Laboratory Evaluation and Field Feasibility of Micro-Encapsulated Insecticide Effect on Rhodnius prolixus and Triatoma dimidiata Mortality in Rural Households in Boyacá, Colombia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
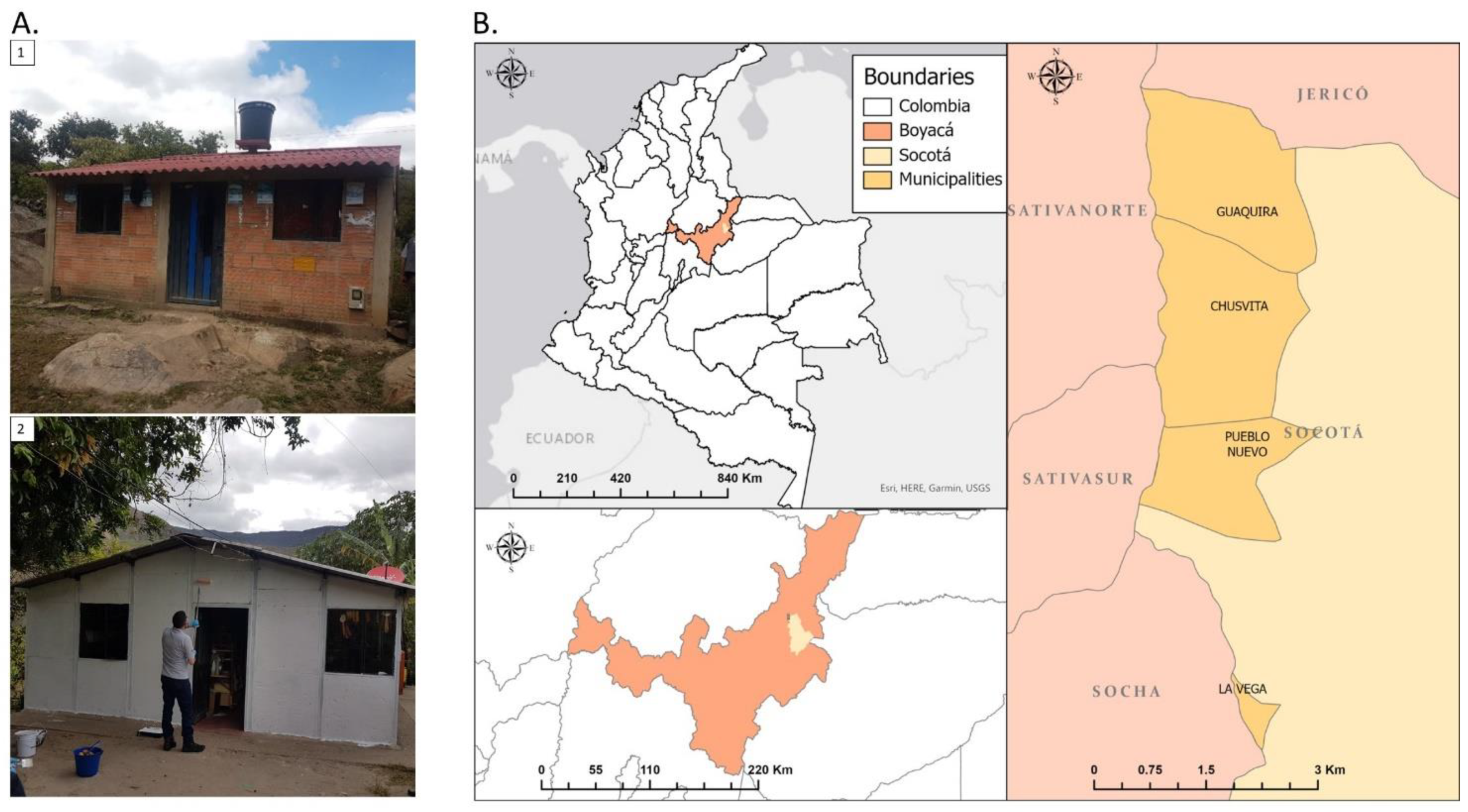
2.1. Study Area
2.2. Insects
2.3. Insecticide Paint
2.4. Laboratory Bioassay
2.5. Field Application
2.6. Ethics Statement
3. Results
3.1. Laboratory Bioassay
3.2. Field Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Echeverria, L.E.; Morillo, C.A. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am. 2019, 33, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Olivera, M.J.; Fory, J.A.; Porras, J.F.; Buitrago, G. Prevalence of Chagas disease in Colombia: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0210156. [Google Scholar] [CrossRef] [PubMed]
- Moncayo, Á.; Silveira, A.C. 4—Current epidemiological trends of Chagas disease in Latin America and future challenges in epidemiology, surveillance, and health policy. Mem. Inst. Oswaldo Cruz. 2009, 104 (Suppl. 1), 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivera, M.J.; Buitrago, G. Economic costs of Chagas disease in Colombia in 2017: A social perspective. Int. J. Infect. Dis. 2020, 91, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Olivera, M.J.; Porras Villamil, J.F.; Toquica Gahona, C.C.; Rodríguez Hernández, J.M. Barriers to Diagnosis Access for Chagas Disease in Colombia. J. Parasitol. Res. 2018, 2018, 4940796. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.C. Chagas’ disease and its control in Latin America: An analysis of possibilities. Cad Saude Publica 1993, 9, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Yamagata, Y.; Nakagawa, J. Control of Chagas disease. Adv. Parasitol. 2006, 61, 129–165. [Google Scholar] [CrossRef]
