Flightless Females in the Neotropical Moth Genus Cataspilates Warren (Lepidoptera: Geometridae) †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Molecuar Analysis
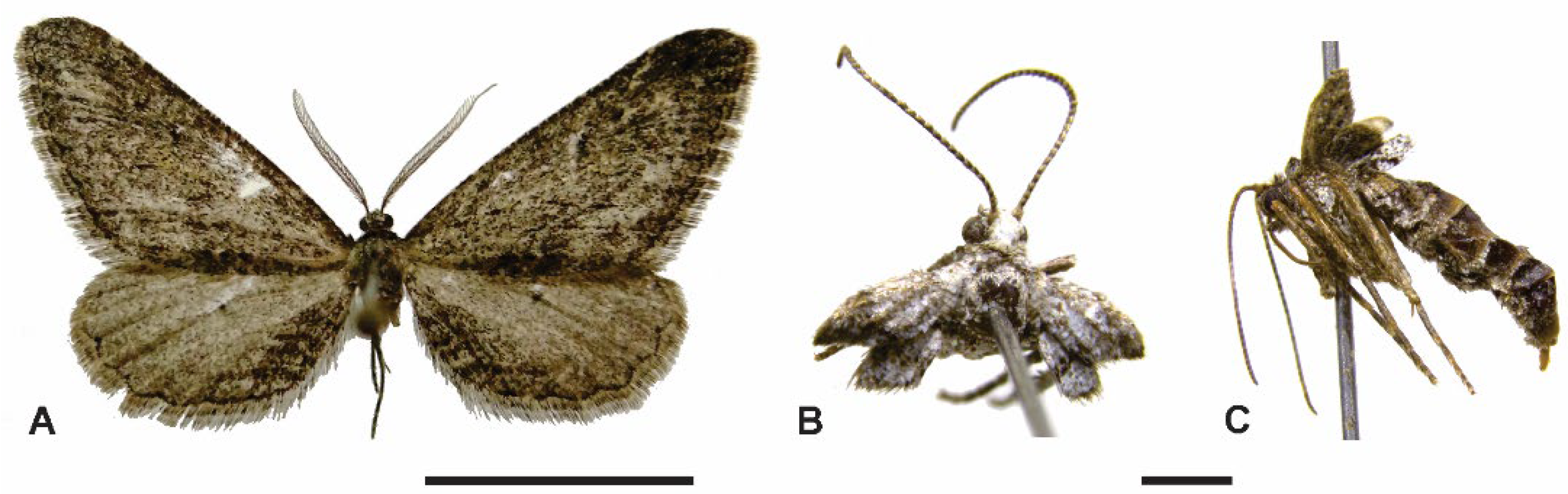
3.2. Taxonomy
4. Discussion
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heppner, J.B. Brachyptery and aptery in Lepidoptera. Trop. Lepid. 1991, 2, 11–40. [Google Scholar]
- Sattler, K. A review of wing reduction in Lepidoptera. Bull. Br. Mus. Nat. Hist. Entomol. 1991, 60, 243–288. [Google Scholar]
- Yamamoto, S.; Sota, T. Phylogeny of the Geometridae and the evolution of winter moths inferred from a simultaneous analysis of mitochondrial and nuclear genes. Mol. Phylogenet. Evol. 2007, 44, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Petschenka, G.; Tavakoli, M.; Trusch, R. Description of the unknown female of Agriopis beschkovi Ganev, 1987 (Geometridae: Ennominae), and illustration of the larvae. Nota Lepidopterol. 2006, 29, 27–35. [Google Scholar]
- Liu, S.-P.; Wipfler, B.; Niitsu, S.; Beutel, R.G. The thoracic anatomy of the male and female winter moth Nyssiodes lefuarius (Lepidoptera: Geometridae) and evolutionary changes in the thorax of moths and butterflies. Org. Divers. Evol. 2017, 17, 565–594. [Google Scholar] [CrossRef]
- Snäll, N.; Tammaru, T.; Wahlberg, N.; Viidalepp, J.; Ruohomaki, K.; Savontaus, M.L.; Huoponen, K. Phylogenetic relationships of the tribe Operophterini (Lepidoptera, Geometridae): A case study of the evolution of female flightlessness. Biol. J. Linn. Soc. 2007, 92, 241–252. [Google Scholar] [CrossRef]
- Murillo-Ramos, L.; Chazot, N.; Sihvonen, P.; Õunap, E.; Jiang, N.; Han, H.; Clarke, J.T.; Davis, R.B.; Tammaru, T.; Wahlberg, N. Molecular phylogeny, classification, biogeography and diversification patterns of a diverse group of moths (Geometridae: Boarmiini). Mol. Phylogenet. Evol. 2021, 162, 107198. [Google Scholar] [CrossRef]
- Wahlberg, N.; Snäll, N.; Viidalepp, J.; Ruohomäki, K.; Tammaru, T. The evolution of female flightlessness among Ennominae of the Holarctic forest zone (Lepidoptera, Geometridae). Mol. Phylogenet. Evol. 2010, 55, 929–938. [Google Scholar] [CrossRef]
- Pitkin, L.M. Neotropical Ennominae moths: A review of the genera (Lepidoptera: Geometridae). Zool. J. Linn. Soc. 2002, 135, 121–401. [Google Scholar] [CrossRef]
- Vargas, H.A.; Hausmann, A. Additions to the geometrid fauna (Lepidoptera: Geometridae) of Chile. Neotrop. Entomol. 2008, 37, 167–168. [Google Scholar] [CrossRef] [Green Version]
- Hebert, P.N.; Cywinska, A.; Ball, S.; de Waard, J. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- McDunnough, J. Studies in North American Cleorini (Geometridae). Can. Dept. Agric. Bull. 1920, 18, 1–64. [Google Scholar]
