Effects of Arbuscular Mycorrhizal Fungi-Colonized Populus alba × P. berolinensis Seedlings on the Microbial and Metabolic Status of Gypsy Moth Larvae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment of P. alba × P. berolinensis Seedlings
2.2. Insect Rearing
2.3. Gut Microbiota Analysis
2.4. Metabolomic Analysis of Larval Fat Body
3. Results
3.1. Root Colonization by AMF
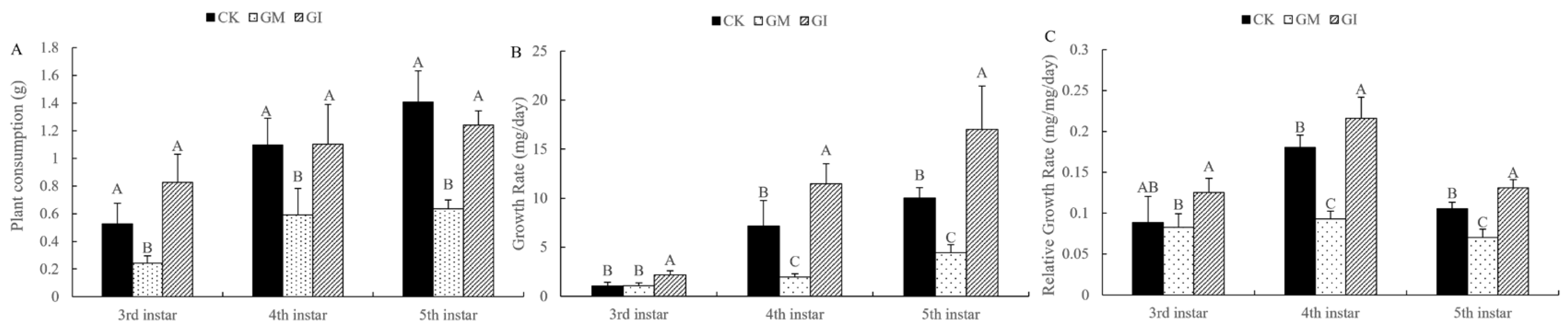
3.2. Analysis of Growth and Development Indexes of Gypsy Moth Larvae
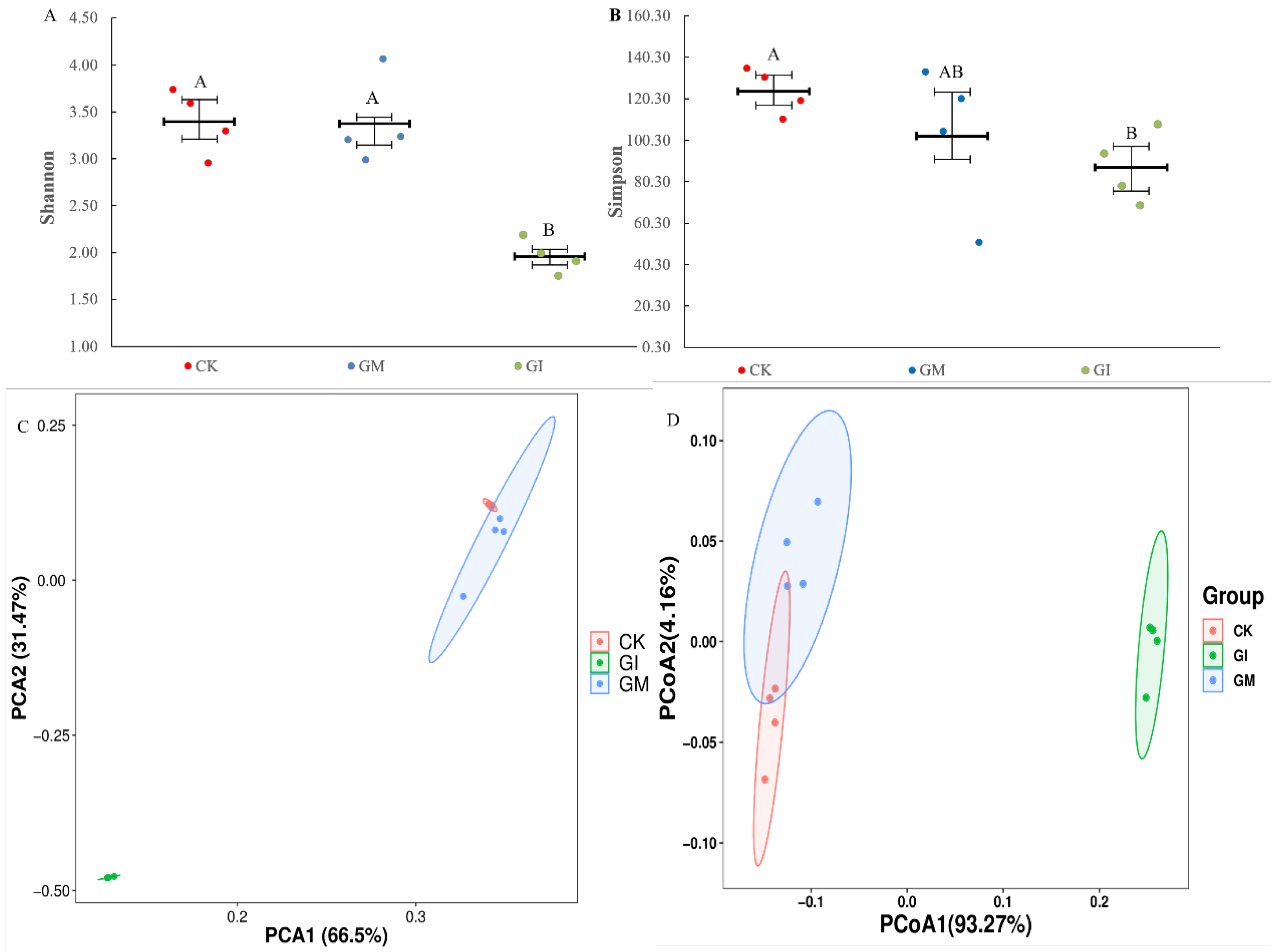
3.3. Diversity Analysis of Gut Microbial Community
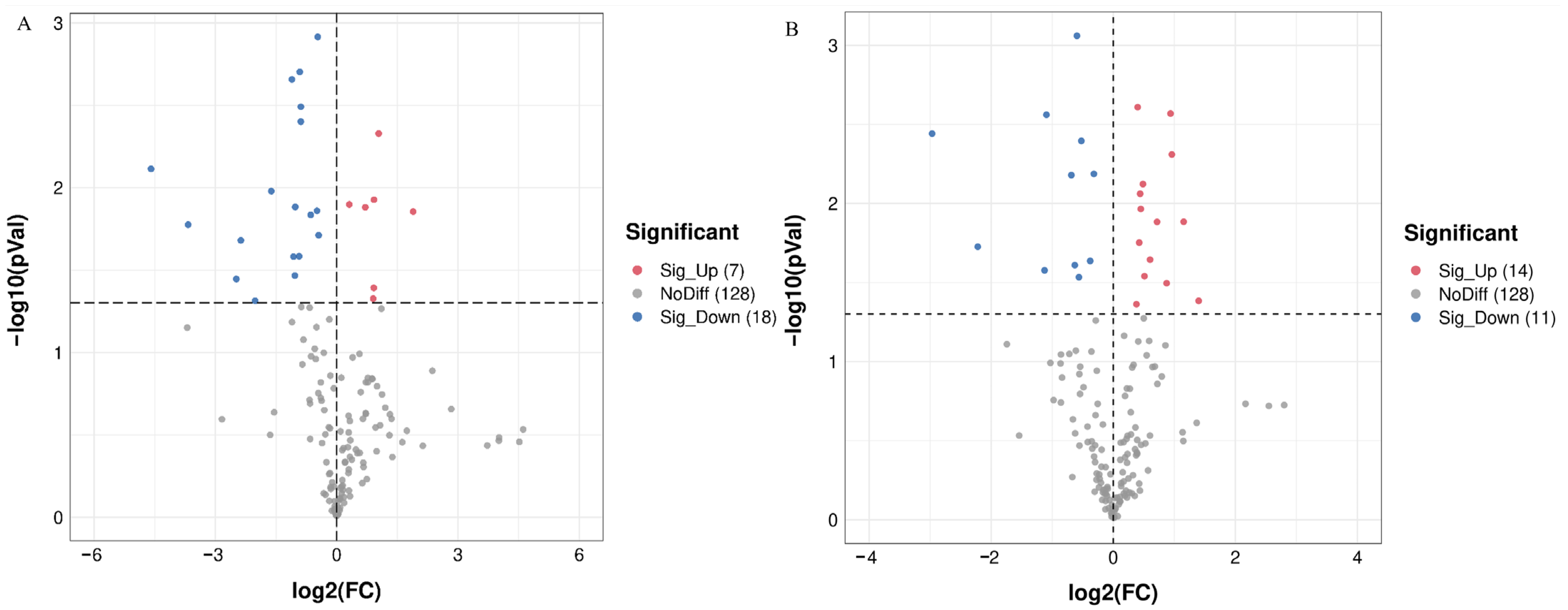
3.4. Differential Gut Microflora Analysis
3.5. Analysis of Gut Microbial Function
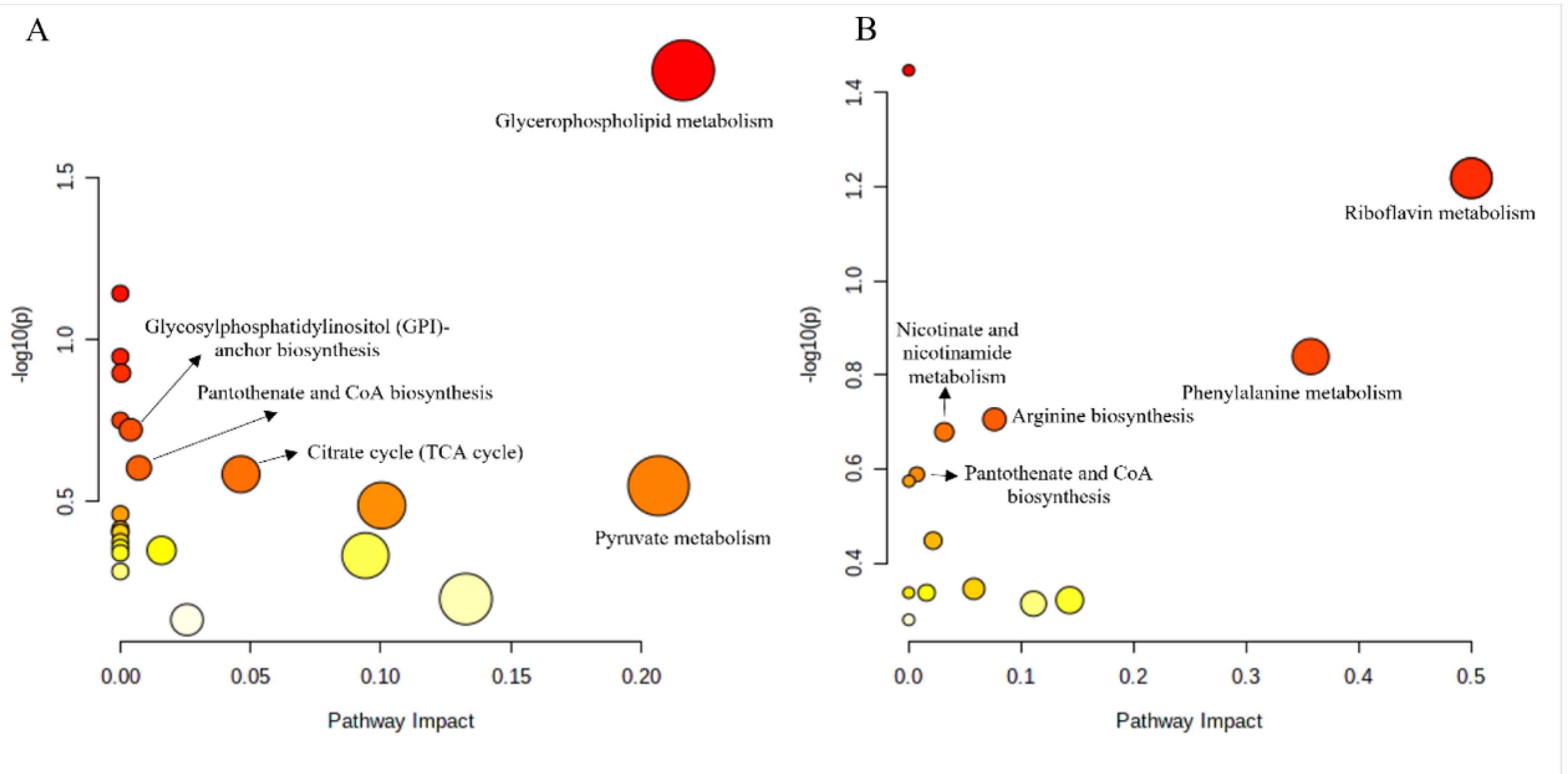
3.6. Metabonomics Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meier, A.R.; Hunter, M.D. Variable effects of mycorrhizal fungi on predator–prey dynamics under field conditions. J. Anim. Ecol. 2021, 90, 1341–1352. [Google Scholar] [CrossRef]
- Xie, L.; Bi, Y.; Ma, S.; Shang, J.; Hu, Q.; Christie, P. Combined inoculation with dark septate endophytes and arbuscular mycorrhizal fungi: Synergistic or competitive growth effects on maize? BMC Plant. Biol. 2021, 21, 498. [Google Scholar] [CrossRef] [PubMed]
- Kavadia, A.; Omirou, M.; Fasoula, D.A.; Louka, F.; Ehaliotis, C.; Ioannides, I.M. Co-inoculations with rhizobia and arbuscular mycorrhizal fungi alters mycorrhizal composition and lead to synergistic growth effects in cowpea that are fungal combination-dependent. Appl. Soil. Ecol. 2021, 167, 104013. [Google Scholar] [CrossRef]
