Electrophysiological and Behavioral Responses of Apis mellifera and Bombusterrestris to Melon Flower Volatiles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Plants
2.3. Identification of Volatile Compounds
2.4. EAG Recording
2.5. Behavior Tests
2.6. Statistical Analysis
3. Results
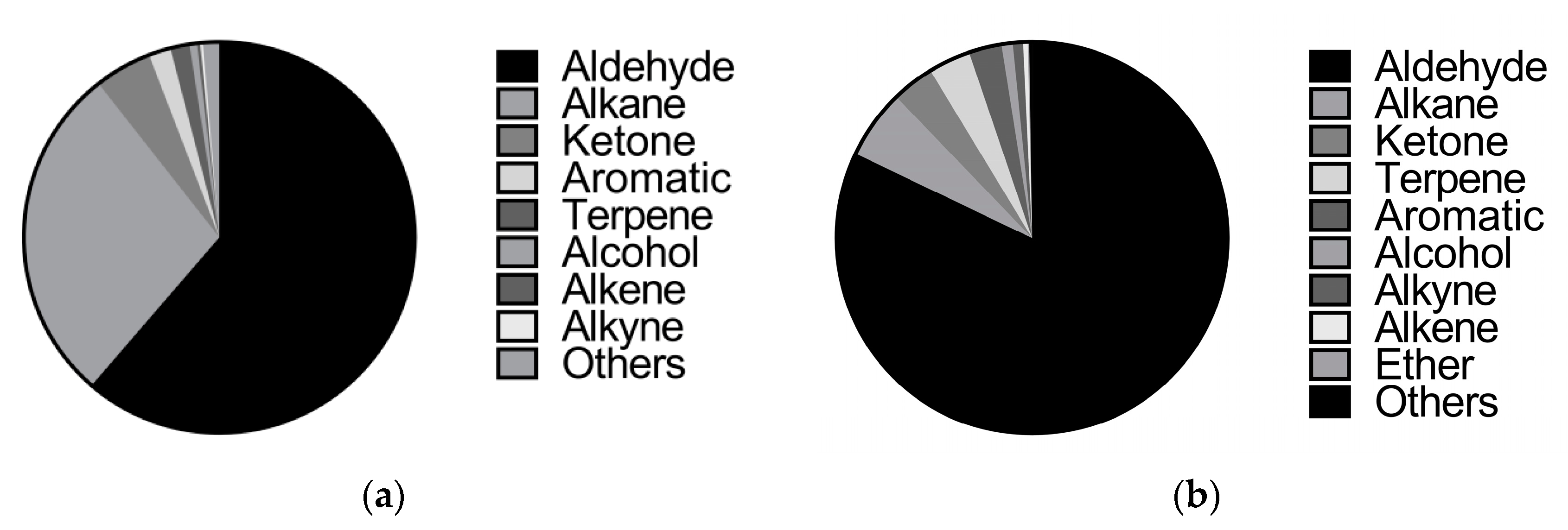
3.1. Identification of Volatile Compounds
3.2. EAG Response of Bees to Volatiles
3.2.1. EAG Response of A. mellifera
3.2.2. EAG Response of B. terrestris
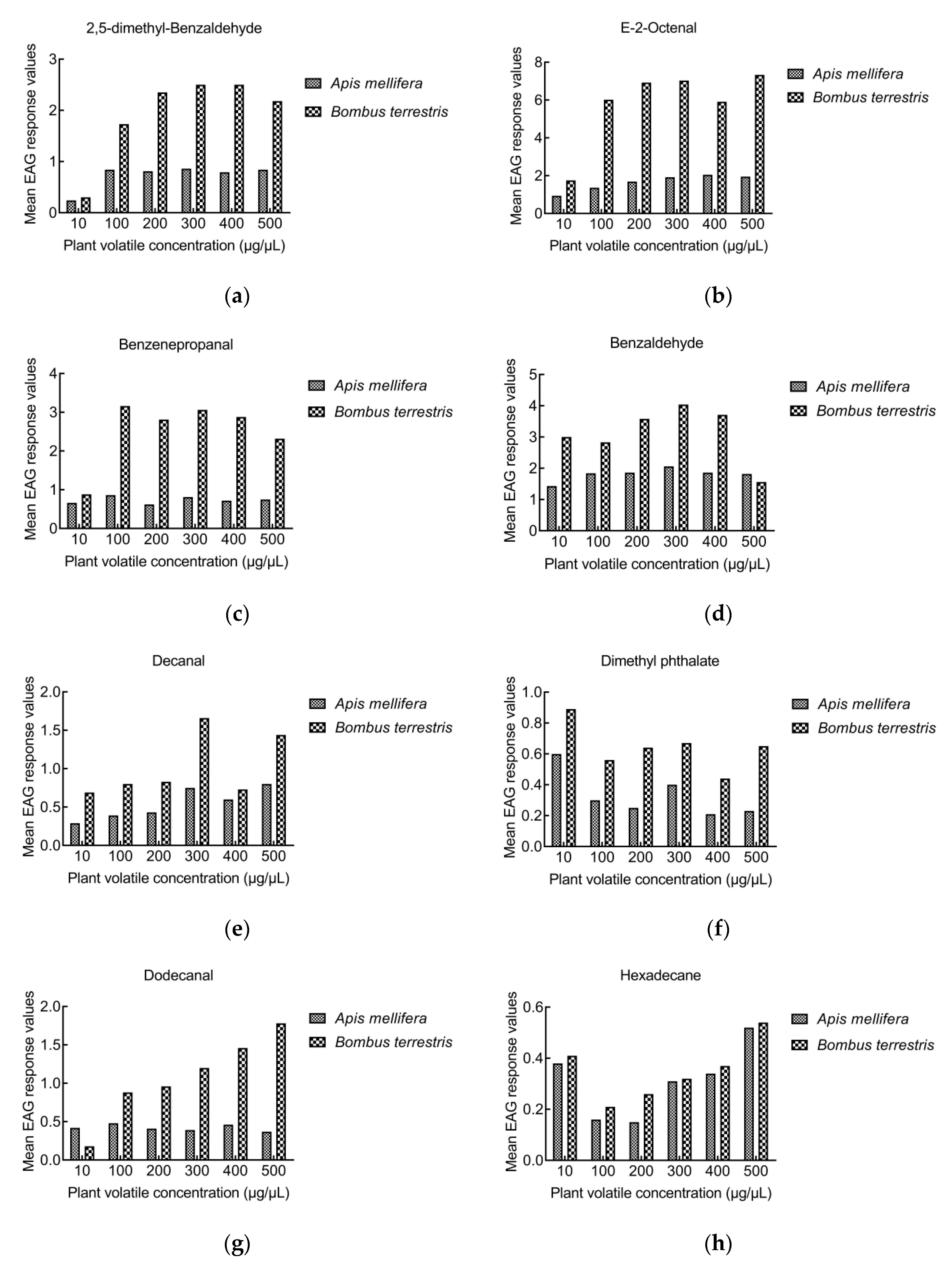
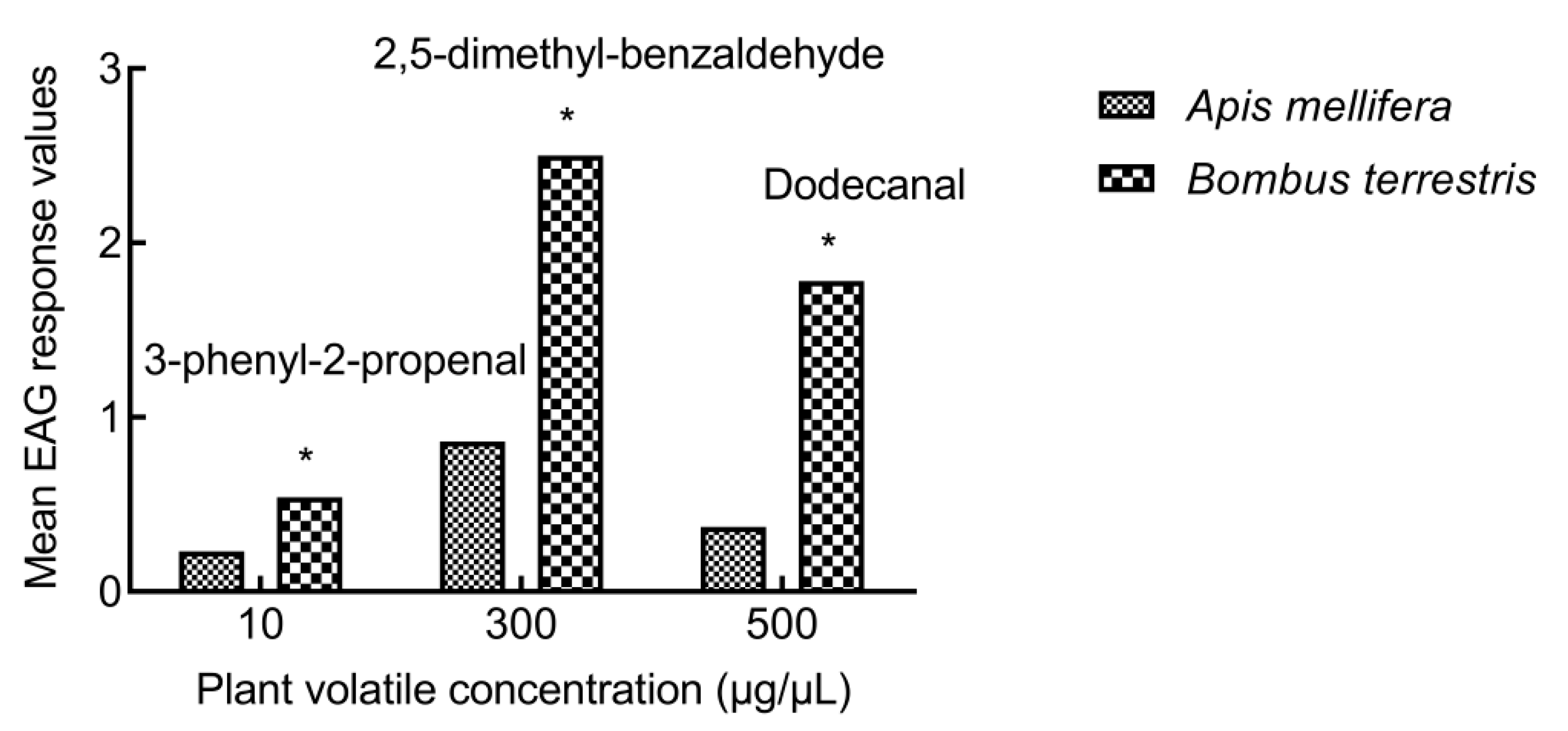
3.2.3. Comparison of EAG responses between A. mellifera and B. terrestris
3.3. Behavior Tests
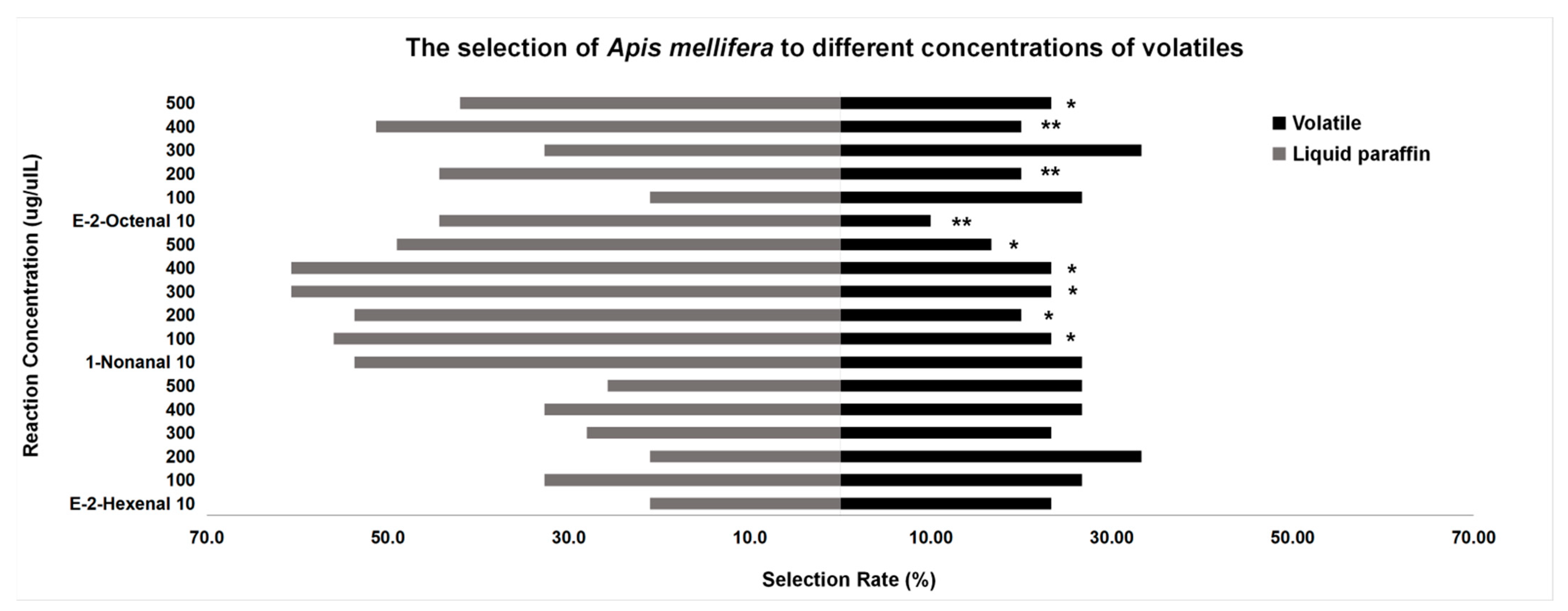
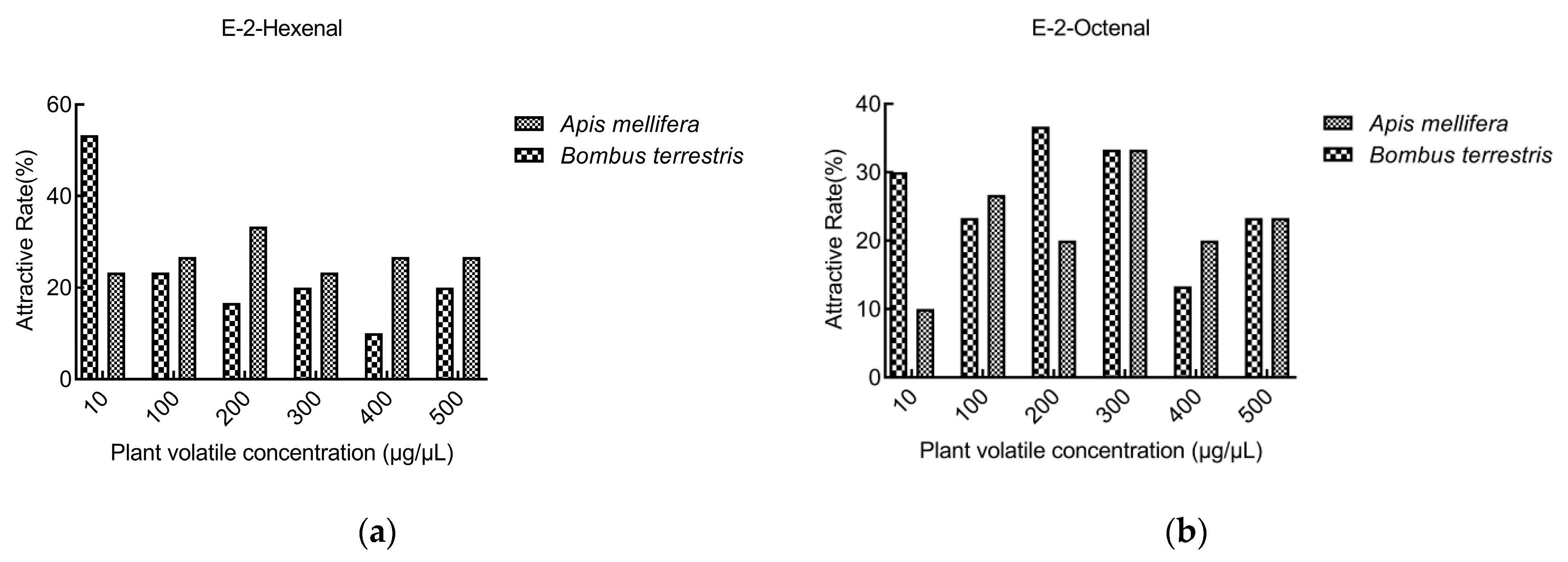
3.3.1. Behavior Tests of A. mellifera
3.3.2. Behavior Tests of B. terrestris
3.3.3. Comparison of Behavior Tests between A. mellifera and B. terrestris
4. Discussion
4.1. Identification of Volatile Compounds from Melon Flowers
4.2. EAG Response of Bees to Volatiles
4.3. Behavior Tests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compounds | Relative EAG Response Values (rEAG) | |||||
|---|---|---|---|---|---|---|
| 10 μg/μL | 100 μg/μL | 200 μg/μL | 300 μg/μL | 400 μg/μL | 500 μg/μL | |
| heptadecane | 0.84 ± 0.33 Aabcdef | 0.31 ± 0.08 Bcd | 0.26 ± 0.07 Bc | 0.17 ± 0.05 Bd | 0.26 ± 0.07 Bb | 0.44 ± 0.24 ABb |
| 3-phenyl-2-propenal | 0.23 ± 0.07 Bef | 0.24 ± 0.06 Bcd | 0.31 ± 0.09 Bc | 0.18 ± 0.06 Bcd | 0.29 ± 0.10 Bb | 0.63 ± 0.15 Ab |
| hexadecane | 0.38 ± 0.15 Aef | 0.16 ± 0.04 Ad | 0.15 ± 0.07 Ac | 0.31 ± 0.16 Abcd | 0.34 ± 0.15 Ab | 0.52 ± 0.27 Ab |
| 2,5-dimethyl-benzaldehyde | 0.24 ± 0.15 Aef | 0.84 ± 0.24 Abcd | 0.81 ± 0.31 Abc | 0.86 ± 0.33 Abcd | 0.79 ± 0.41 Ab | 0.84 ± 0.50 Ab |
| dimethyl phthalate | 0.60 ± 0.14 Abcdef | 0.30 ± 0.13 ABcd | 0.25 ± 0.11 Bc | 0.40 ± 0.10 ABbcd | 0.21 ± 0.06 Bb | 0.23 ± 0.06 Bb |
| hexadecanoic acid, methyl ester | 0.45 ± 0.17 Adef | 0.15 ± 0.05 Ad | 0.34 ± 0.20 Ac | 0.48 ± 0.28 Abcd | 0.59 ± 0.36 Ab | 0.53 ± 0.30 Ab |
| benzaldehyde | 1.43 ± 0.59 Aabc | 1.84 ± 0.82 Abc | 1.86 ± 0.87 Abc | 2.06 ± 0.85 Abc | 1.86 ± 0.89 Ab | 1.82 ± 0.88 Ab |
| e-2-hexenal | 1.02 ± 0.30 Aabcdef | 2.32 ± 0.76 Ab | 2.42 ± 0.86 Ab | 2.15 ± 0.87 Ab | 2.15 ± 0.80 Ab | 1.84 ± 0.74 Ab |
| benzenepropanal | 0.66 ± 0.28 Aabcdef | 0.86 ± 0.38 Abcd | 0.62 ± 0.20 Ac | 0.81 ± 0.33 Abcd | 0.72 ± 0.26 Ab | 0.75 ± 0.29 Ab |
| benzeneacetaldehyde | 1.51 ± 0.55 Aab | 1.45 ± 0.64 Abcd | 1.24 ± 0.41 Abc | 0.93 ± 0.34 Abcd | 0.97 ± 0.44 Ab | 0.92 ± 0.42 Ab |
| 1-nonanal | 1.58 ± 0.53 Aa | 4.68 ± 2.13 Aa | 5.17 ± 2.34 Aa | 5.71 ± 2.47 Aa | 5.59 ± 2.45 Aa | 5.96 ± 2.48 Aa |
| 1,3-bis(1,1-dimethylethyl)-benzene | 0.50 ± 0.19 Acdef | 0.41 ± 0.14 Acd | 0.37 ± 0.09 Ac | 0.49 ± 0.09 Abcd | 0.47 ± 0.16 Ab | 0.52 ± 0.15 Ab |
| trans-ß-ionone | 0.36 ± 0.10 Aef | 0.49 ± 0.18 Acd | 0.48 ± 0.14 Ac | 0.78 ± 0.23 Abcd | 0.87 ± 0.33 Ab | 0.65 ± 0.34 Ab |