- Organizacion Panamericana de la Salud. Iniciativa de Salud del Cono del Sur (INCOSUR): Comisión Intergubernamental del Cono Sur para la eliminacion del Triatoma infestants y la interrupcion de la Tripanosomiasis Américana Transfusional: Quinta Evaluación del Programa Nacional de Control de la Enfermedad de Chagas de Chile/Fifth Evaluation of National Program of Chagas Disease Control of Chile. In Proceedings of the Iniciativa de la Salud del Cono del Sur (INCOSUR), Santiago de Chile, Chile, 1–6 November 1999. [Google Scholar]
- Salvatella, R. Andean subregional Chagas disease area and the Andean initiative of Chagas disease. Mem. Inst. Oswaldo Cruz 2007, 102, 39–40. [Google Scholar] [CrossRef] [Green Version]
- Schofield, C.J.; Dujardin, J.P. Chagas disease vector control in Central America. Parasitol. Today 1997, 13, 141–144. [Google Scholar] [CrossRef]
- Aguilar, H.M.; Abad-Franch, F.; Dias, J.C.; Junqueira, A.C.; Coura, J.R. Chagas disease in the Amazon region. Mem. Inst. Oswaldo Cruz 2007, 102 (Suppl. 1), 47–56. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). Guidelines for the Diagnosis and Treatment of Chagas Disease; Pan American Health Organization: Washington, DC, USA, 2019; p. 176. [Google Scholar]
- Pan American Health Organization. Chagas Disease. Available online: https://www.paho.org/en/topics/chagas-disease (accessed on 12 July 2022).
