Climate Change Impacts on the Potential Distribution of Apocheima cinerarius (Erschoff) (Lepidoptera: Geometridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials
2.1. CLIMEX Model
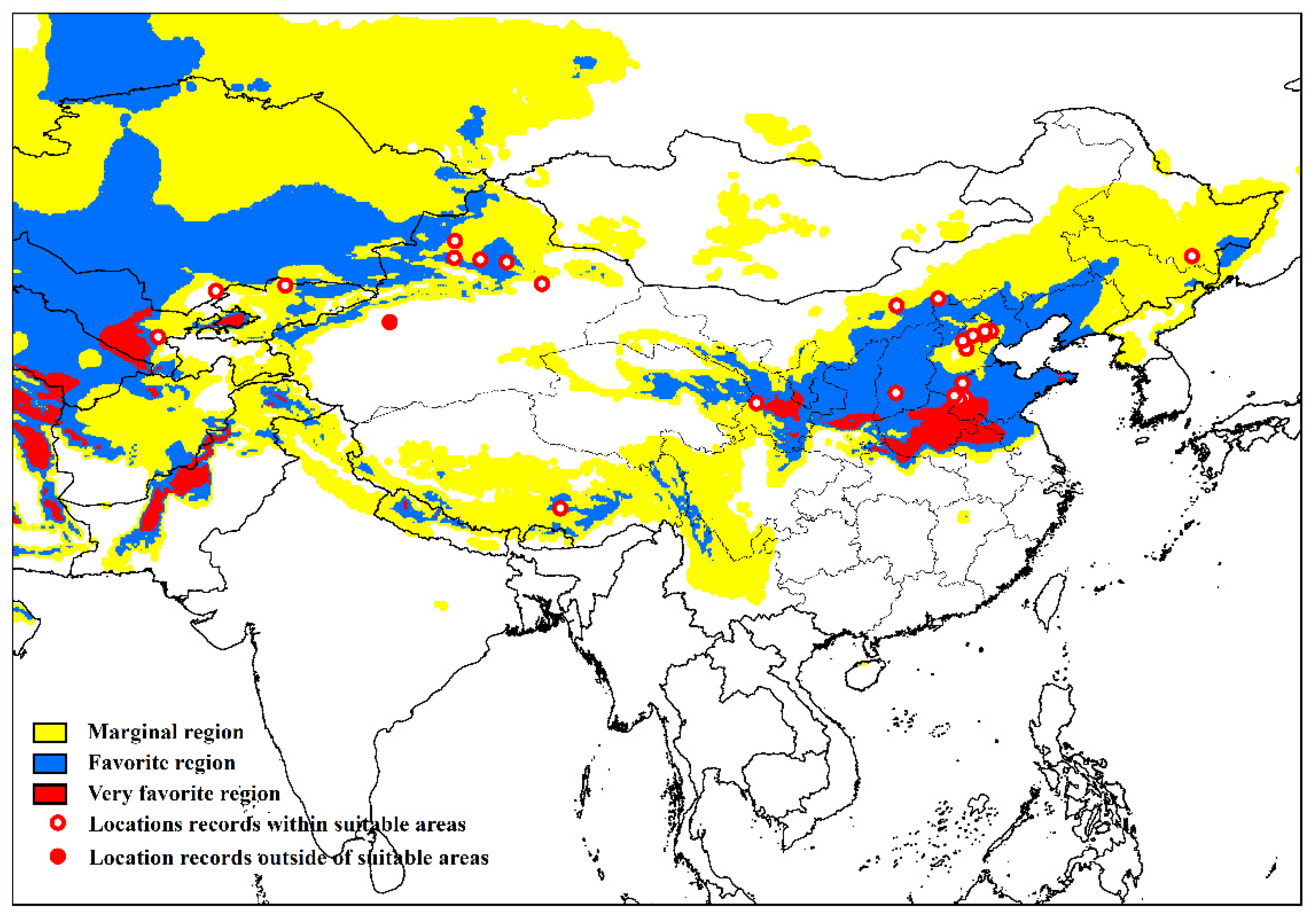
2.2. Known Distribution Data
2.3. Meteorological Data and Irrigation
2.4. CLIMEX Parameter
2.4.1. Temperature Index
2.4.2. Moisture Index
2.4.3. Stresses
2.4.4. Effective Accumulated Temperature
2.4.5. Diapause
2.5. Classification of EI Values
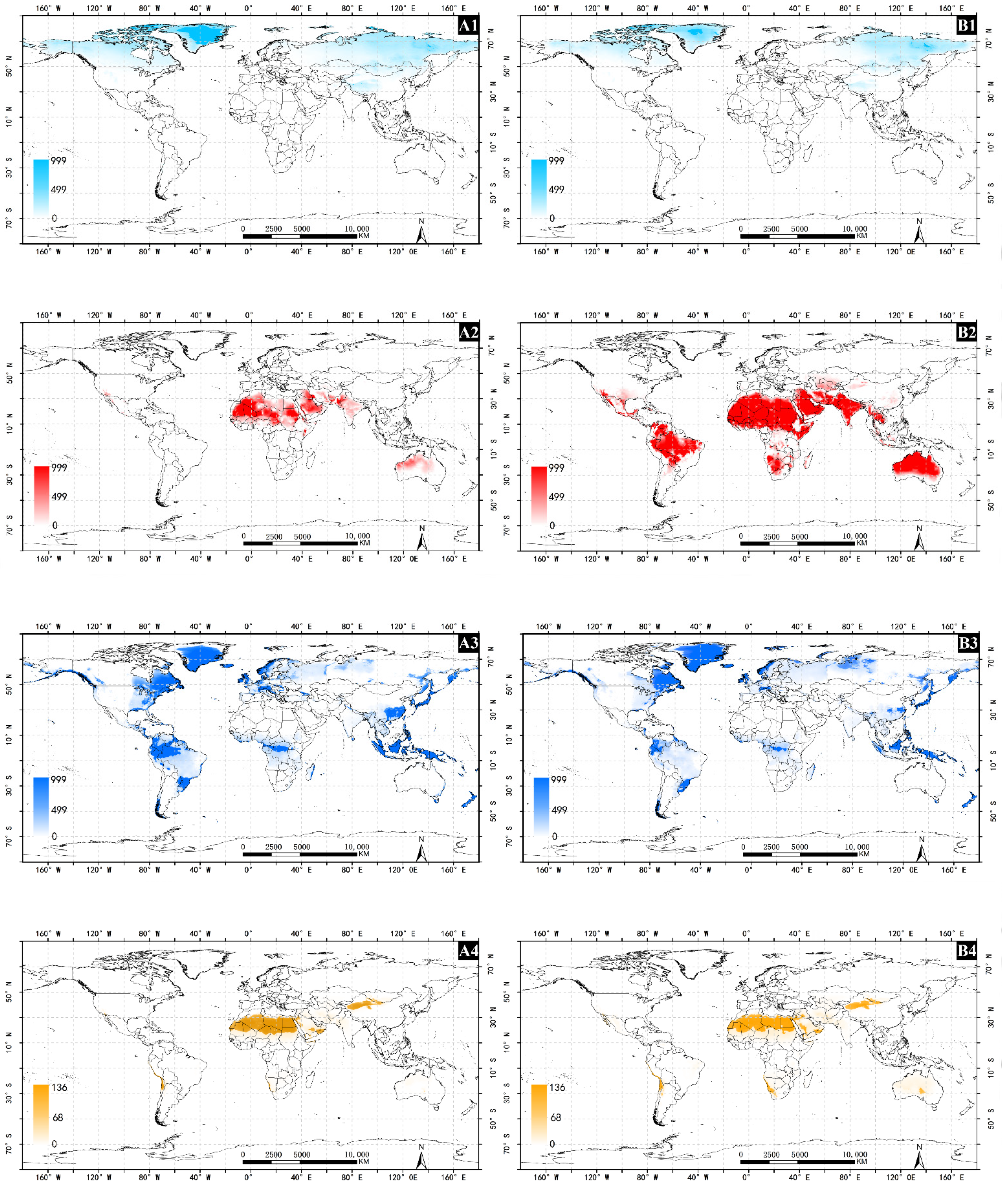
3. Results
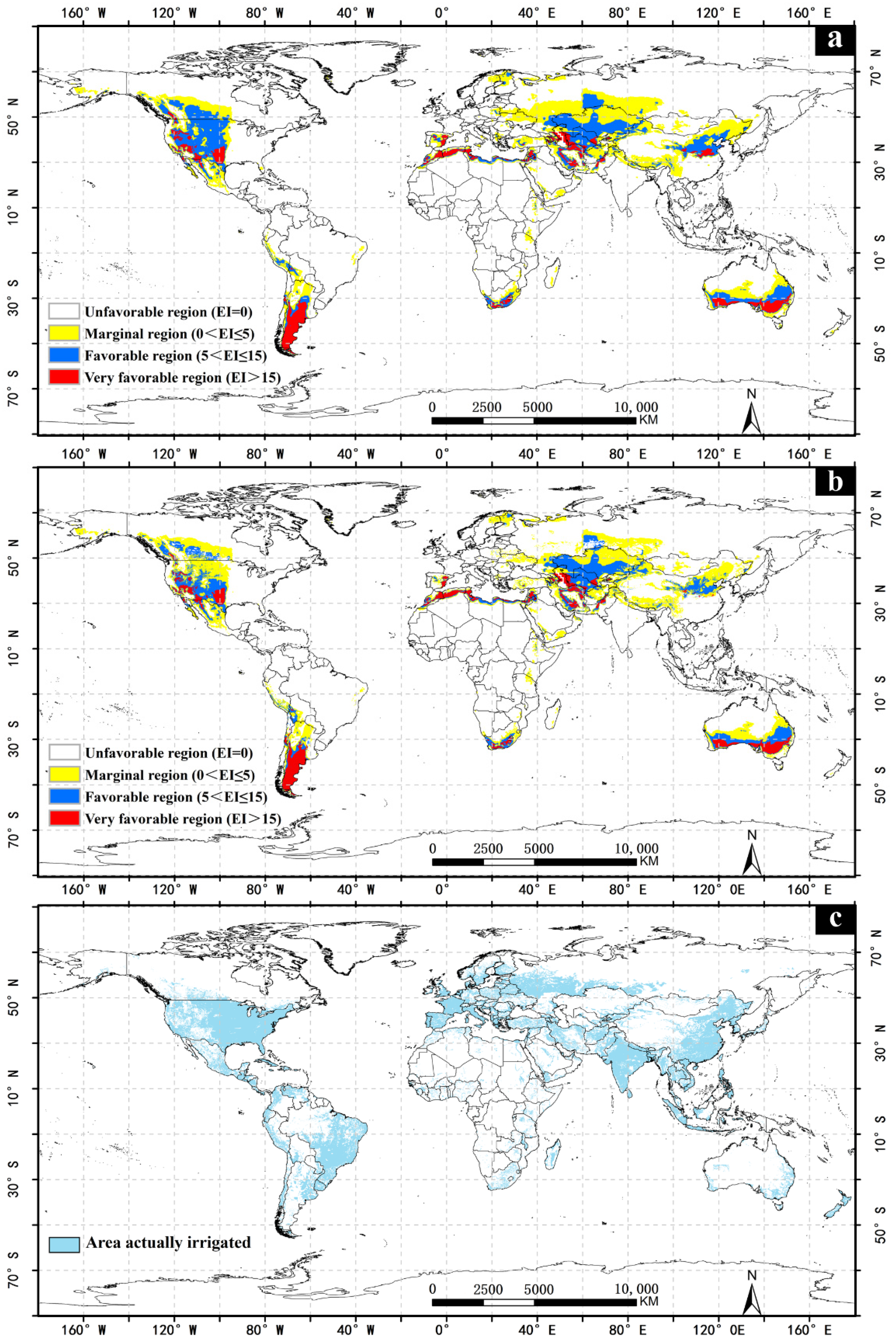
3.1. The Impact of Irrigation
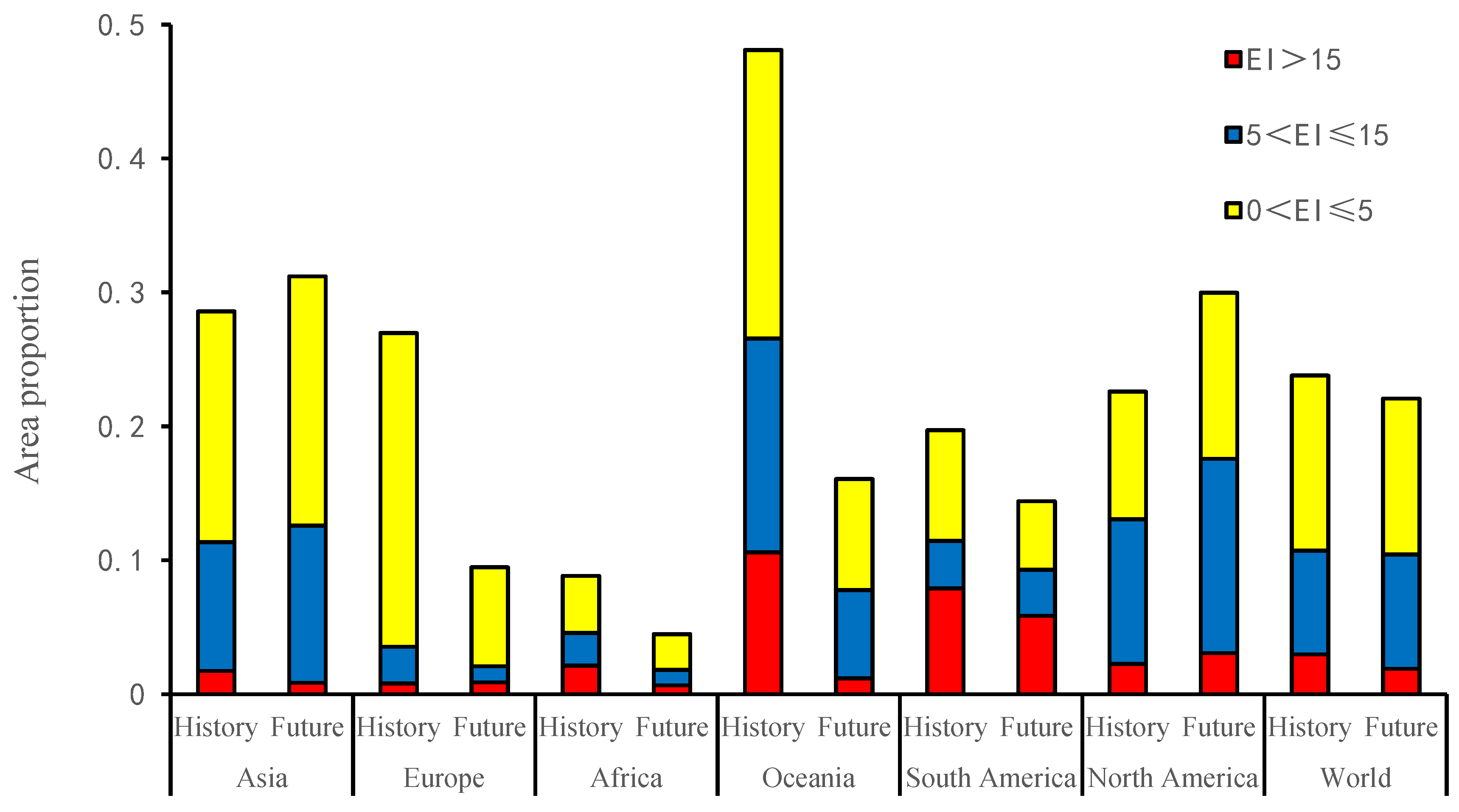
3.2. America
3.3. Africa
3.4. Asia
3.5. Australia
3.6. Europe
3.7. Bioclimatology
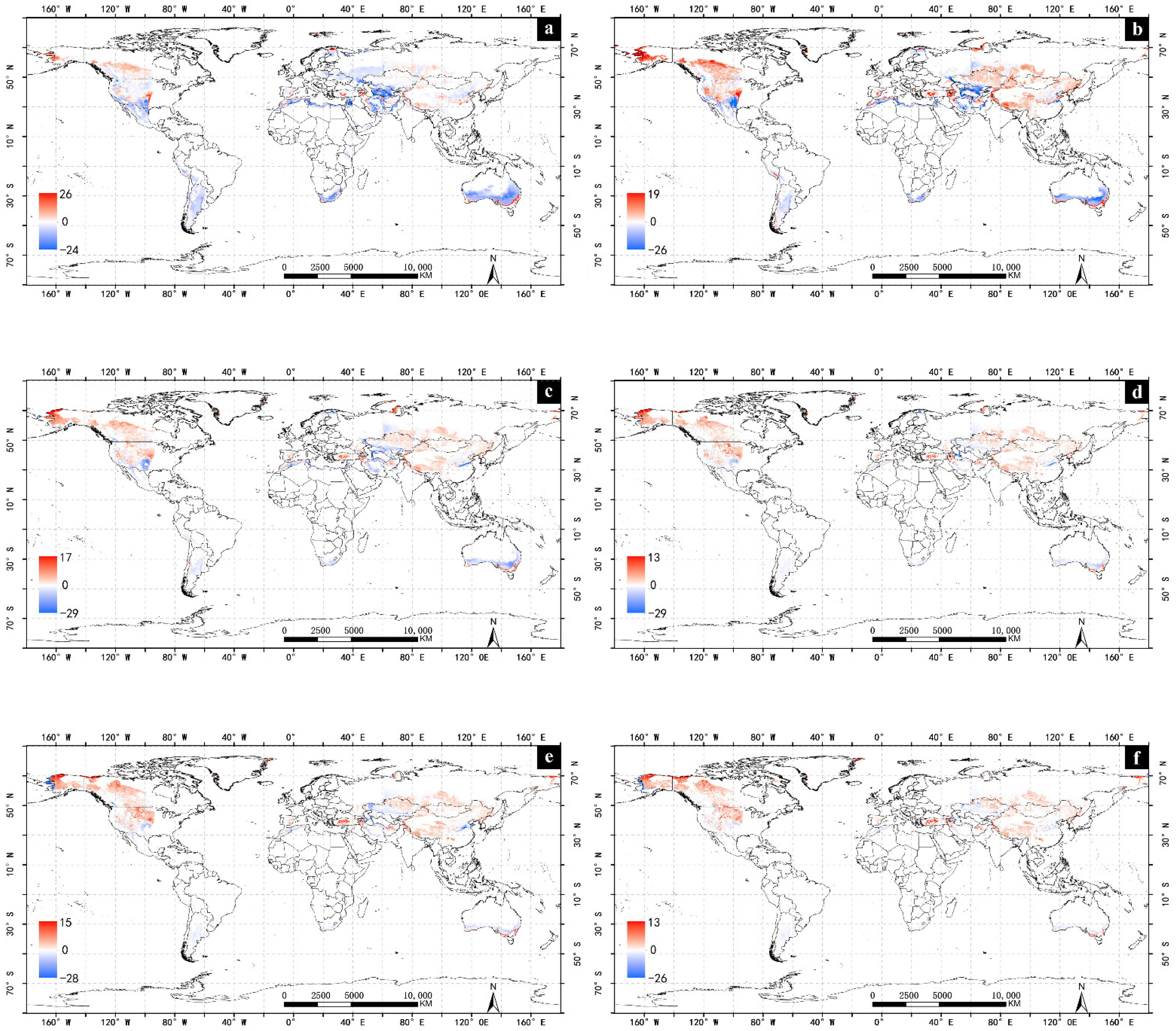
3.8. Driving Variables Limiting the Potential Distribution
4. Discussion
4.1. Impacts of Climate Change
4.2. Effects of Host Distribution
4.3. Process of Diapause
4.4. Limitations of the CLIMEX Model
4.5. Strategies and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, J.J.; Tian, Y.; Han, Y.; Li, L.; Zhang, B. Field evaluation of insect-resistant transgenic Populus nigra trees. Euphytica 2001, 121, 123–127. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, W. Occurrence regularity of Apocheima cinerarius Erschoff in Jilin. For. Pest Dis. 2010, 29, 24–25. (In Chinese) [Google Scholar]
- Shuxian, L.; Nan, J.; Dayong, X.; Rui, C.; Yanhua, Q.; Xinxin, L.; Fumin, L.; Hongxiang, H. Evolutionary history of Apocheima cinerarius(Lepidoptera: Geometridae), a female flightless moth in northern China. Zool. Scr. 2016, 45, 160–174. [Google Scholar] [CrossRef]
- Pang, J.G.; He, W.W.; Xiong, C.R. Research Progress of Important Forest Pest: Apocheima cinerarius. Hans J. Agric. Sci. 2018, 8, 461–467. [Google Scholar] [CrossRef]
