.45 | 3.00 | 0.13 | 9.32 | 0.07 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Multini, L.C.; Oliveira-Christe, R.; Medeiros-Sousa, A.R.; Evangelista, E.; Barrio-Nuevo, K.M.; Mucci, L.F.; Ceretti-Junior, W.; Camargo, A.A.; Wilke, A.B.B.; Marrelli, M.T. The Influence of the pH and Salinity of Water in Breeding Sites on the Occurrence and Community Composition of Immature Mosquitoes in the Green Belt of the City of São Paulo, Brazil. Insects 2021, 12, 797. https://doi.org/10.3390/insects12090797
Multini LC, Oliveira-Christe R, Medeiros-Sousa AR, Evangelista E, Barrio-Nuevo KM, Mucci LF, Ceretti-Junior W, Camargo AA, Wilke ABB, Marrelli MT. The Influence of the pH and Salinity of Water in Breeding Sites on the Occurrence and Community Composition of Immature Mosquitoes in the Green Belt of the City of São Paulo, Brazil. Insects. 2021; 12(9):797. https://doi.org/10.3390/insects12090797
Chicago/Turabian StyleMultini, Laura Cristina, Rafael Oliveira-Christe, Antônio Ralph Medeiros-Sousa, Eduardo Evangelista, Karolina Morales Barrio-Nuevo, Luis Filipe Mucci, Walter Ceretti-Junior, Amanda Alves Camargo, André Barretto Bruno Wilke, and Mauro Toledo Marrelli. 2021. "The Influence of the pH and Salinity of Water in Breeding Sites on the Occurrence and Community Composition of Immature Mosquitoes in the Green Belt of the City of São Paulo, Brazil" Insects 12, no. 9: 797. https://doi.org/10.3390/insects12090797
APA StyleMultini, L. C., Oliveira-Christe, R., Medeiros-Sousa, A. R., Evangelista, E., Barrio-Nuevo, K. M., Mucci, L. F., Ceretti-Junior, W., Camargo, A. A., Wilke, A. B. B., & Marrelli, M. T. (2021). The Influence of the pH and Salinity of Water in Breeding Sites on the Occurrence and Community Composition of Immature Mosquitoes in the Green Belt of the City of São Paulo, Brazil. Insects, 12(9), 797. https://doi.org/10.3390/insects12090797