- Rindge, F.H. A revision of the Nearctic species of the genus Glena (Lepidoptera, Geometridae). Bull. Am. Mus. Nat. Hist. 1965, 129, 265–306. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. Available online: http://www.barcodinglife.org (accessed on 1 September 2022). [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Xia, X. DAMBE7: New and improved tools for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2018, 35, 1550–1552. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.; Schmidt, H.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [Green Version]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1.4.4. 2018. Available online: https://github.com/rambaut/figtree/releases (accessed on 1 September 2022).
- Brehm, G.; Hebert, P.D.N.; Colwell, R.K.; Adams, M.O.; Bodner, F.; Friedemann, K.; Möckel, L.; Fiedler, K. Turning up the heat on a hotspot: DNA barcodes reveal 80% more species of geometrid moths along an Andean elevational gradient. PLoS ONE 2016, 11, e0150327. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, A.; Parra, L.E. An unexpected hotspot of moth biodiversity in Chilean northern Patagonia (Lepidoptera, Geometridae). Zootaxa 2009, 1989, 23–38. [Google Scholar] [CrossRef]
- Moraes, S.S.; Montebello, Y.; Stanton, M.A.; Yamaguchi, L.F.; Kato, M.J.; Freitas, A.V.L. Description of three new species of Geometridae (Lepidoptera) using species delimitation in an integrative taxonomy approach for a cryptic species complex. PeerJ 2021, 9, e11304. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Ramos, L.; Sihvonen, P.; Brehm, G.; Ríos-Malaver, I.C.; Wahlberg, N. A database and checklist of geometrid moths (Lepidoptera) from Colombia. Biodivers. Data J. 2021, 9, e68693. [Google Scholar] [CrossRef]
- Strutzenberger, P.; Brehm, G.; Fiedler, K. DNA barcoding-based species delimitation increases species count of Eois (Geometridae) moths in a well-studied tropical mountain forest by up to 50%. Insect Sci. 2011, 18, 349–362. [Google Scholar] [CrossRef]
- Gossner, M.M.; Hausmann, A. DNA barcoding enables the identification of caterpillars feeding on native and alien oak (Lepidoptera: Geometridae). Mitt. Munch. Entomol. Ges. 2009, 99, 135–140. [Google Scholar]
- Guerrero, J.J.; Hausmann, A.; Rubio, R.M.; Garre, M.; Ortiz, A.S. First description of the male and DNA barcode of Euphyia vallantinaria (Oberthür, 1890) from the Iberian Peninsula (Lepidoptera, Geometridae, Larentiinae). Nota Lepidopterol. 2022, 45, 33–39. [Google Scholar] [CrossRef]
- Hausmann, A.; Haszprunar, G.; Hebert, P.D.N. DNA barcoding the geometrid fauna of Bavaria (Lepidoptera): Successes, surprises and questions. PLoS ONE 2011, 6, e17134. [Google Scholar] [CrossRef]
- Vargas, H.A.; Solis, M.A.; Vargas-Ortiz, M. The South American moth Rheumaptera mochica (Dognin, 1904) (Lepidoptera, Geometridae, Larentiinae) rediscovered after more than a century of anonymity. ZooKeys 2022, 1085, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Wanke, D.; Hausmann, A.; Rajaei, H. An integrative taxonomic revision of the genus Triphosa Stephens, 1829 (Geometridae: Larentiinae) in the Middle East and Central Asia, with description of two new species. Zootaxa 2019, 4603, 39–65. [Google Scholar] [CrossRef] [PubMed]