- Liu, S.X.; Xue, D.Y.; Cheng, R.; Han, H.X. The complete mitogenome of Apocheima cinerarius (Lepidoptera: Geometridae: Ennominae) and comparison with that of other lepidopteran insects. Gene 2014, 547, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Ding, X.; Zhu, X.; Chen, S.; Chen, M.; Jia, X.; Lai, F.; Zhang, X. Assessment of Poplar Looper (Apocheima cinerarius Erschoff) Infestation on Euphrates (Populus euphratica) Using Time-Series MODIS NDVI Data Based on the Wavelet Transform and Discriminant Analysis. Remote Sens. 2021, 13, 2345. [Google Scholar] [CrossRef]
- Aishan, T.; Halik, Ü.; Betz, F.; Gärtner, P.; Cyffka, B. Modeling height–diameter relationship for Populus euphratica in the Tarim riparian forest ecosystem, Northwest China. J. For. Res. 2016, 27, 889–900. [Google Scholar] [CrossRef]
- Lang, P.; Jeschke, M.; Wommelsdorf, T.; Backes, T.; Lv, C.; Zhang, X.; Thomas, F.M. Wood harvest by pollarding exerts long-term effects on Populus euphratica stands in riparian forests at the Tarim River, NW China. For. Ecol. Manag. 2015, 353, 87–96. [Google Scholar] [CrossRef]
- Fengchun, Y.; Lifang, Q.; Hongli, Y. Observation and Control of Emergemce(Eclosion) of Spring Cankerworm(Apocheima Cinerius Erschoff). Xinjiang Agric. Sci. 2002, 41, 385–387. (In Chinese) [Google Scholar]
- Yao, D.U.; Chun-Sen, M.A.; Zhao, Q.H.; Gang, M.A. Effects of heat stress on physiological and biochemical mechanisms of insects: A literature review. Acta Ecol. Sin. 2007, 27, 1565–1572. [Google Scholar]
- Chen, Y.; Ma, C. Effect of global warming on insect: A literature review. Acta Ecol. Sin. 2010, 30, 2159–2172. [Google Scholar]
- Harrington, R.; Fleming, R.A.; Woiwod, I.P. Climate change impacts on insect management and conservation in temperate regions: Can they be predicted? Agric. Entomol. 2001, 3, 233–240. [Google Scholar] [CrossRef]
