Polyandrous Mexican Fruit Flies: Second Male Paternity and Biological Attributes of Transgenic Strains
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Attributes
2.2. Male Sexual Competition
2.3. Paternity
2.4. Induction of Sterility by Irradiated Males
2.5. Statistical Analysis
3. Results
3.1. Biological Attributes
3.2. Male Sexual Competitiveness
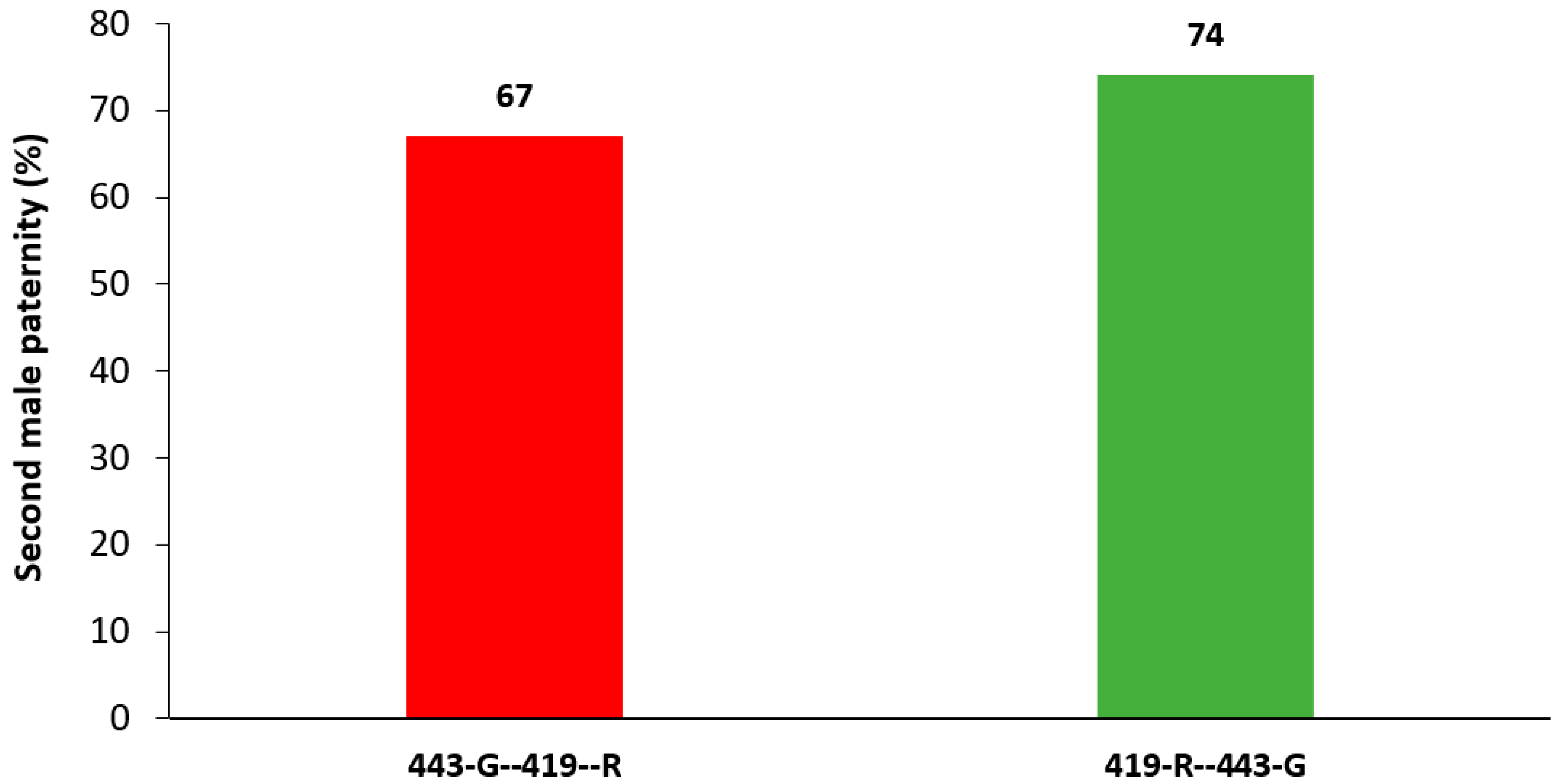
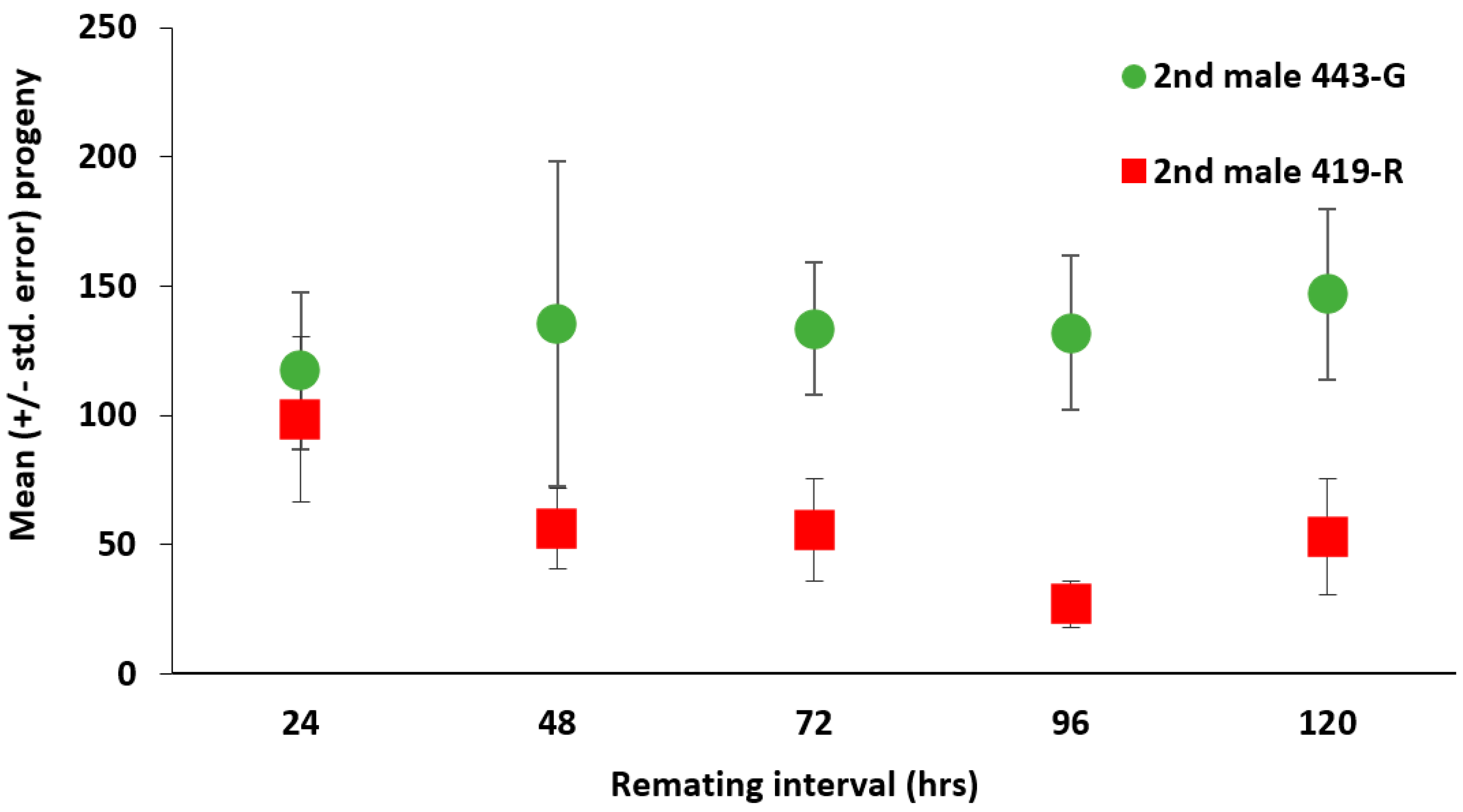
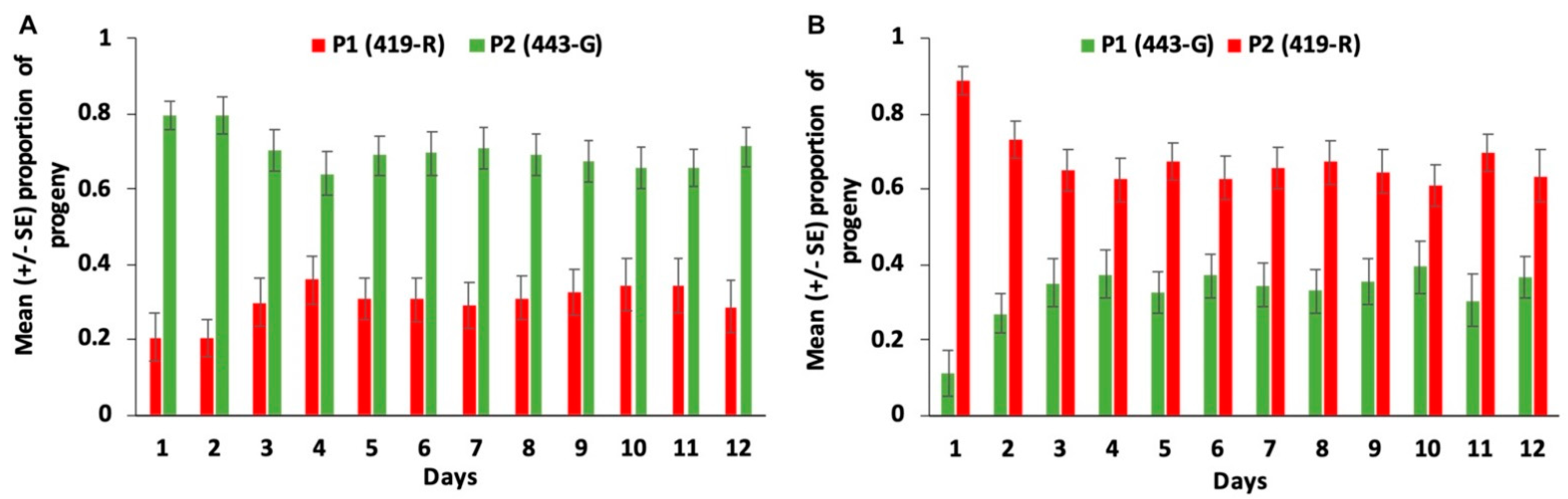
3.3. Second Male Paternity
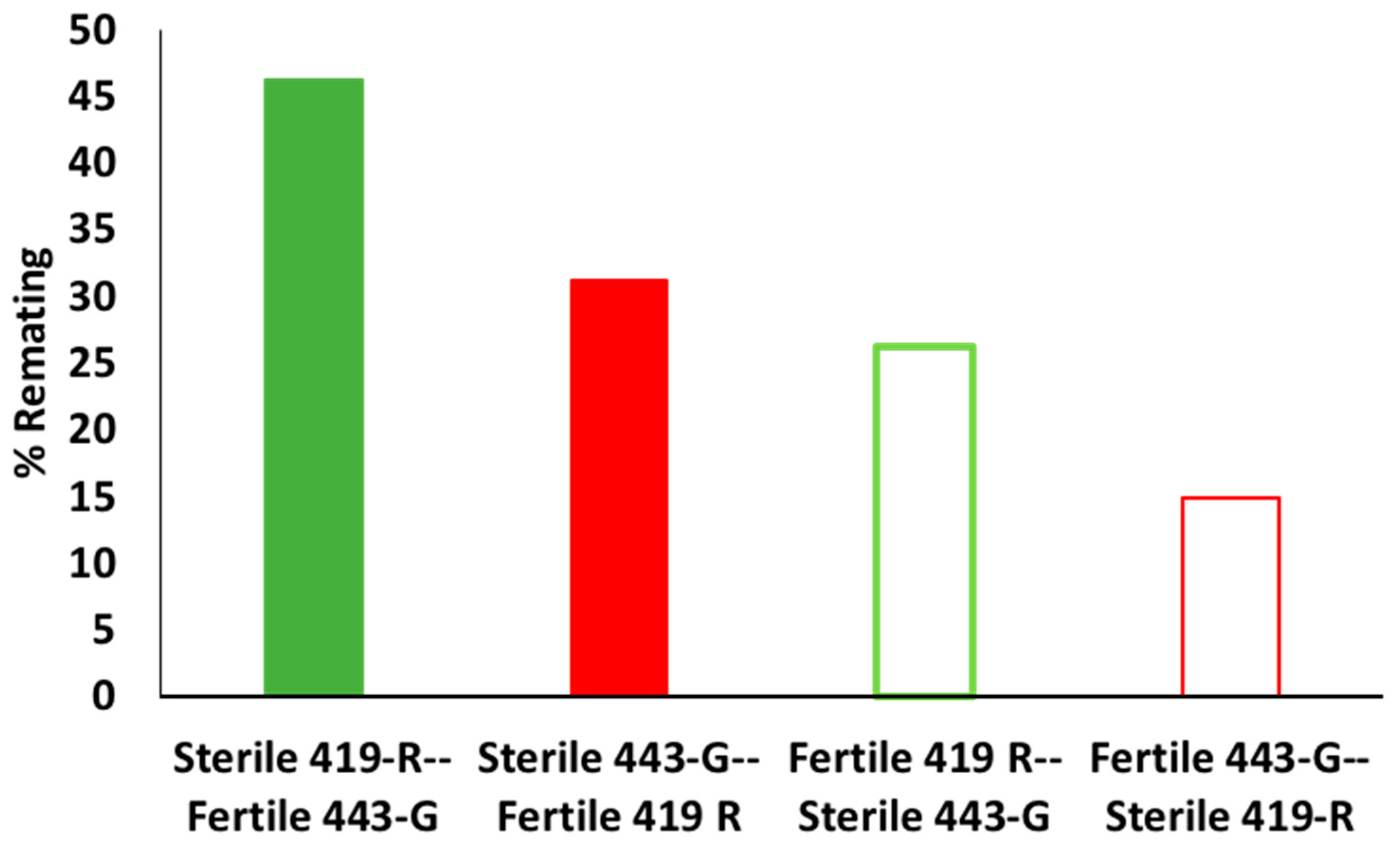
3.4. Induction of Sterility by Irradiated Males
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orozco, D.; Domínguez, J.; Reyes, J.; Villaseñor, A.; Gutiérrez, J. SIT and biological control of Anastrepha fruit flies in Mexico. In Proceedings of the 6th International Fruit Fly Symposium, Stellenbosch, South Africa, 6–10 May 2002; pp. 245–249. [Google Scholar]
- Orozco-Dávila, D.; Quintero, L.; Hernández, E.; Solís, E.; Artiaga, T.; Hernández, R.; Ortega, C.; Montoya, P. Mass rearing and sterile insect releases for the control of Anastrepha spp. pests in Mexico—A review. Entomol. Exp. Appl. 2017, 164, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Knipling, E.F. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 1955, 48, 459–462. [Google Scholar] [CrossRef]
- Krafsur, E.S. Sterile insect technique for suppressing and eradicating insect population: 55 years and counting. J. Agric. Entomol. 1998, 15, 303–317. [Google Scholar]
- Robinson, A. Genetic Basis of the Sterile Insect Technique: Dominant Letal Mutations. In Sterile Insect Technique Principles and Practice in Area-Wide Integrated Pest Managament, 2nd ed.; Dyck, V., Hendrichs, J., Robison, A., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 146–150. [Google Scholar]
- SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). Manual Técnico Para las Operaciones de Campo de la Campaña Nacional Contra Moscas de la Fruta; Sección V Control Autocida; SAGARPA-SENASICA: Mexico City, Mexico, 2012; pp. 6–31. [Google Scholar]
- Hagler, J.; Jackson, C. Methods for marking insects: Current techniques and future prospects. Annu. Rev. Entomol 2001, 46, 511–543. [Google Scholar] [CrossRef] [Green Version]
- Dominiak, B.; Schinagl, L.; Nicol, H. Impact of fluorescent marker dyes on emergence of sterile Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). J. Appl. Entomol. 2000, 29, 45–47. [Google Scholar]
- Weldon, C. Marking Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) with fluorescent pigments: Pupal emergence, adult mortality, and visibility and persistence of marks. J. Appl. Entomol. 2005, 34, 7–13. [Google Scholar]
- Scheteling, M.F.; Targovska, A.; Meza, J.S.; Bourtzis, K.; Handelr, A. Tetracycline-suppressible female lethality and sterility in the Mexican fruit fly, Anastrepha ludens. Insect Mol. Biol. 2016, 24, 1–8. [Google Scholar]
- Handler, A.; Harrell, R. Transformation of the Caribbean fruit fly, Anastrepha suspensa, with a piggyBac vector marked with polyubiquitin-regulated GFP. Insect Biochem. Mol. Biol. 2001, 31, 199–205. [Google Scholar] [CrossRef]
- Orozco-Dávila, D.; Hernández, R.; Meza, S.; Domínguez, J. Sexual competitiveness and compatibility between mass-reared sterile flies and wild populations of Anastrepha ludens (Diptera: Tephritidae) from different regions in Mexico. Fla. Entomol. 2007, 90, 19–26. [Google Scholar] [CrossRef]
- Pérez-Staples, D.; Shelly, T.; Yuval, B. Female mating failure and the failure of mating in sterile insect programs. Entomol. Exp. Appl. 2013, 146, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Lance, D.; Mcinnis, D. Biological basis of the sterile insect technique: Biology and sterile insect production. In Sterile Insect Technique Principles and Practice in Area-Wide Integrated Pest Managament, 2nd ed.; Dyck, V., Hendrichs, J., Robison, A., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 124–125. [Google Scholar]