| 3-nonen-2-one | 1.15 ± 0.30 Aabcde | 1.57 ± 0.61 Abcd | 1.57 ± 0.66 Abc | 1.40 ± 0.60 Abcd | 1.44 ± 0.50 Ab | 1.24 ± 0.49 Ab |
| nonadecane | 0.51 ± 0.31 Acdef | 0.44 ± 0.28 Acd | 0.30 ± 0.17 Ac | 0.98 ± 0.44 Abcd | 0.71 ± 0.39 Ab | 0.49 ± 0.32 Ab |
| 2,2,4-trimethyl-1,3-pentanediol diisobutyrate | 0.32 ± 0.11 Aef | 0.24 ± 0.12 Acd | 0.44 ± 0.07 Ac | 0.22 ± 0.09 Acd | 0.29 ± 0.10 Ab | 0.48 ± 0.20 Ab |
| e-2-decenal | 0.29 ± 0.10 Bef | 1.11 ± 0.32 ABbcd | 1.32 ± 0.48 ABbc | 1.47 ± 0.46 ABbcd | 1.54 ± 0.44 Ab | 1.76 ± 0.53 Ab |
| hexadecanoic acid, ethyl ester | 0.18 ± 0.07 Af | 0.18 ± 0.07 Ad | 0.18 ± 0.06 Ac | 0.15 ± 0.04 Ad | 0.31 ± 0.11 Ab | 0.34 ± 0.10 Ab |
| e-2-octenal | 0.94 ± 0.45 Aabcdef | 1.36 ± 0.59 Abcd | 1.69 ± 0.74 Abc | 1.92 ± 0.65 Abcd | 2.05 ± 0.90 Ab | 1.95 ± 0.70 Ab |
| decanal | 0.29 ± 0.05 Aef | 0.39 ± 0.12 Acd | 0.43 ± 0.14 Ac | 0.75 ± 0.27 Abcd | 0.60 ± 0.30 Ab | 0.80 ± 0.37 Ab |
| phytol | 0.46 ± 0.16 Adef | 0.56 ± 0.22 Acd | 1.04 ± 0.49 Abc | 1.04 ± 0.61 Abcd | 1.15 ± 0.64 Ab | 1.16 ± 0.84 Ab |
| 1,2-benzenedicarboxylic acid, butyl octyl ester | 0.90 ± 0.55 Abcdfe | 0.53 ± 0.21 Acd | 0.89 ± 0.45 Abc | 0.85 ± 0.34 Abcd | 0.86 ± 0.49 Ab | 1.03 ± 0.61 Ab |
| dodecanal | 0.42 ± 0.22 Adef | 0.48 ± 0.22 Acd | 0.41 ± 0.18 Ac | 0.39 ± 0.18 Abcd | 0.46 ± 0.12 Ab | 0.37 ± 0.13 Ab |
| 1,2-dimethoxy-4-(1-propenyl)-benzene | 1.35 ± 0.83 Aabcd | 1.60 ± 0.88 Abc | 1.31 ± 0.91 Abc | 1.72 ± 0.99 Abcd | 2.14 ± 1.03 Ab | 1.91 ± 1.06 Ab |
| Compounds | Relative EAG Response Values (rEAG) | |||||
|---|---|---|---|---|---|---|
| 10 μg/μL | 100 μg/μL | 200 μg/μL | 300 μg/μL | 400 μg/μL | 500 μg/μL | |
| heptadecane | 0.30 ± 0.09 Ac | 0.17 ± 0.04 Ae | 0.35 ± 0.12 Ac | 0.38 ± 0.17 Acd | 0.42 ± 0.22 Ac | 0.14 ± 0.02 Ac |
| 3-phenyl-2-propenal | 0.54 ± 0.03 Abc | 0.48 ± 0.17 Ae | 0.42 ± 0.26 Ac | 0.43 ± 0.16 Acd | 0.58 ± 0.16 Ac | 0.54 ± 0.21 Abc |
| hexadecane | 0.41 ± 0.21 Abc | 0.21 ± 0.09 Ae | 0.26 ± 0.09 Ac | 0.32 ± 0.12 Acd | 0.37 ± 0.22 Ac | 0.54 ± 0.29 Abc |
| 2,5-dimethyl-benzaldehyde | 0.30 ± 0.18 Bc | 1.73 ± 0.63 ABbcde | 2.35 ± 0.94 ABbc | 2.50 ± 0.50 Abcd | 2.50 ± 0.86 Abc | 2.18 ± 0.77 ABbc |
| dimethyl phthalate | 0.89 ± 0.21 Abc | 0.56 ± 0.25 Acde | 0.64 ± 0.32 Ac | 0.67 ± 0.29 Acd | 0.44 ± 0.15 Ac | 0.65 ± 0.29 Abc |
| hexadecanoic acid, methyl ester | 0.46 ± 0.15 Abc | 0.25 ± 0.06 Ae | 0.33 ± 0.12 Ac | 0.30 ± 0.10 Acd | 0.13 ± 0.03 Ac | 0.34 ± 0.11 Abc |
| benzaldehyde | 3.00 ± 1.99 Aa | 2.83 ± 2.07 Abcd | 3.58 ± 2.69 Ab | 4.04 ± 2.77 Ab | 3.71 ± 2.90 Aab | 1.56 ± 1.26 Abc |
| e-2-hexenal | 0.78 ± 0.38 Abc | 2.85 ± 1.13 Abc | 2.41 ± 1.13 Abc | 2.63 ± 1.24 Abcd | 2.70 ± 1.28 Abc | 2.78 ± 1.25 Ab |
| benzenepropanal | 0.88 ± 0.51 Abc | 3.16 ± 1.72 Ab | 2.81 ± 1.66 Abc | 3.06 ± 1.72 Abc | 2.88 ± 1.69 Abc | 2.32 ± 1.29 Abc |
| benzeneacetaldehyde | 0.84 ± 0.18 Abc | 0.54 ± 0.21 Ade | 0.38 ± 0.19 Ac | 0.32 ± 0.06 Acd | 1.11 ± 0.62 Abc | 0.57 ± 0.06 Abc |