- Bergant, K.; Trdan, S.; Žnidarčič, D.; Črepinšek, Z.; Kajfež-Bogataj, L. Impact of Climate Change on Developmental Dynamics ofThrips tabaci(Thysanoptera: Thripidae): Can It Be Quantified? Environ. Entomol. 2005, 34, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Yu, Y.; Fang, G.; Zhang, X.; Yu, Z.; Zhang, X. Effects of meteorological factors on the defoliation dynamics of the larch caterpillar (Dendrolimus superans Butler) in the Great Xing’an boreal forests. J. For. Res. 2021, 32, 2683–2697. [Google Scholar] [CrossRef]
- Chapman, D.; Purse, B.V.; Roy, H.E.; Bullock, J.M. Global trade networks determine the distribution of invasive non-native species. Glob. Ecol. Biogeogr. 2017, 26, 907–917. [Google Scholar] [CrossRef]
- Perrings, C.; Dehnen-Schmutz, K.; Touza, J.; Williamson, M. How to manage biological invasions under globalization. Trends Ecol. Evol. 2005, 20, 212–215. [Google Scholar] [CrossRef]
- Ward, N.L.; Masters, G.J. Linking climate change and species invasion: An illustration using insect herbivores. Glob. Change Biol. 2007, 13, 1605–1615. [Google Scholar] [CrossRef]
- Franklin, J. Predictive vegetation mapping: Geographic modelling of biospatial patterns in relation to environmental gradients. Prog. Phys. Geogr. 1995, 19, 474–499. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Maywald, G.F. A computerised system for matching climates in ecology. Agric. Ecosyst. Environ. 1985, 13, 281–299. [Google Scholar] [CrossRef]
- Stockwell, D.; Peters, D.P. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geogr. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Soares, J.R.S.; da Silva, R.S.; Ramos, R.S.; Picanco, M.C. Distribution and invasion risk assessments of Chrysodeixis includens (Walker, [1858]) (Lepidoptera: Noctuidae) using CLIMEX. Int. J. Biometeorol. 2021, 65, 1137–1149. [Google Scholar] [CrossRef]
- Lei, W.; Lei, L.; Ping, L.; Chen, H.X.; Qin, Q.; Qi, G.Y.; Guang, L.X. Potential Risk Zone Analysis of Apocheima cinerarius Erschoff disaster inXinjiang Featured Forest Fruits. Xinjiang Agric. Sci. 2019, 56, 1691–1700. (In Chinese) [Google Scholar] [CrossRef]
