Toxicity of Some Essential Oils Constituents against Oriental Fruit Fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil Constituents (EOCs)
2.2. Bioassays
2.3. Bactrocera Dorsalis Colonies
2.4. Fumigation Toxicity Bioassays
2.5. Ingestion Toxicity Bioassays
2.6. Pupal Toxicity Bioassays
2.7. Larval Toxicity Bioassays
2.8. Oviposition-Deterrent Activities
2.9. Repellency Test for the EOCs
2.10. Data Analysis
3. Results
3.1. Fumigation Toxicity Bioassays
3.2. Ingestion Toxicity Bioassays
3.3. Pupal Toxicity Bioassays
3.4. Larval Toxicity Bioassays
3.5. Oviposition-Deterrent Activities
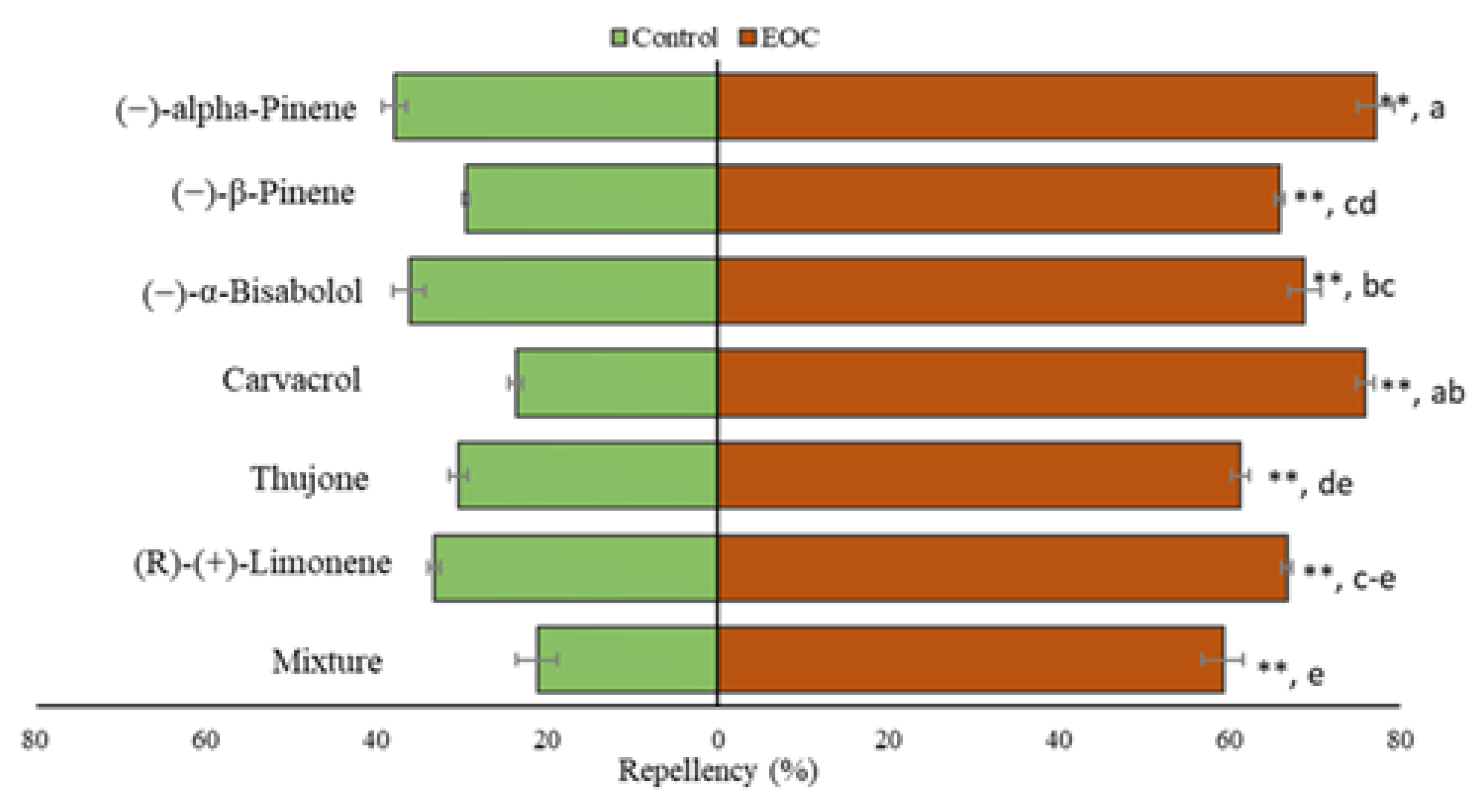
3.6. Repellency Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, B.; Siddiqui, J.A.; Xu, Y. Vertically transmitted gut bacteria and nutrition influence the immunity and fitness of Bactrocera dorsalis larvae. Front. Microbiol. 2020, 11, 596352. [Google Scholar] [CrossRef]
- Chang, C.; Huang, C.-Y.; Dai, S.-M.; Atlihan, R.; Chi, H. Genetically Engineered Ricin Suppresses Bactrocera dorsalis (Diptera: Tephritidae) based on Demographic Analysis of Group-Reared Life Table. J. Econ. Entomol. 2016, 109, 987–992. [Google Scholar] [CrossRef] [Green Version]
- Perry, A.S.; Yamamoto, I.; Ishaaya, I.; Perry, R.Y. Insecticides in Agriculture and Environment: Retrospects and Prospects; Springer Science & Business Media: Berlin, Germany, 2013; ISBN 13.978-3540627296. [Google Scholar]
- Schreinemachers, P.; Tipraqsa, P. Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy 2012, 37, 616–626. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Preisser, E.L.; Chu, D.; Wang, S.; Wu, Q.; Carrière, Y.; Zhou, X.; Zhang, Y. Insecticides promote viral outbreaks by altering herbivore competition. Ecol. Appl. 2015, 25, 1585–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, P.E. Biochemical aspects of insect immunology. Annu. Rev. Entomol. 1986, 31, 321–339. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Feng, H.-T.; Wu, W.-J. Resistance and synergistic effects of insecticides in Bactrocera dorsalis (Diptera: Tephritidae) in Taiwan. J. Econ. Entomol. 2004, 97, 1682–1688. [Google Scholar] [CrossRef]
- Jin, T.; Zeng, L.; Lin, Y.; Lu, Y.; Liang, G. Insecticide resistance of the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae), in mainland China. Pest Manag. Sci. 2011, 67, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.A.; Akram, W. Trichlorfon and spinosad resistance survey and preliminary determination of the resistance mechanism in Pakistani field strains of Bactrocera dorsalis. Sci. Rep. 2018, 8, 11223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadhwa, G.; Kumar, S.; Chhabra, L.; Mahant, S.; Rao, R. Essential oil–cyclodextrin complexes: An updated review. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 39–58. [Google Scholar] [CrossRef]