- Millar, N.S.; Bennett, A.E. Stressed out symbiotes: Hypotheses for the influence of abiotic stress on arbuscular mycorrhizal fungi. Oecologia 2016, 182, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.J.; Zhang, N.L.; Zhang, L.L.; Zhang, X.L.; Wu, A.P.; Huang, J.Y.; Yu, S.Q.; Wang, Y.H. Mediation of arbuscular mycorrhizal fungi on growth and biochemical parameters of Ligustrum vicaryi in response to salinity. Physiol. Mol. Plant. Pathol. 2020, 112, 101522. [Google Scholar] [CrossRef]
- Rozpadek, P.; Wezowicz, K.; Stojakowska, A.; Malarz, J.; Surowka, E.; Sobczyk, L.; Anielska, A.; Wazny, R.; Miszalski, Z.; Turnau, K. Mycorrhizal fungi modulate phytochemical production and antioxidant activity of Cichorium intybus L. (Asteraceae) under metal toxicity. Chemosphere 2014, 112, 217–224. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhang, J.C.; Xu, G.P.; Zhou, L.W.; Li, Y.Q. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2019, 50, 593–604. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, H.J.; Zhang, Y.X.; Liu, Y.Q.; Zhang, H.Q.; Tang, M. Arbuscular mycorrhizal fungi alter carbohydrate distribution and amino acid accumulation in Medicago truncatula under lead stress. Environ. Exp. Bot. 2020, 171, 103950. [Google Scholar] [CrossRef]
- Tomczak, V.V.; Müller, C. Influence of arbuscuar mycorrhizal stage and plant age on the performance of a generalist aphid. J. Insect Physiol. 2017, 98, 258. [Google Scholar] [CrossRef]
- Formenti, L.; Rasmann, S. Mycorrhizal fungi enhance resistance to herbivores in tomato plants with reduced jasmonic acid production. Agronomy 2019, 9, 131. [Google Scholar] [CrossRef]
- Schoenherr, A.P.; Rizzo, E.; Jackson, N.; Manosalva, P.; Gomez, S.K. Mycorrhiza-induced induced resistance in potato involves priming of defense response against cabbage looper. Environ. Entomol. 2019, 48, 370–381. [Google Scholar] [CrossRef]
