Characterizing Repellencies of Methyl Benzoate and Its Analogs against the Common Bed Bug, Cimex lectularius
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
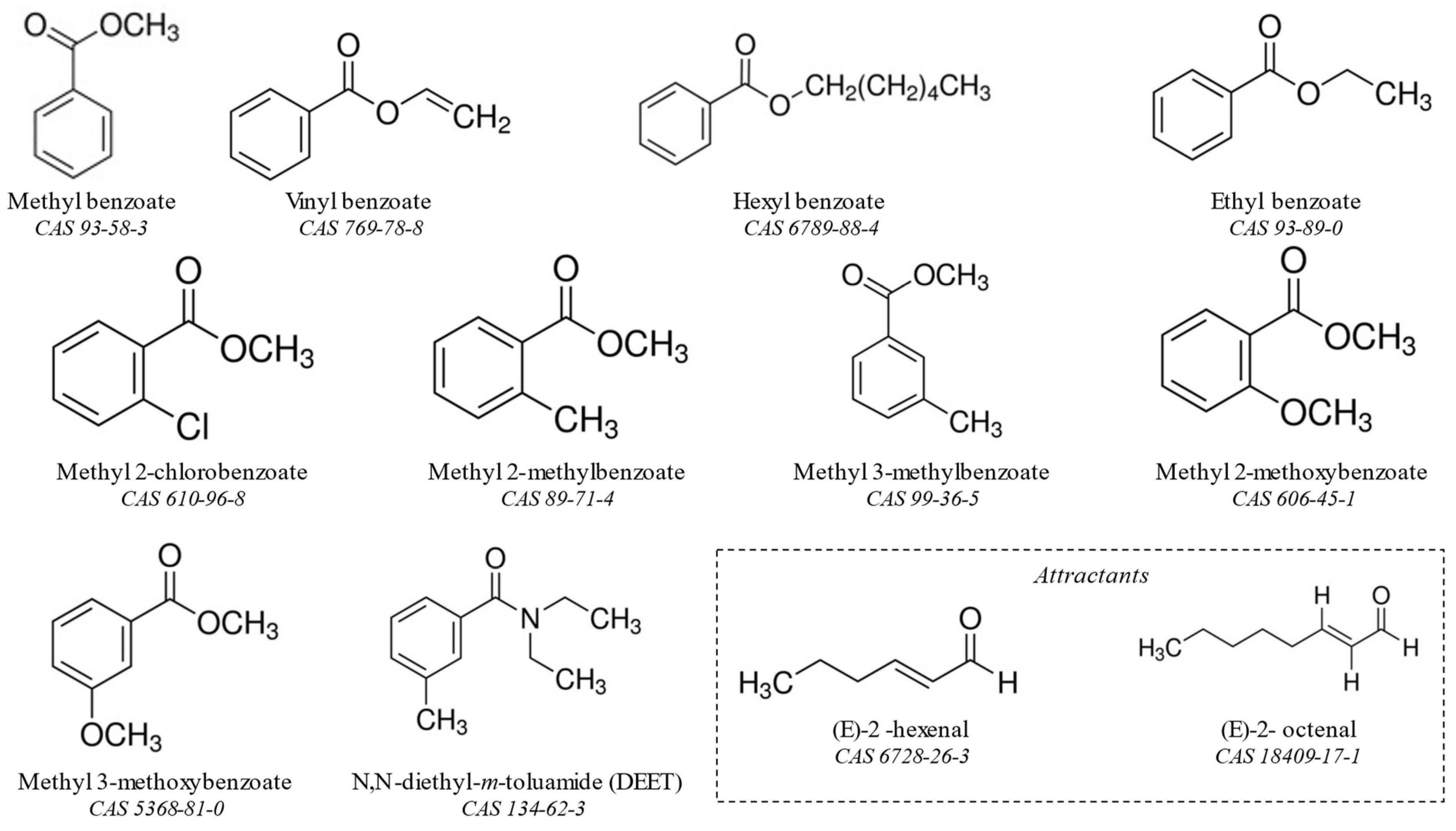
2.2. Chemicals
2.3. Experimental Setup
2.3.1. Repellency Longevity
2.3.2. Repellent Activity in Presence of Aggregation Pheromone Components
2.3.3. Repellent Activity in Presence of Food Source
2.4. Analysis
2.4.1. Repellency Longevity
2.4.2. Repellent Activity in Presence of Aggregation Pheromone Components
2.4.3. Repellent Activity in Presence of Food Source
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potter, M.F. The history of bed bug management- With lessons from the past. Am. Entomol. 2011, 57, 14–25. [Google Scholar] [CrossRef]
- Goddard, J.; De Shazo, R. Psychological effects of bed bug attacks (Cimex lectularius L.). Am. J. Med. 2012, 125, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.L.; Leffler, K. Bedbug infestations in the news: A picture of an emerging public health problem in the United States. J. Environ. Health 2008, 70, 24–27. [Google Scholar]
- Boase, C. Bedbugs-back from the brink. Pestic. Outlook 2001, 12, 159–162. [Google Scholar] [CrossRef]
- Hwang, S.W.; Svoboda, T.J.; De Jong, I.J.; Kabasele, K.J.; Gogosis, E. Bed bug infestations in an urban environment. Emerg. Infect. Dis. 2005, 11, 533–538. [Google Scholar] [CrossRef]
- Pereira, R.M.; Koehler, P.G.; Pfiester, M.; Walker, W. Lethal effects of heat and use of localized heat treatment for control of bed bug infestations. J. Econ. Entomol. 2009, 102, 1182–1188. [Google Scholar] [CrossRef]
- Kells, S.A.; Goblirsch, M.J. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011, 2, 412–422. [Google Scholar] [CrossRef]
- Koganemaru, R.; Miller, D.M. The bed bug problem: Past, present, and future control methods. Pestic. Biochem. Physiol. 2013, 106, 177–189. [Google Scholar] [CrossRef]
- Romero, A.; Sutherland, A.M.; Gouge, D.H.; Spafford, H.; Nair, S.; Lewis, V.; Choe, D.H.; Li, S.; Young, D. Pest management strategies for bed bugs (Hemiptera: Cimicidae) in multiunit housing: A literature review on field studies. J. Integr. Pest Manag. 2017, 8, 13. [Google Scholar] [CrossRef]
- Ashbrook, A.R.; Scharf, M.E.; Bennett, G.W.; Gondhalekar, A.D. Bed bugs (Cimex lectularius L.) exhibit limited ability to develop heat resistance. PLoS ONE 2019, 14, e0211677. [Google Scholar] [CrossRef]
- Wang, C.; Eiden, A.; Singh, N.; Zha, C.; Cooper, R.; Wang, D. Dynamics of bed bug infestations in three low-income housing communities with various bed bug management programs. Pest Manag. Sci. 2018, 74, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Gibb, T.; Bennett, G.W. Evaluation of two least toxic integrated pest management programs for managing bed bugs (Heteroptera: Cimicidae) with discussion of a bed bug intercepting device. J. Med. Entomol. 2009, 46, 566–571. [Google Scholar] [CrossRef] [PubMed]
- HomeAdvisor, How Much Do Bed Bug Exterminators Cost? 2002. Available online: https://www.homeadvisor.com/cost/environmental-safety/bed-bug-treatment/ (accessed on 28 September 2022).
- Liu, B.; Pennington-Gray, L. Bed bugs bite the hospitality industry? A framing analysis of bed bug news coverage. Tour. Manag. 2015, 48, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.J.; Kim, H.; Pennington-Gray, L. Responding to the bed bug crisis in social media. Int. J. Hosp. Manag. 2015, 47, 76–84. [Google Scholar] [CrossRef]