| 1-nonanal | 0.77 ± 0.46 Abc | 1.40 ± 1.09 Abcde | 1.44 ± 1.23 Abc | 1.80 ± 1.49 Abcd | 1.98 ± 1.45 Abc | 1.97 ± 1.44 Abc |
| 1,3-bis(1,1-dimethylethyl)-benzene | 0.37 ± 0.09 Abc | 0.21 ± 0.09 Ae | 0.50 ± 0.24 Ac | 0.63 ± 0.08 Acd | 0.50 ± 0.09 Ac | 0.59 ± 0.10 Abc |
| trans-ß-ionone | 0.57 ± 0.29 Abc | 0.32 ± 0.14 Ae | 0.52 ± 0.22 Ac | 0.19 ± 0.11 Acd | 0.35 ± 0.12 Ac | 0.28 ± 0.09 Abc |
| 3-nonen-2-one | 0.48 ± 0.13 Abc | 0.26 ± 0.08 Ae | 0.31 ± 0.12 Ac | 0.26 ± 0.11 Acd | 0.45 ± 0.09 Ac | 0.46 ± 0.19 Abc |
| nonadecane | 0.44 ± 0.16 Abc | 0.26 ± 0.04 Ae | 0.22 ± 0.08 Ac | 0.33 ± 0.20 Acd | 0.26 ± 0.05 Ac | 0.15 ± 0.08 Ac |
| 2,2,4-trimethyl-1,3-pentanediol diisobutyrate | 0.36 ± 0.15 Ac | 0.48 ± 0.26 Ae | 0.36 ± 0.12 Ac | 0.46 ± 0.10 Acd | 0.45 ± 0.04 Ac | 0.22 ± 0.13 Ac |
| e-2-decenal | 0.37 ± 0.05 Abc | 0.95 ± 0.42 Abcde | 1.11 ± 0.59 Abc | 1.20 ± 0.56 Abcd | 1.41 ± 0.47 Abc | 1.36 ± 0.62 Abc |
| hexadecanoic acid, ethyl ester | 0.42 ± 0.08 Abc | 0.62 ± 0.12 Acde | 0.35 ± 0.22 Ac | 0.22 ± 0.11 Acd | 0.36 ± 0.11 Ac | 0.30 ± 0.16 Abc |
| e-2-octenal | 1.75 ± 0.81 Aab | 6.02 ± 2.17 Aa | 6.92 ± 2.70 Aa | 7.03 ± 2.96 Aa | 5.91 ± 2.78 Aa | 7.33 ± 3.19 Aa |
| decanal | 0.69 ± 0.33 Abc | 0.80 ± 0.45 Acde | 0.83 ± 0.37 Ac | 1.66 ± 0.83 Abcd | 0.73 ± 0.39 Ac | 1.44 ± 0.41 Abc |
| phytol | 0.31 ± 0.15 Ac | 0.24 ± 0.15 Ae | 0.27 ± 0.11 Ac | 0.17 ± 0.04 Ad | 0.54 ± 0.28 Ac | 0.28 ± 0.08 Abc |
| 1,2-benzenedicarboxylic acid, butyl octyl ester | 0.41 ± 0.19 bAc | 0.30 ± 0.11 Ae | 0.38 ± 0.13 Ac | 0.30 ± 0.20 Acd | 0.50 ± 0.23 Ac | 0.71 ± 0.35 Abc |
| dodecanal | 0.18 ± 0.14 Bc | 0.88 ± 0.57 ABbcde | 0.96 ± 0.40 ABbc | 1.20 ± 0.49 ABbcd | 1.46 ± 0.74 ABbc | 1.78 ± 0.61 Abc |
| 1,2-dimethoxy-4-(1-propenyl)-benzene | 0.30 ± 0.09 Bc | 0.22 ± 0.12 Be | 0.34 ± 0.19 ABc | 0.55 ± 0.09 ABcd | 0.66 ± 0.20 Ac | 0.43 ± 0.09 ABbc |
References
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Jung, C.E. Economic value of honeybee pollination on major fruit and vegetable crops in Korea. J. Apic. 2008, 23, 147–152. [Google Scholar]
- Williams, P.H.; Osborne, J.L. Bombus vulnerability and conservation world-wide. Apidologie 2009, 40, 367–387. [Google Scholar] [CrossRef] [Green Version]
- Aizen, M.A.; Aguiar, S.; Biesmeijer, J.C.; Garibaldi, L.A.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Morales, C.L.; Ngo, H.; et al. Global agricultural productivity is threatened by increasing pollinator dependence without a parallel increase in crop diversification. Glob. Chang. Biol. 2019, 25, 3516–3527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahengbam, J.; Raut, A.M.; Satinder, P.; Najitha Banu, A. Role of bumble bee in pollination. Ann. Biol. 2019, 35, 290–295. [Google Scholar]
- Sajid, Z.; Ramzan, M.; Akhtar, N. A review: Foraging behavior and pollination ecology of bumblebee and honey bee in pakistan. J. Innov. Sci. 2020, 6, 126–131. [Google Scholar] [CrossRef]