- Hughes, R.D.; Maywald, G.F. Forecasting the favourableness of the Australian environment for the Russian wheat aphid, Diuraphis noxia (Homoptera: Aphididae), and its potential impact on Australian wheat yields. Bull. Entomol. Res. 1990, 80, 165–175. [Google Scholar] [CrossRef]
- Sutherst, R.W. A Climate Model of the Red Imported Fire Ant, Solenopsis invicta Buren (Hymenoptera: Formicidae): Implications for Invasion of New Regions, Particularly Oceania. Environ. Entomol. 2005, 34, 317–335. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.J.; Ge, X.Z.; Guo, S.W.; Li, X.; Chen, L.H.; Wang, T.; Zong, S.X. Prediction of the Long-Term Potential Distribution of Cryptorhynchus lapathi (L.) under Climate Change. Forests 2019, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Kriticos, D.J.; Maywald, G.F.; Yonow, T.; Zurcher, E.J.; Sutherst, R.W. CLIMEX Version 4: Exploring the Effects of Climate on Plants, Animals and Diseases. CSIRO 2015, 4, 184. [Google Scholar]
- Wang, Y.; Meng, X.W.; Han, C.J. Occurrence and control techniques of Apocheima cinerarius. For. Sci. Technol. 1999, 24, 22–23. (In Chinese) [Google Scholar]
- Wu, X.H. The Occurrence Dynamics and Control Techniques of Apocheima cinerarius (Lepidoptera: Geometridae) In Northern Xinjiang. Ph.D. Thesis, Shihezi University, Shihezi, China, 2017. [Google Scholar]
- Kriticos, D.J.; Webber, B.L.; Leriche, A.; Ota, N.; Macadam, I.; Bathols, J.; Scott, J.K. CliMond: Global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Kanle Satishchandra, N.; Geerts, S. Modeling the Distribution of the Invasive Alien Cycad Aulacaspis Scale in Africa Under Current and Future Climate Scenarios. J. Econ. Entomol. 2020, 113, 2276–2284. [Google Scholar] [CrossRef]
- Qin, W.; Shatar, A.; Yan, W.B. Study on the Biological Characteristics of Apocheima cinerarius. Acta Appl. Entomol. 2016, 53, 174–184. [Google Scholar]