- Frew, A.; Wilson, B.A.L. Different mycorrhizal fungal communities differentially affect plant phenolic-based resistance to insect herbivory. Rhizosphere 2021, 19, 100365. [Google Scholar] [CrossRef]
- Pozo, M.J.; Azcon-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant. Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef]
- Tomczak, V.V.; Schweiger, R.; Müller, C. Effect of arbuscular mycorrhiza on plant chemistry and the development and behavior of a generalist herbivore. J. Chem. Ecol. 2016, 42, 1247–1258. [Google Scholar] [CrossRef]
- Real-Santillan, R.O.; del-Val, E.; Cruz-Ortega, R.; Contreras-Cornejo, H.A.; Gonzalez-Esquivel, C.E.; Larsen, J. Increased maize growth and P uptake promoted by arbuscular mycorrhizal fungi coincide with higher foliar herbivory and larval biomass of the fall armyworm Spodoptera frugiperda. Turk. J. Vet. Anim. Sci. 2019, 44, 714–719. [Google Scholar] [CrossRef]
- Minton, M.M.; Barber, N.A.; Gordon, L.L. Effects of arbuscular mycorrhizal fungi on herbivory defense in two Solanum (Solanaceae) species. Plant. Ecol. Evol. 2016, 149, 157–164. [Google Scholar] [CrossRef]
- Jiang, D.; Tan, M.; Wu, S.; Zheng, L.; Wang, Q.; Wang, G.; Yan, S. Defense response of arbuscular mycorrhizal fungus-colonized poplar seedlings against the gypsy moth larvae: A multi-omics study. Hortic. Res. 2021, 8, 245. [Google Scholar] [CrossRef]
- Jiang, D.; Lin, R.X.; Tan, M.T.; Yan, J.X.; Yan, S.C. The mycorrhizal-induced growth promotion and insect resistance reduction in Populus alba × P. berolinensis seedling: A multi-omics study. Tree Physiol. 2022, 42, 1059–1069. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular arbuscular fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Milanovic, S.; Milenkovic, I.; Dobrosavljevic, J.; Popovic, M.; Solla, A.; Tomsovsky, M.; Jankovsky, L. Growth rates of Lymantria dispar larvae and Quercus robur seedlings at elevated CO2 concentration and Phytophthora plurivora infection. Forests 2020, 11, 1059. [Google Scholar] [CrossRef]
- Wang, C.; Tian, B.L.; Yu, Z.Z.; Ding, J.Q. Effect of different combinations of phosphorus and nitrogen fertilization on arbuscular mycorrhizal fungi and aphids in wheat. Insects 2020, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Razak, N.A.; Gange, A.C. Multitrophic interactions between arbuscular mycorrhizal fungi, foliar endophytic fungi and aphids. Microb. Ecol. 2021, 21, 1937. [Google Scholar] [CrossRef]
- Simon, A.L.; Wellham, P.A.D.; Aradottir, G.I.; Gange, A.C. Unravelling mycorrhiza-induced wheat susceptibility to the English grain aphid Sitobion avenae. Sci. Rep. 2017, 7, 46497. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The gut microbiota of insects-diversity in structure and function. Fems. Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, X.Q.; Zhou, W.; Liu, G.Y.; Wan, Y.J. Isolation and characterization of lipase-producing bacteria in the intestine of the silkworm, Bombyx mori, reared on different forage. J. Insect Sci. 2011, 11, 135. [Google Scholar] [CrossRef]
- Jiang, D.; Wu, S.; Tan, M.; Wang, Q.; Zheng, L.; Yan, S. The high adaptability of Hyphantria cunea larvae to cinnamic acid involves in detoxification, antioxidation and gut microbiota response. Pestic. Biochem. Phys. 2021, 174, 104805. [Google Scholar] [CrossRef]
- Briones-Roblero, C.I.; Rodriguez-Diaz, R.; Santiago-Cruz, J.A.; Zuniga, G.; Rivera-Orduna, F.N. Degradation capacities of bacteria and yeasts isolated from the gut of Dendroctonus rhizophagus (Curculionidae: Scolytinae). Folia Microbiol. 2017, 62, 1–9. [Google Scholar] [CrossRef]