- Brown, M.; Brow, M.J.F. Nectar preferences in male bumblebees. Insectes Sociaux 2020, 67, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Howell, A.D.; Alarcón, R. Osmia bees (Hymenoptera: Megachilidae) can detect nectar-rewarding flowers using olfactory cues. Anim. Behav. 2007, 74, 199–205. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, W.; Zhang, D.; Wang, G.; Cao, F. Flowering Stage and Daytime Affect Scent Emission of Malus ioensis “Prairie Rose”. Molecules 2019, 24, 2356. [Google Scholar] [CrossRef] [Green Version]
- Dötterl, S.; Vereecken, N.J. The chemical ecology and evolution of bee flower interactions: A review and perspectives. Can. J. Zool. 2010, 88, 668–697. [Google Scholar] [CrossRef]
- Wakamura, S.; Arakaki, N.; Moriyama, D.; Kanayama, S.; Oike, M.; Kimura, A.; Wajima, S.; Ono, H.; Yasui, H. Does the orchid Luisia teres attract its male chafer pollinators (Scarabaeidae: Protaetia pryeri pryeri) by sexual deception? Chemoecology 2020, 30, 49–57. [Google Scholar] [CrossRef]
- Dötterl, S.; Milchreit, K.; Schäffler, I. Behavioural plasticity and sex differences in host finding of a specialized bee species. J. Comp. Physiol. A 2011, 197, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Long, D.; Wang, Y.; Li, X.; Huang, J.; Shen, J.; Su, W.; Jiang, Y.; Li, J. Electrophysiological and behavioral responses of Asian and European honeybees to pear flower volatiles. J. Asia Pac. Entomol. 2021, 24, 221–228. [Google Scholar] [CrossRef]
- Būda, V.; Radžiutė, S.; Apšegaitė, V.; Blažytė-Čereškienė, L.; Čepulytė, R.; Bumbulytė, G.; Mozūraitis, R. Electroantennographic and behavioural responses of European cherry fruit fly, Rhagoletis cerasi, to the volatile organic compounds from sour cherry, Prunus cerasus, fruit. Insects 2022, 13, 114. [Google Scholar] [CrossRef]
- Akar, Z. Chemical compositions by using LC-MS/MS and GC-MS and antioxidant activities of methanolic extracts from leaf and flower parts of Scabiosa columbaria subsp. columbaria var. columbaria L. Saudi J. Biol. Sci. 2021, 28, 6639–6644. [Google Scholar] [CrossRef]
- Parachnowitsch, A.L.; Kessler, A. Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytol. 2010, 188, 393–402. [Google Scholar] [CrossRef]
- Moghbeli Gharaei, A.; Ziaaddini, M.; Frérot, B.; Nejad Ebrahimi, S.; Jalali, M.A.; Reddy, G.V. Identification and evaluation of four cucurbitaceous host plant volatiles attractive to Diaphania indica (Saunders) (Lep.: Pyralidae). Chemoecology 2020, 30, 173–182. [Google Scholar] [CrossRef]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef]
- Andersson, S.; Dobson, H.E. Behavioral foraging responses by the butterfly Heliconius melpomene to Lanatana camara floral scent. Chem. Ecol. 2003, 29, 2303–2318. [Google Scholar] [CrossRef]