- Bocaz, P.A.; Parra, L.E. Revisión y bionomía del género Syncirsodes Butler 1882 (Lepidoptera: Geometridae). Rev. Chil. Hist. Nat. 2005, 78, 89–111. [Google Scholar] [CrossRef] [Green Version]
- Bodner, F.; Brehm, G.; Homeier, J.; Strutzenberger, P.; Fiedler, K. Caterpillars and host plant records for 59 species of Geometridae (Lepidoptera) from a montane rainforest in southern Ecuador. J. Insect Sci. 2010, 10, 67. [Google Scholar] [CrossRef]
- Brehm, G. Host-plant records and illustrations of the larvae of 19 geometrid moth species from a montane rain forest in Ecuador (Lepidoptera: Geometridae). Nachr. Entomol. Ver. Apollo 2003, 24, 29–34. [Google Scholar]
- Marconato, G.; Dias, M.M.; Penteado-Dias, M.A. Larvas de Geometridae (Lepidoptera) e seus parasitoides, associadas à Erythroxylum microphyllum St.-Hilaire (Erythroxylaceae). Rev. Bras. Entomol. 2008, 52, 296–299. [Google Scholar] [CrossRef]
- Vargas, H.A. On the natural history of Cosmophyga cortesi Vargas (Lepidoptera: Geometridae), a little-known geometrid moth of the Atacama Desert. Rev. Bras. Entomol. 2021, 65, e20210040. [Google Scholar] [CrossRef]
- Wanke, D.; Feizpour, S.; Hausmann, A.; Viidalepp, J.; Rajaei, H. Taxonomy and systematics of the enigmatic emerald moth Xenochlorodes graminaria (Kollar, 1850) (Lepidoptera: Geometridae), and its assignment to a new genus. Integr. Syst. 2022, 5, 61–71. [Google Scholar] [CrossRef]
- Brehm, G.; Murillo-Ramos, L.; Sihvonen, P.; Hausmann, A.; Schmidt, B.C.; Õunap, E.; Moser, A.; Mörtter, R.; Bolt, D.; Bodner, F.; et al. New World geometrid moths (Lepidoptera: Geometridae): Molecular phylogeny, biogeography, taxonomic updates and description of 11 new tribes. Arthropod Syst. Phylogeny 2019, 77, 457–486. [Google Scholar] [CrossRef]




| Species | BOLD Accession |
|---|---|
| Anavitrinella pampinaria (Guenée, 1858) * | DUNLP059-08 |
| Cataspilates marceloi sp. nov. | CATMA001-22 |
| Cataspilates marceloi sp. nov. | CATMA002-22 |
| Glena cognataria (Hübner, 1831) * | LNAUS3126-13 |
| Glenoides texanaria (Hulst, 1888) * | BBLSW899-09 |
| Odysia excurvaria (Warren, 1907) | NGEOB458-10 |
| Physocleora pauper Warren, 1897 * | BCIGE031-10 |
| “Physocleora” sp. | GEOCO032-20 |
| Tornos abjectarius (Hulst, 1887) | TXLEP1124-20 |
| Tornos scolopacinaria (Guenée, 1858) | GWNC094-07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, H.A.
Flightless Females in the Neotropical Moth Genus Cataspilates Warren (Lepidoptera: Geometridae)
Vargas HA.
Flightless Females in the Neotropical Moth Genus Cataspilates Warren (Lepidoptera: Geometridae)
Vargas, Héctor A.
2022. "Flightless Females in the Neotropical Moth Genus Cataspilates Warren (Lepidoptera: Geometridae)