- Hashimoto, K.; Schofield, C.J. Elimination of Rhodnius prolixus in Central America. Parasit. Vectors 2012, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Cantillo-Barraza, O.; Medina, M.; Zuluaga, S.; Valverde, C.; Motta, C.; Ladino, A.; Osorio, M.I.; Jaimes-Dueñez, J.; Triana-Chávez, O. Eco-epidemiological study reveals the importance of Triatoma dimidiata in the Trypanosoma cruzi transmission, in a municipality certified without transmission by Rhodnius prolixus in Colombia. Acta Trop. 2020, 209, 105550. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Zuluaga, S.; Martínez, M.F.; Bermúdez, J.C.; Hernández, C.; Beltrán, V.; Velásquez-Ortiz, N.; Muñoz, M.; Ramírez, J.D.; Triana, O.; et al. Interrogating the transmission dynamics of Trypanosoma cruzi (Trypanosomatida, Trypanosomatidae) by Triatoma venosa (Hemiptera: Reduviidae) after the elimination of vector transmission by Rhodnius prolixus in Boyacá eastern Colombia. Front. Cell. Infect. Microbiol. 2022, 12, 998202. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Cordon-Rosales, C.; Trampe, R.; Kawabata, M. Impact of single and multiple residual sprayings of pyrethroid insecticides against Triatoma dimidiata (Reduviiade; Triatominae), the principal vector of Chagas disease in Jutiapa, Guatemala. Am. J. Trop. Med. Hyg. 2006, 75, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Palacio, A.; Triana, O. Molecular evidence of demographic expansion of the chagas disease vector Triatoma dimidiata (Hemiptera, Reduviidae, Triatominae) in Colombia. PLoS Negl. Trop. Dis. 2014, 8, e2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurgel-Gonçalves, R.; Galvão, C.; Costa, J.; Peterson, A.T. Geographic distribution of chagas disease vectors in Brazil based on ecological niche modeling. J. Trop. Med. 2012, 2012, 705326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guhl, F.; Aguilera, G.; Pinto, N.; Vergara, D. [Updated geographical distribution and ecoepidemiology of the triatomine fauna (Reduviidae: Triatominae) in Colombia]. Biomedica 2007, 27 (Suppl. 1), 143–162. [Google Scholar] [CrossRef] [Green Version]
- Velásquez-Ortiz, N.; Hernández, C.; Cantillo-Barraza, O.; Medina, M.; Medina-Alfonso, M.; Suescún-Carrero, S.; Muñoz, M.; Vega, L.; Castañeda, S.; Cruz-Saavedra, L. Estimating the genetic structure of Triatoma dimidiata (Hemiptera: Reduviidae) and the transmission dynamics of Trypanosoma cruzi in Boyacá, eastern Colombia. PLOS Negl. Trop. Dis. 2022, 16, e0010534. [Google Scholar] [CrossRef]
- Paez Restrepo, V.; Rubio Sandoval, C.; Ardila Roldan, S.C.; Tibaduiza Zacipa, T.E. Capítulo 4. Vigilancia De Triatominae (Hemiptera: Reduviidae) En Boyacá. In Vigilancia de Triatominae (Hemiptera: Reduviidae) en Colombia, 1st ed.; Parra-Henao, G.F.M., M. Angulo Silva, V., Eds.; Sic Editorial: Bucaramanga, Colombia, 2015; pp. 27–37. [Google Scholar]
- Pan American Health Organization. Métodos de Vigilancia Entomológica y Control de los Principales Vectores en las Américas; PAHO: Washington, DC, USA, 2021; p. 122. [Google Scholar]
- Calderón, J.M.; Fuya, P.; Santacoloma, L.; González, C. Deltamethrin resistance in Chagas disease vectors colonizing oil palm plantations: Implications for vector control strategies in a public health-agriculture interface. Parasites Vectors 2020, 13, 163. [Google Scholar] [CrossRef] [Green Version]