- Wright, G.A.; Schiestl, F.P. The evolution of floral scent: The influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct. Ecol. 2009, 23, 841–851. [Google Scholar] [CrossRef] [Green Version]
- Goulson, D.; Chapman, J.W.; Hughes, W.O.H. Discrimination of unrewarding flowers by bees; direct detection of rewards and use of repellent scent marks. Insect Behav. 2001, 14, 669–678. [Google Scholar] [CrossRef]
- Majetic, C.J.; Raguso, R.A.; Ashman, T.L. The sweet smell of success: Floral scent affects pollinator attraction and seed fitness in Hesperis matronalis. Funct. Ecol. 2009, 23, 480–487. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of Plant Volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farré-Armengol, G.; Fernández-Martínez, M.; Filella, I.; Junker, R.R.; Peñuelas, J. Deciphering the biotic and climatic factors that influence floral scents: A systematic review of floral volatile emissions. Front. Plant Sci. 2020, 11, 1154. [Google Scholar] [CrossRef]
- Fernandes, N.D.S.; Silva, F.A.N.; De Aragão, F.A.S.; Zocolo, G.; Freitas, B.M. Volatile organic compounds role in selective pollinator visits to commercial melon types. J. Agric. Sci. 2019, 11, 93–108. [Google Scholar] [CrossRef]
- Silva, F.A.N.; da Silva, A.A.; de Sousa Fernandes, N.; Rodrigues, T.H.; Canuto, K.M.; do Nascimento, R.F.; de Brito, E.S.; de Aragão, F.A.S.; Freitas, B.M.; Zocolo, G.J. Evaluation of Headspace Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry for the characterization of volatile organic compounds from Melon (Cucumis melo L.) Flowers. Chromatographia 2018, 81, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Huang, Z.Y.; Li, K.; Chen, X.; Chen, Y.; Sun, Y. EAG responses of Apis cerana to floral compounds of a biodiesel plant, Jatropha curcas (Euphorbiaceae). Econ. Entomol. 2013, 106, 1653–1658. [Google Scholar] [CrossRef]
- Liu, Y.B.; Zeng, Z.J.; Barron, A.B.; Ma, Y.; He, Y.Z.; Liu, J.F.; Li, Z.; Yan, W.Y.; He, X.J. The involvement of a floral scent in plant-honeybee interaction. Sci. Nat. 2022, 109, 30. [Google Scholar] [CrossRef]
- Twidle, A.M.; Barker, D.; Seal, A.G.; Fedrizzi, B.; Suckling, D.M. Identification of floral volatiles and pollinator responses in kiwifruit cultivars, actinidia chinensis var. chinensis. Chem. Ecol. 2018, 44, 406–415. [Google Scholar] [CrossRef]
- Ren, L.L.; Balakrishnan, K.; Luo, Y.Q.; Schütz, S. EAG response and behavioral orientation of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae) to synthetic host-associated volatiles. PLoS ONE 2017, 12, e0190067. [Google Scholar] [CrossRef]
- Wickham, J.D.; Xu, Z.; Teale, S.A. Evidence for a female-produced, long range pheromone of Anoplophora glabripennis (Coleoptera: Cerambycidae). Insect Sci. 2012, 19, 355–371. [Google Scholar] [CrossRef]
- Zhuge, P.P.; Luo, S.L.; Wang, M.Q.; Zhang, G. Electrophysiological responses of Batocera horsfieldi (Hope) adults to plant volatiles. J. Appl. Entomol. 2010, 134, 600–607. [Google Scholar] [CrossRef]