- Rizzo, R.; Verde, G.L.; Sinacori, M.; Maggi, F.; Cappellacci, L.; Petrelli, R.; Vittori, S.; Morshedloo, M.R.; Benelli, G. Developing green insecticides to manage olive fruit flies? Ingestion toxicity of four essential oils in protein baits on Bactrocera oleae. Ind. Crops Prod. 2020, 143, 111884. [Google Scholar] [CrossRef]
- Hillocks, R.J. Farming with fewer pesticides: EU pesticide review and resulting challenges for UK agriculture. Crop Prot. 2012, 31, 85–93. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides in the twenty-first century—fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeni, V.; Benelli, G.; Campolo, O.; Giunti, G.; Palmeri, V.; Maggi, F.; Rizzo, R.; Lo Verde, G.; Lucchi, A.; Canale, A. Toxics or lures? Biological and behavioral effects of plant essential oils on tephritidae fruit flies. Molecules 2021, 26, 5898. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Burčul, F.; Blažević, I.; Radan, M.; Politeo, O. Terpenes, phenylpropanoids, sulfur and other essential oil constituents as inhibitors of cholinesterases. Curr. Med. Chem. 2020, 27, 4297–4343. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kader, M.; El-Mougy, N.; Lashin, S. Essential oils and Trichoderma harzianum as an integrated control measure against faba bean root rot pathogens. J. Plant Prot. Res. 2011, 51, 3. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Ebadollahi, A.; Jalali Sendi, J. A review on recent research results on bio-effects of plant essential oils against major Coleopteran insect pests. Toxin Rev. 2015, 34, 76–91. [Google Scholar] [CrossRef]
- Segura, D.F.; Belliard, S.A.; Vera, M.T.; Bachmann, G.E.; Ruiz, M.J.; Jofre-Barud, F.; Fernández, P.C.; López, M.L.; Shelly, T.E. Plant chemicals and the sexual behavior of male tephritid fruit flies. Ann. Entomol. Soc. Am. 2018, 111, 239–264. [Google Scholar] [CrossRef]
- Diongue, A.; Yen, T.B.; Lai, P.-Y. Bioassay studies on the effect of essential oils on the female oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Pest Manag. Hortic. Ecsyst. 2010, 16, 91–102. [Google Scholar]
- Akter, M.; Theary, K.; Kalkornsurapranee, E.; Prabhakar, C.S.; Thaochan, N.M. The effects of methyl eugenol, cue lure and plant essential oils in rubber foam dispenser for controlling Bactrocera dorsalis and Zeugodacus cucurbitae. Asian J. Agric. Biol. 2021, 9, 356–367. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; Ling, S.; Tian, F.; Xie, F.; Zeng, X. Toxicity and enzyme inhibition activities of the essential oil and dominant constituents derived from Artemisia absinthium L. against adult Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Ind. Crops Prod. 2018, 121, 468–475. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; Ling, S.; Zeng, X. Seriphidium brevifolium essential oil: A novel alternative to synthetic insecticides against the dengue vector Aedes albopictus. Environ. Sci. Pollut. Res. 2020, 27, 31863–31871. [Google Scholar] [CrossRef] [PubMed]