- Aluja, M.; Rull, J.; Sivinski, J.; Trujillo, G.; Pérez-Staples, D. Male and female condition influence mating performance ans sexual receptivity in two tropical fruit flies (Diptera: Tephritidae) with contrasting life histories. J. Insect Physiol. 2009, 55, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Manier, K.; Belote, J.; Berben, K.; Novikov, D.; Stuart, W.; Pitnick, S. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science 2010, 328, 354–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scolari, F.; Yuval, B.; Gomulski, L.M.; Schetelig, M.F.; Gabrieli, P.; Bassetti, F.; Wimmer, E.A.; Malacrida, A.R.; Gasperi, G. Polyandry in the medfly—Shifts in paternity mediated by sperm stratification and maxing. BMC Genet. 2014, 15, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Harari, A.; Sharon, R.; Weintraub, P.G. Manipulation of Insect Reproductive Systems as a Tool in Pest Control. In Advances in Insect Control and Resistance Management; Horowitz, A., Ishaaya, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 93–119. [Google Scholar]
- Meza, J.S.; Nirmala, X.; Zimowska, G.; Zepeda, S.; Handler, A. Development of transgenic strains for the biological control of the Mexican fruit fly, Anastrepha ludens. Genetica 2011, 139, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zavala López, J.; Domínguez, G.; Gómez, Y.; Moreno, P. Mass Rearing of the Mexican Fruit Fly, Anastrepha Ludens, at the Fruit Fly Biofactory in Metapa de Dominguez, Chiapas, Mexico. (IEAE-TECDOC—1064); International Atomic Energy Agency (IAEA): Vienna, Austria, 1999; pp. 43–51. [Google Scholar]