- Santo-Orihuela, P.L.; Vassena, C.V.; Carvajal, G.; Clark, E.; Menacho, S.; Bozo, R.; Gilman, R.H.; Bern, C.; Marcet, P.L. Toxicological, enzymatic, and molecular assessment of the insecticide susceptibility profile of Triatoma infestans (Hemiptera: Reduviidae, Triatominae) populations from rural communities of Santa Cruz, Bolivia. J. Med. Entomol. 2017, 54, 187–195. [Google Scholar] [CrossRef]
- Granada, Y.; Mejía-Jaramillo, A.M.; Zuluaga, S.; Triana-Chávez, O. Molecular surveillance of resistance to pyrethroids insecticides in Colombian Aedes aegypti populations. PLoS Negl. Trop. Dis. 2021, 15, e0010001. [Google Scholar] [CrossRef] [PubMed]
- Maia, P.C.R.; La Corte, R.; Pires, L.B.; Banfield, L.; Logan, J.G.; Lima-Camara, T.N. Increased Repellent Effect of DEET on Aedes aegypti (Diptera: Culicidae) Field Population. J. Med. Entomol. 2022, 59, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Palomino, M.; Pinto, J.; Yañez, P.; Cornelio, A.; Dias, L.; Amorim, Q.; Martins, A.J.; Lenhart, A.; Lima, J.B.P. First national-scale evaluation of temephos resistance in Aedes aegypti in Peru. Parasit Vectors 2022, 15, 254. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, G.C.; Vinãs, P.A.; Rosa, A.C.; Diotaiuti, L. History of insecticide resistance of Triatominae vectors. Rev. Soc. Bras. Med. Trop. 2015, 48, 380–389. [Google Scholar] [CrossRef] [Green Version]
- Mougabure-Cueto, G.; Picollo, M.I. Insecticide resistance in vector Chagas disease: Evolution, mechanisms and management. Acta Trop. 2015, 149, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Maloney, K.M.; Ancca-Juarez, J.; Salazar, R.; Borrini-Mayori, K.; Niemierko, M.; Yukich, J.O.; Naquira, C.; Keating, J.A.; Levy, M.Z. Comparison of insecticidal paint and deltamethrin against Triatoma infestans (Hemiptera: Reduviidae) feeding and mortality in simulated natural conditions. J. Vector Ecol. 2013, 38, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Llácer, E.; Dembilio, O.; Jacas, J.A. Evaluation of the efficacy of an insecticidal paint based on chlorpyrifos and pyriproxyfen in a microencapsulated formulation against Rhynchophorus ferrugineus (Coleoptera: Curculionidae). J. Econ. Entomol. 2010, 103, 402–408. [Google Scholar] [CrossRef]
- Mosqueira, B.; Chabi, J.; Chandre, F.; Akogbeto, M.; Hougard, J.M.; Carnevale, P.; Mas-Coma, S. Efficacy of an insecticide paint against malaria vectors and nuisance in West Africa—Part 2: Field evaluation. Malar. J 2010, 9, 341. [Google Scholar] [CrossRef] [Green Version]
- Alarico, A.G.; Romero, N.; Hernández, L.; Catalá, S.; Gorla, D. Residual effect of a micro-encapsulated formulation of organophosphates and piriproxifen on the mortality of deltamethrin resistant Triatoma infestans populations in rural houses of the Bolivian Chaco region. Mem. Inst. Oswaldo Cruz 2010, 105, 752–756. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.C.P.; Jemmio, A. Sobre uma pintura inseticida para o controle de Triatoma infestans, na Bolívia. Rev. Soc. Bras. Med. Trop. 2008, 41, 79–81. [Google Scholar] [CrossRef]