- Penn, J.; Hu, W. Reports of bed bugs on hotel selection: A choice experiment. Int. J. Hosp. Manag. 2020, 89, 102568. [Google Scholar] [CrossRef]
- Boase, C. Bed Bugs and the Law in the United Kingdom. In Advances in the Biology and Management of Modern Bed Bugs; Doggett, S.L., Miller, D.M., Lee, C.-Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 397–402. [Google Scholar]
- Cains, T.; Lilly, D.G.; Doggett, S.L. Bed bugs and the jaw in Australia. In Advances in the Biology and Management of Modern Bed Bugs; Doggett, S.L., Miller, D.M., Lee, C.-Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 403–408. [Google Scholar]
- Ho-Ohara, A.; Lee, C.-Y. Bed bugs and the law in Asia. In Advances in the Biology and Management of Modern Bed Bugs; Doggett, S.L., Miller, D.M., Lee, C.-Y., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 409–412. [Google Scholar]
- Lipman, J.; Miller, D.M. Bed bugs and the law in the USA. In Advances in the Biology and Management of Modern Bed Bugs; Doggett, S.L., Miller, D.M., Lee, C.-Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 385–396. [Google Scholar]
- Doggett, S.L.; Miller, D.M.; Vail, K.; Wilson, M.S. Fiscal impacts. In Advances in the Biology and Management of Modern Bed Bugs; Doggett, S.L., Miller, D.M., Lee, C.-Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 139–148. [Google Scholar]
- Cooper, R.A.; Wang, C.; Singh, N. Evaluation of a model community-wide bed bug management program in affordable housing. Pest Manag. Sci. 2016, 72, 45–56. [Google Scholar] [CrossRef]
- Ryan, E.T. Illness after international travel. N. Engl. J. Med. 2002, 347, 505–516. [Google Scholar] [CrossRef]
- Vasievich, M.P.; Villarreal, J.D.M.; Tomecki, K.J. Got the travel bug? A review of common infections, infestations, bites, and stings among returning travelers. Am. J. Chin. Dematol. 2016, 17, 451–462. [Google Scholar] [CrossRef]
- Wang, C.; Lu, L.; Zhang, A.; Chaofeng, L. Repellency of selected chemicals against the bed bug (Hemiptra: Cimicidae). J. Econ. Entomol. 2013, 106, 2522–2529. [Google Scholar] [CrossRef]
- Soukand, R.; Kalle, R.; Svanberg, I. Uninvited guests: Traditional insect repellents in Estonia used against the clothes moth Tineola bisselliella, human flea Pulex irritans and bedbug Cimex lectularius. J. Insect Sci. 2010, 10, 150. [Google Scholar] [CrossRef]
- Liu, F.; Haynes, K.F.; Appel, A.G.; Liu, N. Antennal olfactory sensilla responses to insect chemical repellents in the common bed bug, Cimex lectularius. J. Chem. Ecol. 2014, 40, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Wang, C.; Cooper, R. Potential of essential oil-based pesticides and detergents for bed bug control. J. Econ. Entomol. 2014, 107, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.; Ferrandino, F.J.; Vasil, M.P.; Bedoukian, R.H.; Maher, M.; McKenzie, K. Repellency of naturally occurring or related compounds, DEET, and para-menthane-3,8-diol to bed bugs (Hemiptera: Cimicidae). J. Med. Entomol. 2018, 55, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Kruger, A.; Schmolz, E.; Vander Pan, A. Methods for testing repellents against bed bugs (Hemiptera: Cimicidae). J. Econ. Entomol. 