- Langsi, J.D.; Nukenine, E.N.; Oumarou, K.M.; Moktar, H.; Fokunang, C.N.; Mbata, G.N. Evaluation of the insecticidal activities of α-Pinene and 3-Carene on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Insects 2020, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Feng, Y.; Wang, Y.; Li, J.; Zou, K.; Liu, H.; Hu, Y.; Xue, Y.; Yang, L.; Du, S. Investigation of pesticidal effects of Peucedanum terebinthinaceum essential oil on three stored-product insects. Rec. Nat. Prod. 2020, 14, 177–189. [Google Scholar] [CrossRef]
- Pajaro-Castro, N.; Caballero-Gallardo, K.; Olivero-Verbel, J. Neurotoxic effects of linalool and β-pinene on Tribolium castaneum Herbst. Molecules 2017, 22, 2052. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Flamini, G.; Canale, A.; Cioni, P.L.; Conti, B. Toxicity of some essential oil formulations against the Mediterranean fruit fly Ceratitis capitata (Wiedemann)(Diptera Tephritidae). Crop Prot. 2012, 42, 223–229. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G.; Conti, B.; Lenzi, G.; Flamini, G.; Francini, A.; Cioni, P.L. Ingestion toxicity of three Lamiaceae essential oils incorporated in protein baits against the olive fruit fly, Bactrocera oleae (Rossi)(Diptera Tephritidae). Nat. Prod. Res. 2013, 27, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; He, P.; Xue, J.-P.; Guo, Q.; Zhu, X.-Y.; Fang, L.-P.; Li, J.-B. Insecticidal activities and biochemical properties of Pinellia ternata extracts against the beet armyworm Spodoptera exigua. J. Asia Pac. Entomol. 2017, 20, 469–476. [Google Scholar] [CrossRef]
- Wangrawa, D.W.; Badolo, A.; Guelbéogo, W.M.; Nébié, R.C.H.; Sagnon, N.F.; Borovsky, D.; Sanon, A. Larvicidal, oviposition-deterrence, and excito-repellency activities of four essential oils: An eco-friendly tool against malaria vectors Anopheles coluzzii and Anopheles gambiae (Diptera: Culicidae). Int. J. Trop. Insect Sci. 2021, 41, 1771–1781. [Google Scholar] [CrossRef]
- Kramer, W.L.; Mulla, M.S. Oviposition attractants and repellents of mosquitoes: Oviposition responses of Culex mosquitoes to organic infusions. Environ. Entomol. 1979, 8, 1111–1117. [Google Scholar] [CrossRef]
- Zaka, S.M.; Zeng, X.N.; Holford, P.; Beattie, G.A.C. Repellent effect of guava leaf volatiles on settlement of adults of citrus psylla, Diaphorina citri Kuwayama, on citrus. Insect Sci. 2010, 17, 39–45. [Google Scholar] [CrossRef]
- Jaleel, W.; He, Y.; Lü, L. The response of two Bactrocera species (Diptera: Tephritidae) to fruit volatiles. J. Asia Pac. Entomol. 2019, 22, 758–765. [Google Scholar] [CrossRef]
- Lima, A.; Arruda, F.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Variations in Essential Oil Chemical Composition and Biological Activities of Cryptomeria japonica (Thunb. ex L.f.) D. Don from Different Geographical Origins & mdash; A Critical Review. Appl. Sci. 2021, 11, 11097. [Google Scholar] [CrossRef]
- Enan, E.E. Molecular response of Drosophila melanogaster tyramine receptor cascade to plant essential oils. Insect Biochem. Mol. Biol. 2005, 35, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Can Baser, K. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. 2008, 14, 3106–3119. [Google Scholar] [CrossRef] [PubMed]