- Chuit, R.; Paulone, I.; Wisnivesky-Colli, C.; Bo, R.; Perez, A.; Sosa-Stani, S.; Segura, E. Result of a first step toward community-based surveillance of transmission of Chagas’ disease with appropriate technology in rural areas. Am. J. Trop. Med. Hyg. 1992, 46, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.; Barbeau, B.; McCaffrey, K.; Gruninger, R.; Eason, J.; Burnett, C.; Dunn, A.; Dimond, M.; Harbour, J.; Rossi, A.; et al. Human Rabies—Utah, 2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 121–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amelotti, I.; Catalá, S.S.; Gorla, D.E. Experimental evaluation of insecticidal paints against Triatoma infestans (Hemiptera: Reduviidae), under natural climatic conditions. Parasites Vectors 2009, 2, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorla, D.E.; Vargas Ortiz, R.; Catalá, S.S. Control of rural house infestation by Triatoma infestans in the Bolivian Chaco using a microencapsulated insecticide formulation. Parasites Vectors 2015, 8, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra-Henao, G.; Angulo, V.M.; Osorio, L.; Jaramillo-O, N. Geographic distribution and ecology of Triatoma dimidiata (Hemiptera: Reduviidae) in Colombia. J. Med. Entomol. 2016, 53, 122–129. [Google Scholar] [CrossRef]
- Schiøler, K.L.; Alifrangis, M.; Kitron, U.; Konradsen, F. Insecticidal paints: A realistic approach to vector control? PLoS Negl. Trop. Dis. 2016, 10, e0004518. [Google Scholar] [CrossRef] [Green Version]
- Hites, R.A. The Rise and Fall of Chlorpyrifos in the United States. Environ. Sci. Technol. 2021, 55, 1354–1358. [Google Scholar] [CrossRef]
- Simón-Moya, V.; Rodríguez-García, M. Introduction and Empirical Framework. In The Emergence of Social Entrepreneurship; Springer: Berlin/Heidelberg, Germany, 2021; pp. 135–143. [Google Scholar]
- Parra-Henao, G.; Quirós-Gómez, O.; Jaramillo-O, N.; Cardona, Á.S. Environmental determinants of the distribution of Chagas disease vector Triatoma dimidiata in Colombia. Am. J. Trop. Med. Hyg. 2016, 94, 767. [Google Scholar] [CrossRef] [Green Version]
- Ferral, J.; Chavez-Nuñez, L.; Euan-Garcia, M.; Ramirez-Sierra, M.J.; Najera-Vazquez, M.R.; Dumonteil, E. Comparative field trial of alternative vector control strategies for non-domiciliated Triatoma dimidiata. Am. J. Trop. Med. Hyg. 2010, 82, 60. [Google Scholar] [CrossRef]
- Avila Montes, G.A.; Ponce, C.; Ponce, E.; Martínez Hernández, M.; Flores, M. Insecticidal paint and fumigant canisters for Chagas’ disease control: Community acceptance in Honduras. Rev. Panam. Salud Publica 1999, 6, 311–320. [Google Scholar] [CrossRef]
- Buttenheim, A.M.; Levy, M.Z.; Castillo-Neyra, R.; McGuire, M.; Toledo Vizcarra, A.M.; Mollesaca Riveros, L.M.; Meza, J.; Borrini-Mayori, K.; Naquira, C.; Behrman, J.; et al. A behavioral design approach to improving a Chagas disease vector control campaign in Peru. BMC Public Health 2019, 19, 1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lardeux, F.; Depickère, S.; Aliaga, C.; Chavez, T.; Zambrana, L. Experimental control of Triatoma infestans in poor rural villages of Bolivia through community participation. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Castro-Arroyave, D.; Monroy, M.C.; Irurita, M.I. Integrated vector control of Chagas disease in Guatemala: A case of social innovation in health. Infect. Dis. Poverty 2020, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Sarquis, O.; Sposina, R.; de Oliveira, T.G.; Mac Cord, J.R.; Cabello, P.H.; Borges-Pereira, J.; Lima, M.M. Aspects of peridomiciliary ecotopes in rural areas of northeastern Brazil associated to triatomine (Hemiptera, Reduviidae) infestation, vectors of chagas disease. Mem. Inst. Oswaldo Cruz 2006, 101, 143–147. [Google Scholar] [CrossRef] [PubMed]