2021, 114, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Klun, J.A.; Wang, S.; Carroll, J.F.; Debboun, M. Isolongifolenone: A novel sesquiterpene repellent of ticks and mosquitoes. J. Med. Entomol. 2009, 46, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.G.E.; Field, L.M.; Williamson, M.S. The re-emergence of the bed bug as a nuisance pest: Implications of resistance to the pyrethroid insecticides. Med. Vet. Entomol. 2012, 26, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, A. A floral fragrance, methyl benzoate, is an efficient green pesticide. Sci. Rep. 2017, 7, 42168. [Google Scholar]
- Feng, Y.; Chen, J.; Zhang, A. Commercially available natural benzyl esters and their synthetic analogs exhibit different toxicities against insect pests. Sci. Rep. 2018, 8, 7902. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.H.; Gao, J.; Zhang, Y.J.; Li, X.; Zhou, J.J.; Liang, P.; Gao, X.W.; Gu, S.H. Plant volatile compound methyl benzoate is highly effective against Spodoptera frugiperda and safe to non-target organisms as an eco-friendly botanical-insecticide. Ecotoxicol. Environ. Saf. 2022, 245, 114101. [Google Scholar] [CrossRef]
- Chen, J.; Rashid, T.; Feng, G.; Feng, Y.; Zhang, A.; Grodowitz, M.J. Insecticidal activity of methyl benzoate analogs against red imported fire ants, Solenopsis invicta (Hymenoptera: Formicidae). J. Econ. Entomol. 2019, 112, 691–698. [Google Scholar] [CrossRef]
- Morrison, W.R.; Larson, N.L.; Brabec, D.; Zhang, A.; Mahroof, R. Methyl benzoate as a putative alternative, environmentally friendly fumigant for the control of stored product Insects. J. Econ. Entomol. 2019, 112, 2458–2468. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.B.; Feng, Y.; Zhang, A. Methyl benzoate fumigation for control of post-harvest pests and its effects on apple quality. J. Appl. Entomol. 2020, 144, 191–200. [Google Scholar] [CrossRef]
- Larson, N.; Strickland, J.; Zhang, A.; Feldlaufer, M. Behavioral activity of methyl benzoate against bed bug adults. J. Entomol. Sci. 2020, 55, 344–349. [Google Scholar] [CrossRef]
- Moore, D.J.; Miller, D.M. Laboratory evaluations of insecticide product efficacy for control of Cimex lectularius. J. Econ. Entomol. 2006, 99, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Domingue, M.J.; Kramer, M.; Feldlaufer, M.F. Sexual dimorphism of arrestment and gregariousness in the bed bug (Cimex lectularius) in response to cuticular extracts from nymphal exuviae. Physiol. Entomol. 2010, 35, 203–213. [Google Scholar] [CrossRef]
- Gries, R.; Britton, R.; Holmes, M.; Zhai, H.; Draper, J.; Gries, G. Bed bug aggregation pheromone finally identified. Angew. Chem. Int. Ed. 2015, 54, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.; Ferrandino, F.J.; Vasil, M.P.; Bedoukian, R.H.; Maher, M.; McKenzie, K.; Johnson, R. Relatively small quantities of CO2, ammonium bicarbonate, and a blend of (E)-2-hexenal plus (E)-2-octenal attract bed bugs (Hemiptera: Cimicidae). J. Med. Entomol. 2018, 54, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Siljander, E.; Gries, R.; Khaskin, G.; Gries, G. Identification of the airborne aggregation pheromone of the common bed bug, Cimex lectularius. J. Chem. Ecol. 2008, 34, 708–718. [Google Scholar] [CrossRef]
- Liu, F.; Xiong, C.; Liu, N. Chemoreception to aggregation pheromones in the common bed bug, Cimex lectularius. Insect Biochem. Mol. Biol. 2017, 82, 62–73. [Google Scholar] [CrossRef]