- Paulson, A.R.; Von Aderkas, P.; Perlman, S.J. Bacterial associates of seed-parasitic wasps (Hymenoptera: Megastigmus). BMC Microbiol. 2014, 14, 224. [Google Scholar] [CrossRef]
- Muhammad, A.; Fang, Y.; Hou, Y.M.; Shi, Z.M. The gut entomotype of red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae) and their effect on host nutrition metabolism. Front. Microbiol. 2017, 8, 2291. [Google Scholar] [CrossRef]
- Bog, E.S.; Erturk, O.; Yaman, M. Pathogenicity of aerobic bacteria isolated from honeybees (Apis mellifera) in Ordu Province. Turk. J. Vet. Anim. Sci. 2020, 44, 714–719. [Google Scholar] [CrossRef]
- Dijokaite, A.; Humbert, M.V.; Borkowski, E.; La Ragione, R.M.; Christodoulides, M. Establishing an invertebrate Galleria mellonella greater wax moth larval model of Neisseria gonorrhoeae infection. Virulence 2021, 12, 1900–1920. [Google Scholar] [CrossRef]
- He, Y.H.; Qin, Q.J.; Dilegge, M.J.; Vivanco, J.M. Isolation of Klebsiella pneumoniae and Pseudomonas aeruginosa from entomopathogenic nematode-insect host relationship to examine bacterial pathogenicity on Trichoplusia ni. Microb. Pathog. 2019, 135, 103606. [Google Scholar] [CrossRef]
- Chouaia, B.; Goda, N.; Mazza, G.; Alali, S.; Florian, F.; Gionechetti, F.; Callegari, M.; Gonella, E.; Magoga, G.; Fusi, M.; et al. Developmental stages and gut microenvironments influence gut microbiota dynamics in the invasive beetle Popillia japonica Newman (Coleoptera: Scarabaeidae). Environ. Microbiol. 2019, 21, 4343–4359. [Google Scholar] [CrossRef]