- Carey, J.R.; Liedo, P.; Muller, H.-G.; Wang, J.-L.; Senturk, D.; Harshman, L. Biodemography of a long-lived tephritid: Reproduction and longevity in a large cohort of female Mexican fruit flies, Anastrepha ludens. Exper. Gerontol. 2005, 40, 793–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resilva, S.; Pereira, R. Age- and temperature-related pupal eye colour changes in various tephritid fruit fly species with a view to optimizing irradiation timing. Int. J. Trop. Insect Sci. 2014, 34, 59–65. [Google Scholar] [CrossRef]
- Meza, J.S.; Cáceres, C.; Bourtzis, K. Slow larvae mutant and its potential to improve the pupal color-based genetic sexing system in Mexican fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2019, 112, 1604–1610. [Google Scholar] [CrossRef]
- Orozco, D.; Meza, S.; Zepeda, S.; Solís, E.; Quintero, J. Tapachula-7, a new genetic sexing strain of the Mexican fruit fly (Diptera: Tephritidae): Sexual compatibility and competitiveness. J. Econ. Entomol. 2013, 106, 735–741. [Google Scholar] [CrossRef]
- Morrison, N.I.; Segura, D.F.; Stainton, K.C.; Fu, G.; Donnelly, C.A.; Alphey, L.S. Sexual competitiveness of a transgenic sexing strain of the Mediterranean fruit fly, Ceratitis capitata. Entomol. Exp. Appl. 2009, 133, 146–153. [Google Scholar] [CrossRef]
- Ant, T.; Koukidou, M.; Rempoulakis, P.; Gong, H.-F.; Economopoulos, A.; Vontas, J.; Alphey, L. Control of the olive fruit fly using genetics-enhanced sterile insect technique. BMC Biol. 2012, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Opp, S.; Ziegner, J.; Bui, N.; Prokopy, R. Factors influencing estimates of sperm competition in Rhagoletis pomonella (Walsh) (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1990, 83, 521–526. [Google Scholar] [CrossRef]
- Collins, S.; Pérez-Staples, D.; Taylor, P. A role for copula duration in fertility of Queensland fruit fly females mated by irradiated and unirradiated males. J. Insect Physiol. 2012, 58, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Bertín, S.; Scolari, F.; Guglielmino, R.C.; Bonizzoni, M.; Bonomi, A.; Marchini, D.; Gomulski, M.L.; Gasperi, G.; Malacrida, R.A.; Matessi, C. Sperm storage and use in polyandrous females of the globally invasive fruit fly, Ceratitis capitata. J. Insect Physiol. 2010, 56, 1542–1551. [Google Scholar] [CrossRef]