- Wong, C.; Crystal, K.; Coats, J. Three molecules found in rosemary or nutmeg essential oils repel ticks (Dermacentor variabilis) more effectively than DEET in a no-human assay. Pest Manag. Sci. 2021, 77, 1348–1354. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, S.; Rao, K.M. Comparative activity of three repellents against bedbugs Cimex hemipterus (Fabr.). Indian J. Med. Res. 1995, 102, 20–23. [Google Scholar] [PubMed]
- Leal, W.S. The enigmatic reception of DEET—The gold standard of insect repellents. Curr. Opin. Insect Sci. 2014, 6, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Vassena, C.V.; Caceres, M.; Santo-Orihuela, P.L. Pyrethroid resistance associated with a decreased DEET repellency in the common bed bug (Hemiptera: Cimicidae). J. Econ. Entomol. 2019, 112, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- FDA, CFR—Code of Federal Regulations. Title 21—Food and Drugs, 2022 U.S. Food and Drug Administration. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=172.515 (accessed on 28 September 2022).
- European-Union, Food Flavourings, 2022 European Commission Health and Consumers. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:sa0006 (accessed on 28 September 2022).
- Fradin, M.S.; Day, J.F. Comparative efficacy of insect repellents against mosquito bites. N. Engl. J. Med. 2002, 347, 13–18. [Google Scholar] [CrossRef]






| 0 h | 24 h | 7 Days | 14 Days | 21 Days | 28 Days | |
|---|---|---|---|---|---|---|
| Blank | NSR p = 0.1160 | NSR p = 0.1877 | NSR p = 0.1572 | NT | NT | NT |
| HB | NSR p = 0.3312 | NSR p = 0.5123 | NSR p = 0.1893 | NT | NT | NT |
| EB | R p = 0.0005 | NSR p = 0.6028 | NSR p = 0.1247 | NT | NT | NT |
| M2MB | R p = 0.0007 | NSR p = 0.5266 | NSR p = 0.4672 | NT | NT | NT |
| VB | R p < 0.0001 | R p = 0.0174 | NSR p = 0.3912 | NT | NT | NT |
| M2CB | R p < 0.0001 | R p < 0.0001 | NSR p = 0.7350 | NT | NT | NT |
| M3MB | R p < 0.0001 | R p < 0.0001 | NSR p = 0.7953 | NT | NT | NT |
| M3MOB | R p = 0.0026 | NSR p = 0.0928 | R p < 0.0001 | NSR p = 0.8232 | NT | NT |
| M2MOB | R p = 0.0002 | R p = 0.0001 | R p = 0.0421 | R p = 0.0022 | R p = 0.0081 | NSR p = 0.7433 |
| DEET | NSR p = 0.2705 | R p = 0.0001 | R p = 0.0006 | R p = 0.0397 | R p = 0.0002 | R p = 0.0067 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strickland, J.; Larson, N.R.; Feldlaufer, M.; Zhang, A. Characterizing Repellencies of Methyl Benzoate and Its Analogs against the Common Bed Bug, Cimex lectularius. Insects 2022, 13, 1060. https://doi.org/10.3390/insects13111060
Strickland J, Larson NR, Feldlaufer M, Zhang A. Characterizing Repellencies of Methyl Benzoate and Its Analogs against the Common Bed Bug, Cimex lectularius. Insects. 2022; 13(11):1060. https://doi.org/10.3390/insects13111060
Chicago/Turabian StyleStrickland, Jaime, Nicholas R. Larson, Mark Feldlaufer, and Aijun Zhang. 2022. "Characterizing Repellencies of Methyl Benzoate and Its Analogs against the Common Bed Bug, Cimex lectularius" Insects 13, no. 11: 1060. https://doi.org/10.3390/insects13111060
APA StyleStrickland, J., Larson, N. R., Feldlaufer, M., & Zhang, A. (2022). Characterizing Repellencies of Methyl Benzoate and Its Analogs against the Common Bed Bug, Cimex lectularius. Insects, 13(11), 1060. https://doi.org/10.3390/insects13111060