- Liang, S.Y.; Wang, C.P.; Ahmed, F.; Yin, X.J.; Hu, Y.; Mo, J.C. Exploring the effect of plant substrates on bacterial community structure in termite fungus-combs. PLoS ONE 2020, 15, e0232329. [Google Scholar] [CrossRef] [PubMed]
- Osawa, R.; Fujisawa, T.; Pukall, R. Lactobacillus apodemi sp. nov., a tannase-producing species isolated from wild mouse faeces. Int. J. Syst. Evol. Micr. 2006, 56, 1693–1696. [Google Scholar] [CrossRef]
- Li, D.; Zheng, X.; Cai, M.; Zhao, S.; Zhu, Y. Cloning and expression of silkworm (Bombyx mori) uridine phosphorylase 1. Chin. J. Appl. Entomol. 2020, 57, 1152–1160. [Google Scholar]
- Barek, H.; Sugumaran, M.; Ito, S.; Wakamatsu, K. Insect cuticular melanins are distinctly different from those of mammalian epidermal melanins. Pigm. Cell Melanoma Res. 2018, 31, 384–392. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Fasae, K.D.; Iwezor, C.E.; Farombi, E.O. D-Penicillamine prolongs survival and lessens copper-induced toxicity in Drosophila melanogaster. Toxicol. Res. 2020, 9, 346–352. [Google Scholar] [CrossRef]
- Cabor, C.R.; Perkins, H.R.; Heitmann, A.T.; Forsburg, Z.R.; Aspbury, A.S. Roundup (TM) with corticosterone functions as an infodisruptor to antipredator response in tadpoles. Front. Ecol. Evol. 2019, 7, 114. [Google Scholar]
- Du, S.; Yang, D.; Wan, C.; Zhao, F.; Qin, Z. Design, synthesis and insecticidal activities of aryloxypyridinyl ethanone oxime ethers. Chin. J. Pestic. Sci. 2021, 26, 476–482. [Google Scholar]
- Ninomiya, Y.; Kurakake, M.; Oda, Y.; Tsuzuki, S.; Hayakawa, Y. Insect cytokine growth-blocking peptide signaling cascades regulate two separate groups of target genes. FEBS J. 2008, 275, 894–902. [Google Scholar] [CrossRef]