- Siebert, S.; Döll, P.; Hoogeveen, J.; Faures, J.M.; Frenken, K.; Feick, S. Development and validation of the global map of irrigation areas. Hydrol. Earth Syst. Sci. 2005, 9, 535–547. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Z.; Wang, Y.; He, G.J.; Wang, J.P.; Zhang, Z.M.; Yin, R.Y.; Zhao, J.J. A Method for Detecting the Damage of Apocheima Cinerarius Erschoff based on MODIS Time Series: Case Studies in Bachu Populus euphratica Forest of Xinjiang Province. Remote Sens. Technol. Appl. 2018, 33, 686–695. [Google Scholar] [CrossRef]
- Qin, W. Research on the Influence of Main Environmental Factors on the Occurrence of Apocheima cinerarius Erschoff. Ph.D. Thesis, Xinjiang Agricultural University, Urumchi, China, 2016. [Google Scholar]
- Liu, Y.H.; Jia, X.; Hou, B.Z.; Chen, S.J.; Huang, T.C.; Lai, F.B. Responses of Populus euphratica looper Development Rhythm to Surface Temperature in the Yeerqiang River Basin. Ecol. Sci. 2019, 38, 119–129. [Google Scholar]
- Qing, W.; Shataer, A.; Yan, W.B. Effects of Soil Factor on the Eclosion of Apocheima cinerareius Erschoff Pupa. J. Xinjiang Agric. Univ. 2016, 39, 406–413. (In Chinese) [Google Scholar]
- Yin, F.C.; Qin, L.F.; Yu, H.L. Discussion on Emerging Monitoring and Control Measures of Apocheima cinerarius. Xinjiang Agric. Sci. 2004, 41, 385–387. (In Chinese) [Google Scholar]
- Chen, M.Y.; Jia, X.; Chen, S.J.; Hou, B.Z.; Liu, Y.H.; Huang, T.C.; Yan, Z.M. The prediction of the occurrence period of Populus euphratica Apocheima cinerius Erschoff in the Yarkant River Basin based on remote sensing. Ecol. Sci. 2020, 39, 145–156. (In Chinese) [Google Scholar]
- Wang, C.R.; Li, E.J.; Wang, S.B.; Wang, Q.H.; Wang, Y.Z.; Zhang, Y.A.; Duan, L.Q. Identification of New Virus Strain of Apocheima cinerarius and Its Synergistic Effect on A. cinerarius Nucleopolyhedrovirus. Chin. J. Biol. Control. 2020, 36, 196–202. (In Chinese) [Google Scholar] [CrossRef]