- Papanastasiou, S.A.; Bali, E.-M.D.; Ioannou, C.S.; Papachristos, D.P.; Zarpas, K.D.; Papadopoulos, N.T. Toxic and hormetic-like effects of three components of citrus essential oils on adult Mediterranean fruit flies (Ceratitis capitata). PLoS ONE 2017, 12, e0177837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, I.-K.; Kim, J.N.; Lee, Y.-S.; Lee, S.-G.; Ahn, Y.-J.; Shin, S.-C. Toxicity of plant essential oils and their components against Lycoriella ingenua (Diptera: Sciaridae). J. Econ. Entomol. 2008, 101, 139–144. [Google Scholar] [CrossRef]
- Ma, W.-B.; Feng, J.-T.; Jiang, Z.-L.; Wu, H.; Ma, Z.-Q.; Zhang, X. Fumigant activity of eleven essential oil compounds and their selected binary mixtures against Culex pipiens pallens (Diptera: Culicidae). Parasitol. Res. 2014, 113, 3631–3637. [Google Scholar] [CrossRef]
- Sekine, N.; Shibutani, S. Chemical structures of p-menthane monoterpenes with special reference to their effect on seed germination and termite mortality. J. Wood Sci. 2013, 59, 229–237. [Google Scholar] [CrossRef]
- Lima, R.K.; Cardoso, M.D.G.; Moraes, J.C.; Carvalho, S.M.; Rodrigues, V.G.; Guimarães, L.G.L. Chemical composition and fumigant effect of essential oil of Lippia sidoides Cham. and monoterpenes against Tenebrio molitor (L.)(Coleoptera: Tenebrionidae). Cienc. Agrotecnol. 2011, 35, 664–671. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.; Benelli, G. Larvicidal potential of carvacrol and terpinen-4-ol from the essential oil of Origanum vulgare (Lamiaceae) against Anopheles stephensi, Anopheles subpictus, Culex quinquefasciatus and Culex tritaeniorhynchus (Diptera: Culicidae). Res. Vet. Sci. 2016, 104, 77–82. [Google Scholar] [CrossRef]
- Park, J.-H.; Jeon, Y.-J.; Lee, C.-H.; Chung, N.; Lee, H.-S. Insecticidal toxicities of carvacrol and thymol derived from Thymus vulgaris Lin. against Pochazia shantungensis Chou & Lu., newly recorded pest. Sci. Rep. 2017, 7, 40902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczepanik, M.; Zawitowska, B.; Szumny, A. Insecticidal activities of Thymus vulgaris essential oil and its components (thymol and carvacrol) against larvae of lesser mealworm, Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae). Allelopathy J. 2012, 30, 129–142. [Google Scholar]
- Park, C.G.; Jang, M.; Yoon, K.A.; Kim, J. Insecticidal and acetylcholinesterase inhibitory activities of Lamiaceae plant essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Ind. Crops Prod. 2016, 89, 507–513. [Google Scholar] [CrossRef]