- Ramírez-Santos, E.; Rendón, P.; Ruíz, L.; Toledo, J.; Liedo, P. Effect of irradiation doses on sterility and biological security in a genetically modified strain of the Mediterranean fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2017, 110, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.H. A single abdominal ganglion in Anastrepha suspensa (Diptera: Tephritidae) and its innervation of the female sperm storage organs. Ann. Entomol. Soc. Am. 2002, 95, 103–108. [Google Scholar] [CrossRef]
- Twig, E.; Yuval, B. Function of multiple sperm storage organs in female Mediterranean fruit flies (Ceratitis capitata, Diptera: Tephritidae). J. Insect Physiol. 2005, 51, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Staples, D.; Córdova-García, G.; Aluja, M. Sperm dynamics and cryptic male choice in tephritid flies. Anim. Behav. 2014, 89, 131–139. [Google Scholar] [CrossRef]
- Thomas, D.B.; Leal, S.N.; Conway, H.E. Copula duration, insemination and sperm allocation in Anastrepha ludens (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2014, 107, 858–865. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Staples, D.; Harmer, A.; Taylor, P. Sperm storage and utilization in female Queensland fruit flies (Bactrocera tryoni). Physiol. Entomol. 2007, 32, 127–135. [Google Scholar] [CrossRef]
- Dhakal, P.; Fritz, A.H.; Fritz, G.N. Sperm storage patterns in doubly mated female Anastrepha suspensa (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2018, 111, 55–61. [Google Scholar] [CrossRef]
- Meza, J.S.; Arredondo, J.; Orozco, D.; Pérez-Staplesand, D. Disparity in sexual behaviour between wild and mass-reared Mexican fruit flies. Physiol. Entomol. 2014, 39, 263–270. [Google Scholar] [CrossRef]