- Lu, P.F.; Qiao, H.L. Peach volatile emission and attractiveness of different host plant volatiles blends to Cydia molesta in adjacent peach and pear orchards. Sci. Rep. 2020, 10, 13658. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zhao, D.X.; Gao, J.L.; Peng, Z.Q.; Li, X.N.; Wang, Y. Electrophysiological and Behavioral Activity of Compounds in Metathoracic Glands of Adults of Tessaratoma Papillosa (Hemiptera: Pentatomidae). Appl. Mech. Mater. 2011, 108, 301–307. [Google Scholar]
- Siderhurst, M.S.; Jang, E.B. Female-biased attraction of oriental fruit fly, Bactrocera dorsalis (Hendel), to a blend of host fruit volatiles from Terminalia catappa L. J. Chem. Ecol. 2006, 32, 2513–2524. [Google Scholar] [CrossRef]
- Wright, G.A.; Skinner, B.D.; Smith, B.H. Ability of honeybee, Apis mellifera, to detect and discriminate odors of varieties of canola (Brassica rapa and Brassica napus) and snapdragon flowers (Antirrhinum majus). J. Chem. Ecol. 2002, 28, 721–740. [Google Scholar] [CrossRef]
- Sun, X.-L.; Wang, G.-C.; Gao, Y.; Chen, Z.-M. Screening and field evaluation of synthetic volatile blends attractive to adults of the tea weevil, Myllocerinus aurolineatus. Chemoecology 2012, 22, 229–237. [Google Scholar] [CrossRef]
- Spaethe, J.; Brockmann, A.; Halbig, C.; Tautz, J. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften 2007, 94, 733–739. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G.; Germinara, G.S.; Fusini, G.; Romano, D.; Rapalini, F.; Desneux, N.; Rotundo, G.; Raspi, A.; Carpita, A. Behavioural and electrophysiological responses to overlooked female pheromone components in the olive fruit fly Bactrocera oleae (Diptera:Tephritidae). Chemoecology 2015, 25, 147–157. [Google Scholar] [CrossRef]
- Williams, L.; Blackmer, J.L.; Rodriguez-Saona, C.; Zhu, S. Plant Volatiles Influence Electrophysiological and Behavioral Responses of Lygus hesperus. J. Chem. Ecol. 2010, 36, 467–478. [Google Scholar] [CrossRef]
- Dötterl, S.; Jürgens, A.; Seifert, K.; Laube, T.; Weißbecker, B.; Schütz, S. Silene latifolia moths nursery pollination: Smell in the antenna and the role of behavioral response. J. New Bot. 2006, 169, 707–718. [Google Scholar]
- Ishiwari, H.; Suzuki, T.; Maeda, T. Essential Compounds in Herbivore-Induced Plant Volatiles that Attract the Predatory Mite Neoseiulus womersleyi. J. Chem. Ecol. 2007, 33, 1670. [Google Scholar] [CrossRef]
- Hern, A.; Dorn, S. A female-specific attractant for the codling moth, Cydia pomonella, from apple fruit volatiles. Naturwissenschaften 2004, 91, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Hilker, M.; Hansson, B.; Bombosch, S.; Klein, B.; Schildknecht, H. Oviposition deterring components in larval frass of Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae), A behavioural and electrophysiological evaluation. J. Insect Physiol. 1993, 39, 129–137. [Google Scholar] [CrossRef]