- Yan, Q.; Wei, W.; Hou, X.L.; Ma, J.X.; AISA, H.A. Extraction and GC-MS identification of active components of sexpheromone from Apocheima cinerarius Erschoff ( Lepidoptera: Geometridae). Acta Entomol. Sin. 2011, 54, 368–372. (In Chinese) [Google Scholar]
- Li, X.; Ge, X.Z.; Chen, L.H.; Zhang, L.J.; Wang, T.; Zong, S.X. Climate change impacts on the potential distribution of Eogystia hippophaecolus in China. Pest Manag. Sci. 2019, 75, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Rogers, B.M.; Jantz, P.; Goetz, S.J. Vulnerability of eastern US tree species to climate change. Glob. Chang. Biol. 2017, 23, 3302–3320. [Google Scholar] [CrossRef]
- Ayres, M.P.; Lombardero, M.J. Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci. Total Environ. 2000, 262, 263–286. [Google Scholar] [CrossRef]
- van Lierop, P.; Lindquist, E.; Sathyapala, S.; Franceschini, G. Global forest area disturbance from fire, insect pests, diseases and severe weather events. For. Ecol. Manag. 2015, 352, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Canelles, Q.; Aquilué, N.; James, P.M.A.; Lawler, J.; Brotons, L. Global review on interactions between insect pests and other forest disturbances. Landsc. Ecol. 2021, 36, 945–972. [Google Scholar] [CrossRef]
- Pernek, M.; Lacković, N.; Lukić, I.; Zorić, N.; Matošević, D. Outbreak of Orthotomicus erosus (Coleoptera, Curculionidae) on Aleppo Pine in the Mediterranean Region in Croatia. South-East Eur. For. 2019, 10, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Alcamo, J.; Roehrl, R.A.; Victor, N. Special Report on Emissions Scenarios. Special Report of Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Jactel, H.; Koricheva, J.; Castagneyrol, B. Responses of forest insect pests to climate change: Not so simple. Curr. Opin. Insect. Sci. 2019, 35, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kolb, T.E.; Fettig, C.J.; Ayres, M.P.; Bentz, B.J.; Hicke, J.A.; Mathiasen, R.; Stewart, J.E.; Weed, A.S. Observed and anticipated impacts of drought on forest insects and diseases in the United States. For. Ecol. Manag. 2016, 380, 321–334. [Google Scholar] [CrossRef]