| a. Rhodnius prolixus | |||||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | |||||
| n | 24 h 1 | 48 h | 24 h 1 | 48 h | 24 h 1 | 48 h | |
| Control | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Replica 1 | 10 | 100 | 100 | 100 | 100 | 100 | 100 |
| Replica 2 | 10 | 100 | 100 | 100 | 100 | 100 | 100 |
| Replica 3 | 10 | 100 | 100 | 100 | 100 | 100 | 100 |
| b. Triatoma dimidiata | |||||||
| T1 | T2 | T3 | |||||
| n | 24 h 1 | 48 h | 24 h 1 | 48 h | 24 h 1 | 48 h | |
| Control | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Replica 1 | 10 | 80 | 100 | 90 | 100 | 100 | 100 |
| Replica 2 | 10 | 100 | 100 | 100 | 100 | 100 | 100 |
| Replica 3 | 10 | 100 | 100 | 100 | 100 | 100 | 100 |
| Description | N * (%) | |
|---|---|---|
| Wall materials | Adobe | 11(52.4) |
| Brick | 10 (47.6) | |
| Wood | 2 (9.5) | |
| Mud and Bahareque | 1 (4.8) | |
| Roof materials | Zinc | 10 (47.6) |
| Mud | 16 (76.2) | |
| Asbestos cement | 6 (28.6) | |
| Floor materials | Ground/soil | 10 (47.6) |
| Cement | 19 (90.5) | |
| Tiles | 4 (19.1) | |
| Wood | 1 (4.8) | |
| Features surrounding the household | Chicken Coop | 19 (90.5) |
| Barn | 3 (14.3) | |
| Pigsty | 6 (28.6) | |
| Oven | 10 (47.6) | |
| Mill | 6 (28.6) | |
| Wood pile | 20 (95.2) | |
| Rock pile | 19 (90.5) | |
| Other | Any sight of wall cracks | 15 (71.4) |
| Average number of bulbs inside the home [Mean ± SD [37]] | 3.76 ± 1.48 | |
| Average number of bulbs outside the home [Mean ± SD] | 1.14 ± 0.91 | |
| Domestic animal presence | Homes with animals | 20 (95.2) |
| Dogs | 19 (90.5) | |
| Chickens | 19 (90.5) | |
| Pigs | 8 (38.1) | |
| Cows | 15 (71.4) | |
| Horses | 9 (42.9) | |
| Cats | 17 (81.0) | |
| Domestic birds | 8 (38.1) | |
| Domestic rabbits | 1 (4.8) | |
| Have seen wild animal in the surroundings | Opossum | 20 (95.2) |
| Rodent | 13 (61.9) | |
| Bat | 5 (23.8) | |
| Pigeon | 8 (38.1) | |
| Rabbit | 2 (9.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gual-Gonzalez, L.; Medina, M.; Valverde-Castro, C.; Beltrán, V.; Caro, R.; Triana-Chávez, O.; Nolan, M.S.; Cantillo-Barraza, O. Laboratory Evaluation and Field Feasibility of Micro-Encapsulated Insecticide Effect on Rhodnius prolixus and Triatoma dimidiata Mortality in Rural Households in Boyacá, Colombia. Insects 2022, 13, 1061. https://doi.org/10.3390/insects13111061
Gual-Gonzalez L, Medina M, Valverde-Castro C, Beltrán V, Caro R, Triana-Chávez O, Nolan MS, Cantillo-Barraza O. Laboratory Evaluation and Field Feasibility of Micro-Encapsulated Insecticide Effect on Rhodnius prolixus and Triatoma dimidiata Mortality in Rural Households in Boyacá, Colombia. Insects. 2022; 13(11):1061. https://doi.org/10.3390/insects13111061
Chicago/Turabian StyleGual-Gonzalez, Lídia, Manuel Medina, César Valverde-Castro, Virgilio Beltrán, Rodrigo Caro, Omar Triana-Chávez, Melissa S. Nolan, and Omar Cantillo-Barraza. 2022. "Laboratory Evaluation and Field Feasibility of Micro-Encapsulated Insecticide Effect on Rhodnius prolixus and Triatoma dimidiata Mortality in Rural Households in Boyacá, Colombia" Insects 13, no. 11: 1061. https://doi.org/10.3390/insects13111061