- Pavela, R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd.(Lep., Noctuidae) larvae. Ind. Crops Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Rao, Y.; Hong, L.; Zhang, D. Efficacy of Origanum vulgare essential oil and carvacrol against the housefly, Musca domestica L. (Diptera: Muscidae). Environ. Sci. Pollut. Res. 2019, 26, 23824–23831. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Li, B.; Zhang, F.; Dong, S.; Wang, Y. Toxicity of Phosphine Fumigation against Bactrocera tau at Low Temperature. J. Econ. Entomol. 2014, 107, 601–605. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Chi, B.; Chen, P.; Liu, Y. Sublethal and transgenerational effects of six insecticides on Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). J. Asia Pac. Entomol. 2021, 24, 14–23. [Google Scholar] [CrossRef]
- Lin, J.; Hao, X.; Yue, G.; Yang, D.; Lu, N.; Cai, P.; Ao, G.; Ji, Q. Efficacy of wax-based bait stations for controlling Bactrocera dorsalis (Diptera: Tephritidae). Pest Manag. Sci. 2022, 78, 3576–3586. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Meeran, M.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective potential of limonene and limonene containing natural products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef] [PubMed]
- Hamraoui, A.; Regnault-Roger, C. Comparaison des activités insecticides des monoterpènes sur deux espèces d’insectes ravageurs des cultures: Ceratitis capitata et Rhopalosiphum padi. Acta Bot. Gall. 1997, 144, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Papachristos, D.; Kimbaris, A.; Papadopoulos, N.; Polissiou, M. Toxicity of citrus essential oils against Ceratitis capitata (Diptera: Tephritidae) larvae. Ann. Appl. Biol. 2009, 155, 381–389. [Google Scholar] [CrossRef]
- Höld, K.M.; Sirisoma, N.S.; Ikeda, T.; Narahashi, T.; Casida, J.E. α-Thujone (the active component of absinthe): γ-aminobutyric acid type A receptor modulation and metabolic detoxification. Proc. Natl. Acad. Sci. USA 2000, 97, 3826–3831. [Google Scholar] [CrossRef] [Green Version]
- Kurtca, M.; Tumen, I.; Keskin, H.; Tabanca, N.; Yang, X.; Demirci, B.; Kendra, P.E. Chemical Composition of Essential Oils from Leaves and Fruits of Juniperus foetidissima and Their Attractancy and Toxicity to Two Economically Important Tephritid Fruit Fly Species, Ceratitis capitata and Anastrepha suspensa. Molecules 2021, 26, 7504. [Google Scholar] [CrossRef]
- Ellis, M.D.; Baxendale, F.P. Toxicity of Seven Monoterpenoids to Tracheal Mites (Acari: Tarsonemidae) and Their Honey Bee (Hymenoptera: Apidae) Hosts When Applied as Fumigants. J. Econ. Entomol. 1997, 90, 1087–1091. [Google Scholar] [CrossRef] [Green Version]
- Pavela, R. Insecticidal properties of several essential oils on the house fly (Musca domestica L.). Phytother Res. 2008, 22, 274–278. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Govindaswamy, M.; Sidhu, G.S.; Singh, S.; Kaur, S.; Chhuneja, P. Ingestion of bacteria expressing dsRNA to maggots produces severe mortality and deformities in fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Egypt. J. Biol. Pest Control 2021, 31, 1. [Google Scholar] [CrossRef]