- Clement, W.M. Flower color, a factor in attractiveness of alfalfa clones for honeybees. Crop Sci. 1965, 5, 267–268. [Google Scholar] [CrossRef] [Green Version]
- Kauffeld, N.M.; Sorensen, E.L. Interrelations of Honeybee Preference and Alfalfa Clones and Flower Color, Aroma, Nectar Volume and Sugar Concentration; Agricultural Experiment Station, Kansas State University of Agriculture and Applied Science: Manhattan, NY, USA, 1971; p. 14. [Google Scholar]
- Balkenius, A.; Rosen, W.; Kelber, A. The relative importance of olfaction and vision in a diurnal and a nocturnal hawkmoth. J. Comp. Physiol. A 2006, 192, 431–437. [Google Scholar] [CrossRef]
- Omura, H.; Honda, K. Priority of color over scent during flower visitation by adult Vanessa indica butterflies. Oecologia 2005, 142, 588–596. [Google Scholar] [CrossRef]
- Burger, H.; Dotterl, S.; Ayasse, M. Host plant finding and recognition by visual and olfactory floral cues in an oligolectic bee. Funct. Ecol. 2010, 24, 1234–1240. [Google Scholar] [CrossRef]
- Milet-Pinheiro, P.; Ayasse, M.; Schlindwein, C.; Dobson, H.E.M.; Dotterl, S. Host location by visual and olfactory floral cues in an oligolectic bee: Innate and learned behavior. Behav. Ecol. 2012, 23, 531–538. [Google Scholar] [CrossRef]
- Dötterl, S.; Glück, U.; Jürgens, A.; Woodring, J.; Aas, G. Floral reward, advertisement and attractiveness to honey bees in dioecious Salix caprea. PLoS ONE 2014, 9, e93421. [Google Scholar] [CrossRef]


| Compounds | CAS Number | Purity (%) | Origin |
|---|---|---|---|
| benzaldehyde | 100-52-7 | ≥99.5 | Aladdin |
| e-2-hexenal | 6728-26-3 | 98 | Aladdin |
| heptadecane | 629-78-7 | ≥99 | Macklin |
| benzenepropanal | 104-53-0 | 95 | Aladdin |
| trans-ß-ionone | 79-77-6 | >90.0 | Aladdin |
| 3-phenyl-2-propenal | 104-55-2 | 98 | Macklin |
| benzeneacetaldehyde | 122-78-1 | 95 | Aladdin |
| hexadecane | 544-76-3 | 99 | Macklin |
| 1-nonanal | 124-19-6 | 96 | Aladdin |
| 1,3-bis(1,1-dimethylethyl)-benzene | 1014-60-4 | >98.0 | Aladdin |
| 2,5-dimethyl-benzaldehyde | 5779-94-2 | 98 | Macklin |
| dimethyl phthalate | 131-11-3 | ≥99.7 | Aladdin |
| hexadecanoic acid, methyl ester | 112-39-0 | 99 | Aladdin |
| 3-nonen-2-one | 14309-57-0 | ≥96 | Macklin |
| nonadecane | 629-92-5 | 98 | Macklin |
| 2,2,4-trimethyl-1,3-pentanediol diisobutyrate | 6846-50-0 | 98.5 | Aladdin |
| e-2-decenal | 3913-81-3 | 98.5 | Aladdin |
| hexadecanoic acid, ethyl ester | 628-97-7 | ≥99 | Aladdin |
| e-2-octenal | 2548-87-0 | 95 | Aladdin |
| decanal | 112-31-2 | 97 | Aladdin |
| phytol | 150-86-7 | >90.0 | Aladdin |
| 1,2-benzene dicarboxylic acid, butyl octyl ester | 84-78-6 | 95 | Aladdin |
| dodecanal | 112-54-9 | 95 | Aladdin |
| 1,2-dimethoxy-4-(1-propenyl)-benzene | 93-16-3 | >98.0 | Aladdin |
| Compounds | CAS Number | Female Flowers | Male Flowers | ||
|---|---|---|---|---|---|
| Retain Time (min) | Relative Content (%) | Retain Time (min) | Relative Content (%) | ||