- Hawkins, E.; Sutton, R. The Potential to Narrow Uncertainty in Regional Climate Predictions. Bull. Am. Meteorol. Soc. 2009, 90, 1095–1107. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.R. Chinese Forest Insects; China Forestry Publishing House: Beijing, China, 1992; pp. 890–892. [Google Scholar]
- Li, J.W.; Gong, S.Z.; Chen, J.L. Discussion on the Occurrence of Apocheima cinerarius in Kuitun City. Mod. Gard. 2015, 3, 51. (In Chinese) [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.Z.; He, S.Y.; Zhu, C.Y.; Wang, T.; Xu, Z.C.; Zong, S.X. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest Manag. Sci. 2019, 75, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.; Sansford, C.E.; Jarvis, C.H.; Cannon, R.; Macleod, A.; Walters, K. The role of climatic mapping in predicting the potential geographical distribution of non-indigenous pests under current and future climates. Agric. Ecosyst. Environ. 2000, 82, 57–71. [Google Scholar] [CrossRef]
- Golding, N.; Purse, B.V.; Warton, D. Fast and flexible Bayesian species distribution modelling using Gaussian processes. Methods Ecol. Evol. 2016, 7, 598–608. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, V.; Griess, V.C.; Keena, M.A. Assessing the Potential Distribution of Asian Gypsy Moth in Canada: A Comparison of Two Methodological Approaches. Sci. Rep. 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljaryian, R.; Kumar, L. Changing global risk of invading greenbug Schizaphis graminum under climate change. Crop Prot. 2016, 88, 137–148. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| CLIMEX Parameter | Description | Value | Unit |
|---|---|---|---|
| DV0 | Lower temperature threshold | −2 | °C |
| DV1 | Lower optimum temperature | 7 | °C |