- Wang, D.; Liang, Q.; Chen, M.; Ye, H.; Liao, Y.; Yin, J.; Lü, L.; Lei, Y.; Cai, D.; Jaleel, W.; et al. Susceptibility of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) pupae to entomopathogenic fungi. Appl. Entomol. Zool. 2021, 56, 269–275. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Brilinger, D.; Wille, C.L.; Da Rosa, J.M.; Franco, C.R.; Boff, M.I.C. Mortality Assessment of Botanical Oils on Anastrepha fraterculus (Wiedemann, 1830) Applied in Fruits Under Laboratory Conditions. J. Agric. Sci. 2019, 11, 287. [Google Scholar] [CrossRef]
- Niassy, S.; Murithii, B.; Omuse, E.R.; Kimathi, E.; Tonnang, H.; Ndlela, S.; Mohamed, S.; Ekesi, S. Insight on fruit fly IPM technology uptake and barriers to scaling in Africa. Sustainability 2022, 14, 2954. [Google Scholar] [CrossRef]
- Sedy, K.; Koschier, E. Bioactivity of carvacrol and thymol against Frankliniella occidentalis and Thrips tabaci. J. Appl. Entomol. 2003, 127, 313–316. [Google Scholar] [CrossRef]
- Malacrinò, A.; Campolo, O.; Laudani, F.; Palmeri, V. Fumigant and repellent activity of limonene enantiomers against Tribolium confusum du Val. Neotrop Entomol. 2016, 45, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; Fouda, M.M.; Hamdy, I.; El-Sawy, S.; Abdel-Mohdy, F. Preparation of durable insect repellent cotton fabric: Limonene as insecticide. Carbohydr. Polym. 2008, 74, 268–273. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, W.; Liang, J.; You, C.; Geng, Z.; Wang, C.; Du, S. Contact and repellent activities of the essential oil from Juniperus formosana against two stored product insects. Molecules 2016, 21, 504. [Google Scholar] [CrossRef] [Green Version]
- Usha Rani, P.; Madhusudhanamurthy, J.; Sreedhar, B. Dynamic adsorption of α-pinene and linalool on silica nanoparticles for enhanced antifeedant activity against agricultural pests. J. Pest Sci. 2014, 87, 191–200. [Google Scholar] [CrossRef]
- Tabari, M.A.; Youssefi, M.R.; Benelli, G. Eco-friendly control of the poultry red mite, Dermanyssus gallinae (Dermanyssidae), using the α-thujone-rich essential oil of Artemisia sieberi (Asteraceae): Toxic and repellent potential. Parasitol. Res. 2017, 116, 1545–1551. [Google Scholar] [CrossRef]
- Mudereri, B.T.; Abdel-Rahman, E.M.; Ndlela, S.; Makumbe, L.D.M.; Nyanga, C.C.; Tonnang, H.E.Z.; Mohamed, S.A. Integrating the Strength of Multi-Date Sentinel-1 and -2 Datasets for Detecting Mango (Mangifera indica L.) Orchards in a Semi-Arid Environment in Zimbabwe. Sustainability 2022, 14, 5741. [Google Scholar] [CrossRef]
| CAS No | EOC Commercial Standard | Chemical Structures | Molecular Formula | Groups | Source (e.g.,) | Solubility | Toxicology | References |
|---|---|---|---|---|---|---|---|---|
| 499-75-2 | Carvacrol 98% | 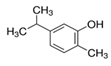 | C10H14O | OM | Aromatic plants (e.g., Origanum spp., Thymus spp., Thymbra capitata etc.) | Ethanol, acetone, diethyl ether, carbon tetrachloride | Insecticidal activity | [25] |
| 23089-26-1 | (−)-α-Bisabolol > 93% |  | C15H26O | OS | Matricaria chamomilla, Myoporum crassifolium etc. | Slightly soluble in ethanol | Insecticidal activity | [25] |