- Reis, F.; Kirsch, R.; Pauchet, Y.; Bauer, E.; Bilz, L.C.; Fukumori, K.; Fukatsu, T.; Kolsch, G.; Kaltenpoth, M. Bacterial symbionts support larval sap feeding and adult folivory in (semi-)aquatic reed beetles. Nat. Commun. 2020, 11, 2964. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Ahmed, S.; Lee, D.H.; Hwang, S.H.; Hammock, B.; Kim, Y. EpOMEs act as immune suppressors in a lepidopteran insect, Spodoptera exigua. Sci. Rep. 2020, 10, 20183. [Google Scholar] [CrossRef]
- Pan, X.H.; Lu, K.; Qi, S.; Zhou, Q.; Zhou, Q. The content of amino acids in artificial diet influences the development and reproduction of brown planthopper, Nilaparvata lugens (STAL). Arch. Insect Biochem. 2014, 86, 75–84. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, M.; Li, Y.; Xu, J.; Yan, S.; Jiang, D. Effects of Arbuscular Mycorrhizal Fungi-Colonized Populus alba × P. berolinensis Seedlings on the Microbial and Metabolic Status of Gypsy Moth Larvae. Insects 2022, 13, 1002. https://doi.org/10.3390/insects13111002
Tan M, Li Y, Xu J, Yan S, Jiang D. Effects of Arbuscular Mycorrhizal Fungi-Colonized Populus alba × P. berolinensis Seedlings on the Microbial and Metabolic Status of Gypsy Moth Larvae. Insects. 2022; 13(11):1002. https://doi.org/10.3390/insects13111002
Chicago/Turabian StyleTan, Mingtao, Yaning Li, Jinsheng Xu, Shanchun Yan, and Dun Jiang. 2022. "Effects of Arbuscular Mycorrhizal Fungi-Colonized Populus alba × P. berolinensis Seedlings on the Microbial and Metabolic Status of Gypsy Moth Larvae" Insects 13, no. 11: 1002. https://doi.org/10.3390/insects13111002
APA StyleTan, M., Li, Y., Xu, J., Yan, S., & Jiang, D. (2022). Effects of Arbuscular Mycorrhizal Fungi-Colonized Populus alba × P. berolinensis Seedlings on the Microbial and Metabolic Status of Gypsy Moth Larvae. Insects, 13(11), 1002. https://doi.org/10.3390/insects13111002