| DV2 | Upper optimum temperature | 15 | °C |
| DV3 | Upper temperature threshold | 25 | °C |
| PDD | Effective accumulated temperature | 650 | DD |
| TTCS | Cold stress temperature threshold | −5 | °C |
| THCS | Cold stress temperature rate | 0.0002 | week−1 |
| TTHS | Heat stress temperature threshold | 35 | °C |
| THHS | Heat stress temperature rate | 0.005 | week−1 |
| SM0 | Lower soil moisture threshold | 0.05 | * |
| SM1 | Lower optimal soil moisture | 0.1 | * |
| SM2 | Upper optimal soil moisture | 0.4 | * |
| SM3 | Upper soil moisture threshold | 0.45 | * |
| SMDS | Dry stress threshold | 0.05 | °C |
| HDS | Dry stress rate | 0.005 | week−1 |
| SMWS | Wet stress threshold | 0.45 | °C |
| HWS | Wet stress rate | 0.003 | week−1 |
| DPD0 | Diapause induction day length | 14 | * |
| DPT0 | Diapause induction temperature | 25 | °C |
| DPT1 | Diapause termination temperature | −5 | °C |
| DPD | Diapause development days | 120 | day |
| DPSW | Diapause summer or winter indicator | 0 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Li, H.; Wen, J. Climate Change Impacts on the Potential Distribution of Apocheima cinerarius (Erschoff) (Lepidoptera: Geometridae). Insects 2022, 13, 59. https://doi.org/10.3390/insects13010059
Ding W, Li H, Wen J. Climate Change Impacts on the Potential Distribution of Apocheima cinerarius (Erschoff) (Lepidoptera: Geometridae). Insects. 2022; 13(1):59. https://doi.org/10.3390/insects13010059
Chicago/Turabian StyleDing, Weicheng, Hongyu Li, and Junbao Wen. 2022. "Climate Change Impacts on the Potential Distribution of Apocheima cinerarius (Erschoff) (Lepidoptera: Geometridae)" Insects 13, no. 1: 59. https://doi.org/10.3390/insects13010059
APA StyleDing, W., Li, H., & Wen, J. (2022). Climate Change Impacts on the Potential Distribution of Apocheima cinerarius (Erschoff) (Lepidoptera: Geometridae). Insects, 13(1), 59. https://doi.org/10.3390/insects13010059