| 1125-12-8 | Thujone (α, β-mixture) > 70.0% | 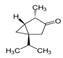 | C10H16O | OMK | Salvia officinalis, Artemisia, Tanacetum vulgare | H2O at 20 °C | Neurotoxin, insecticide | [26] |
| 5989-27-5 | (R)-(+)-Limonene 97% | 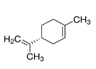 | C10H16 | MH | Citrus | Benzene, chloroform, ether, CS2, soluble in CCl4 | Fumigant, antifeedant | [15] |
| 7785-70-8 | (−)-α-Pinene 98% | 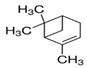 | C10H16 | MH | Mint, holy basil, camphor, bupleurum, and Psidium, coniferous trees | Ethanol, acetone | Insecticidal activity | [27] |
| 18172-67-3 | (−)-β-Pinene 99% |  | C10H16 | MH | Coniferous trees and several other aromatic plants | Alcohol, chloroform, ether | Neurotoxic, insecticide | [29] |
| EOCs a | LC50 (95% CL) b | LC90 (95% CL) b | Slope ± SE c | χ2 (d.f.) d | p-Value |
|---|---|---|---|---|---|
| (−)-alpha-Pinene | 27.94 (24.96–31.20) | 56.28 (50.55–64.31) | 1.26 ± 0.12 | 28.36 (5) | 0.00 |
| (−)-β-Pinene | 29.0 (25.66–34.25) | 71.6 (61.0–83.44) | 0.96 ± 0.11 | 20.76 (5) | 0.001 |
| (−)-α-Bisabolol | 47.31 (40.58–58.29) | 95.84 (79.09–126.47) | 1.24 ± 0.004 | 12.64 (5) | 0.02 |
| Carvacrol | 19.48 (15.23–23.49) | 59.74 (51.47–72.75) | 0.62 ± 0.003 | 35.77 (5) | 0.00 |
| Thujone | 66.25 (55.16–88.03) | 115.25 (92.09–163.04) | 1.71 ± 0.15 | 4.56 (5) | 0.47 |
| (R)-(+)-Limonene | 42.24 (37.07–49.73) | 83.52 (71.09–104.21) | 1.31 ± 0.12 | 16.13 (5) | 0.006 |
| Mixture | 57.52 (49.30–71.87) | 101.63 (85.36–137.86) | 1.67 ± 0.15 | 8.42 (5) | 0.13 |
| EOCs a | LC50 (95% CL) b | LC90 (95% CL) b | Slope ± SE c | χ2 (d.f.) d | p-Value |
|---|---|---|---|---|---|
| (−)-alpha-Pinene | 43.65 (38.11–51.92) | 86.61 (73.19–109.43) | 1.30 ± 0.12 | 14.55 (5) | 0.034 |
| (−)-β-Pinene | 37.89 (33.24–44.24) | 78.86 (67.48–97.41) | 1.18 ± 0.12 | 11.89 (5) | 0.00 |
| (−)-α-Bisabolol | 50.78 (43.77–62.31) | 96.35 (79.98–125.87) | 1.42 ± 0.13 | 7.75 (5) | 0.17 |
| Carvacrol | 41.97 (35.84–51.59) | 92.81 (76.35–123.02) | 1.05 ± 0.11 | 17.33 (5) | 0.004 |
| Thujone | 38.85 (34.38–44.89) | 76.56 (66.17–93.02) | 1.32 ± 0.12 | 20.11 (5) | 0.001 |
| (R)-(+)-Limonene | 37.08 (33.50–41.53) | 67.73 (60.18–78.83) | 1.55 ± 0.13 | 8.77 (5) | 0.11 |
| Mixture | 41.36 (37.74–46.02) | 69.37 (61.96–80.35) | 1.89 ± 0.16 | 19.22 (5) | 0.002 |
| EOCs a | LC50 (95% CL) b | LC90 (95% CL) b | Slope ± SE c | χ2 (d.f.) d | p-Value |
|---|---|---|---|---|---|
| (−)-alpha-Pinene | 11.40 (7.50–14.50) | 40.80 (36.0–47.60) | 0.49 ± 0.10 | 40.59 (5) | 0.45 |
| (−)-β-Pinene | 27.62 (23.85–31.94) | 65.40 (56.91–78.51) | 0.92 ± 0.11 | 16.84 (5) | 0.34 |
| (−)-α-Bisabolol | 23.89 (20.11–27.91) | 62.02 (53.23–74.52) | 0.80 ± 0.10 | 16.22 (5) | 0.33 |
| Carvacrol | 14.89 (12.11–17.52) | 38.52 (34.5–4348) | 0.71 ± 0.11 | 17.94 (5) | 0.23 |
| Thujone | 16.21 (13.50–18.71) | 39.29 (35.39–44.34) | 0.89 ± 0.11 | 25.22 (5) | 0.32 |
| (R)-(+)-Limonene | 16.34 (13.29–19.16) | 43.16 (38.50–49.35) | 0.77 ± 0.13 | 27.33 (5) | 0.12 |