- Fritz, A.; Turner, F. A light and electron microscopical study of the spermathecae and ventral receptacle of Anastrepha suspensa (Diptera: Tephritidae) and implications in female influence of sperm storage. Arthropod Struct. Dev. 2002, 15, 293–313. [Google Scholar] [CrossRef]


| Male Type | Egg Eclosion (%) | Larval Survival | Pupae Produced | Males Produced | Females Produced | Total Offspring |
|---|---|---|---|---|---|---|
| Standard strain | 82.0 a | 70.4 ± 3.02 a | 69.5 ± 2.89 a | 32.1 ± 1.60 | 32.6 ± 1.49 | 64.7 ± 2.70 a |
| 419-R | 86.8 b | 56.5 ± 3.61 b | 55.9 ± 3.60 b | 28.2 ± 1.85 | 25.6 ± 1.96 | 53.7 ± 3.66 b |
| 443-G | 89.4 b | 63.3 ± 4.46 c | 62.7 ± 4.48 c | 31.1 ± 2.38 | 28.5 ± 2.29 | 59.6 ± 4.28 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verónica-Murrieta, B.; Meza, J.S.; Baena, M.L.; Alvarado-Castillo, G.; Pérez-Staples, D. Polyandrous Mexican Fruit Flies: Second Male Paternity and Biological Attributes of Transgenic Strains. Insects 2022, 13, 5. https://doi.org/10.3390/insects13010005
Verónica-Murrieta B, Meza JS, Baena ML, Alvarado-Castillo G, Pérez-Staples D. Polyandrous Mexican Fruit Flies: Second Male Paternity and Biological Attributes of Transgenic Strains. Insects. 2022; 13(1):5. https://doi.org/10.3390/insects13010005
Chicago/Turabian StyleVerónica-Murrieta, Betzabé, José Salvador Meza, Martha L. Baena, Gerardo Alvarado-Castillo, and Diana Pérez-Staples. 2022. "Polyandrous Mexican Fruit Flies: Second Male Paternity and Biological Attributes of Transgenic Strains" Insects 13, no. 1: 5. https://doi.org/10.3390/insects13010005
APA StyleVerónica-Murrieta, B., Meza, J. S., Baena, M. L., Alvarado-Castillo, G., & Pérez-Staples, D. (2022). Polyandrous Mexican Fruit Flies: Second Male Paternity and Biological Attributes of Transgenic Strains. Insects, 13(1), 5. https://doi.org/10.3390/insects13010005