| Mixture | 16.56 (13.47–19.34) | 43.88 (39.29–52.12) | 0.78 ± 0.15 | 29.13 (5) | 0.23 |
| EOCs a | LC50 (95% CL) b | LC90 (95% CL) b | Slope ± SE c | χ2 (d.f.) d | p-Value |
|---|---|---|---|---|---|
| (−)-alpha-Pinene | 26.54 (22.90–30.52) | 62.81 (54.92–74.74) | 0.93 ± 0.11 | 24.68 (5) | 0.00 |
| (−)-β-Pinene | 31.91 (29.03–35.19) | 57.69 (52.20–65.26) | 1.58 ± 0.13 | 6.45 (5) | 0.14 |
| (−)-α-Bisabolol | 33.12 (29.51–37.24) | 69.41 (56.92–75.59) | 1.33 ± 0.12 | 12.52 (5) | 0.15 |
| Carvacrol | 26.19 (23.39–29.21) | 53.31 (48.08–66.03) | 1.23 ± 0.12 | 20.69 (5) | 0.32 |
| Thujone | 31.12 (28.61–33.73) | 51.77 (47.62–57.15) | 1.93 ± 0.15 | 6.35 (5) | 0.32 |
| (R)-(+)-Limonene | 29.12 (26.41–32.23) | 54.23 (49.21–60.92) | 1.49 ± 0.13 | 13.43 (5) | 0.19 |
| Mixture | 38.56 (35.09–42.95) | 67.56 (60.32–78.07) | 1.70 ± 0.14 | 17.23 (5) | 0.24 |
| EOCs | Doses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5% | 4% | 3% | 2% | 1% | 0.5% | |||||||
| EOD (%) | OAI | ER (%) | OAI | ER (%) | OAI | ER (%) | OAI | ER (%) | OAI | ER (%) | OAI | |
| (−)-alpha-Pinene | 100 a, A | –1 | 93.17 b, A | –0.87 | 75.03 c, A | –0.60 | 63.64 d, B | –0.46 | 47.74 e, AB | –0.31 | 36.75 f, B | –0.22 |
| (−)-β-Pinene | 100 a, A | –1 | 97.58 a, A | –0.95 | 78.03 b, A | –0.63 | 60.98 c, BC | –0.43 | 46.33 d, B | –0.30 | 36.60 e, B | –0.22 |
| (−)-α-Bisabolol | 100 a, A | –1 | 97.32 a, A | –0.94 | 78.42 b, A | –0.64 | 67.55 c, AB | –0.51 | 54.09 d, A | –0.37 | 40.55 e, A | –0.25 |
| Carvacrol | 100 a, A | –1 | 95.87 a, A | –0.92 | 68.75 b, B | –0.52 | 43.75 c, D | –0.28 | 22.93 d, E | –0.12 | 12.5 e, E | –0.043 |
| Thujone | 100 a, A | –1 | 94.78 b, A | –0.90 | 84.20 c, A | –0.72 | 73.69 d, A | –0.58 | 39.49 e, C | –0.24 | 31.59 f, C | –0.18 |
| (R)-(+)-Limonene | 100 a, A | –1 | 81.14 b, B | –0.68 | 62.28 c, C | –0.45 | 47.16 d, CD | –0.30 | 26.38 e, D | –0.15 | 16.98 f, D | –0.092 |
| Mixture | 100 a, A | –1 | 97.32 a, A | –0.94 | 72.99 b, AB | –0.57 | 54.09 c, C | –0.37 | 40.55 d, C | –0.25 | 32.44 e, C | –0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaffar, S.; Lu, Y. Toxicity of Some Essential Oils Constituents against Oriental Fruit Fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Insects 2022, 13, 954. https://doi.org/10.3390/insects13100954
Jaffar S, Lu Y. Toxicity of Some Essential Oils Constituents against Oriental Fruit Fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Insects. 2022; 13(10):954. https://doi.org/10.3390/insects13100954
Chicago/Turabian StyleJaffar, Saleem, and Yongyue Lu. 2022. "Toxicity of Some Essential Oils Constituents against Oriental Fruit Fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae)" Insects 13, no. 10: 954. https://doi.org/10.3390/insects13100954
APA StyleJaffar, S., & Lu, Y. (2022). Toxicity of Some Essential Oils Constituents against Oriental Fruit Fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Insects, 13(10), 954. https://doi.org/10.3390/insects13100954