| aldehydes | |||||
| benzaldehyde | 100-52-7 | 7.952 | 48.83 | 7.938 | 74.39 |
| e-2-hexenal | 6728-26-3 | 4.566 | 7.13 | 4.619 | 3.22 |
| benzeneacetaldehyde | 122-78-1 | 11.268 | 1.06 | 11.203 | 1.06 |
| 1-nonanal | 124-19-6 | 13.62 | 0.24 | 13.653 | 1.4 |
| benzenepropanal | 104-53-0 | 16.094 | 2.12 | 16.1 | 0.45 |
| pentadecanal- | 2765-11-9 | 32.562 | 0.4 | 34.003 | 0.04 |
| 2,5-dimethyl-benzaldehyde | 5779-94-2 | 18.442 | 0.13 | — | — |
| 3-phenyl-2-propenal | 104-55-2 | 21.706 | 1.38 | — | — |
| retinal | 116-31-4 | 33.622 | 0.05 | — | — |
| decanal | 112-31-2 | — | — | 17.961 | 0.19 |
| 2,4-dimethyl-benzaldehyde | 15764-16-6 | — | — | 18.42 | 0.3 |
| e-2-decenal, | 3913-81-3 | — | — | 21.067 | 0.39 |
| dodecanal | 112-54-9 | — | — | 26.828 | 0.14 |
| z-13-octadecenal | 58594-45-9 | — | — | 30.962 | 0.09 |
| hexadecanal | 629-80-1 | — | — | 32.563 | 0.16 |
| e-2-octenal | 2548-87-0 | — | — | 11.737 | 0.26 |
| alkanes | |||||
| 2-methyltetracosane | 1560-78-7 | 32.306 | 0.16 | — | — |
| heptadecane | 629-78-7 | 39.445 | 2.88 | — | — |
| 1,1,3-tricyclohexylpropane | 55682-89-8 | 40.856 | 0.16 | — | — |
| hexadecane | 544-76-3 | 41.135 | 1.06 | — | — |
| heneicosane | 629-94-7 | 42.346 | 23.82 | — | — |
| nonadecane | 629-92-5 | — | — | 39.453 | 1.06 |
| 4-propoxy-4’-propyl-1,1’-bicyclohexyl | 98321-58-5 | — | — | 40.627 | 0.03 |
| tetracosane | 646-31-1 | — | — | 42.333 | 4.6 |
| ethers | |||||
| 1,2-dimethoxy-4-(1-propenyl)-benzene | 93-16-3 | — | — | 28.062 | 0.12 |
| alkynes | |||||
| 1-dodecyne | 765-03-7 | 6.157 | 0.1 | — | — |
| 1-octadecyne | 629-89-0 | 26.831 | 0.11 | — | — |
| 4-ethyl-3-nonen-5-yne | 74685-67-9 | — | — | 23.908 | 0.85 |
| alkenes | |||||
| 11-chloro-1-undecene | 872-17-3 | 26.079 | 0.27 | — | — |
| 3-ethyl-cyclohexene | 2808-71-1 | — | — | 9.854 | 0.38 |
| esters | |||||
| dimethyl phthalate | 131-11-3 | 28.012 | 0.37 | — | — |
| hexadecanoic acid, methyl ester | 112-39-0 | 35.686 | 0.37 | — | — |
| docosanoic acid, ethyl ester | 5908-87-2 | 36.978 | 0.55 | 33.688 | 0.05 |
| 11-dodecen-1-ol trifluoroacetate | 128792-46-1 | 40.712 | 0.3 | — | — |
| 1,2,4-benzene tricarboxylic acid, 1,2-dimethyl ester | 54699-35-3 | — | — | 3.966 | 1.4 |
| cyclohexanol, 2-methylene-3-(1-methylethenyl)-, acetate, cis- | 54824-09-8 | — | — | 18.633 | 0.13 |
| borinic acid, diethyl-, 1-ethynylcyclohexyl ester | 55848-34-5 | — | — | 19.548 | 0.14 |
| phenprobamate | 673-31-4 | — | — | 22.921 | 0.34 |
| pentanoic acid, 5-hydroxy-, 2,4-di-t-butyl phenyl esters | 166273-38-7 | — | — | 29.335 | 0.39 |
| 2,2,4-trimethyl-1,3-pentanediol diisobutyrate | 6846-50-0 | — | — | 30.719 | 0.53 |
| 1,2-benzene dicarboxylic acid, butyl octyl ester | 84-78-6 | — | — | 34.843 | 0.14 |
| hexadecanoic acid, ethyl ester | 628-97-7 | — | — | 36.982 | 0.36 |
| alcohols | |||||
| 3,6,6-trimethyl-2-norpinanol | 29548-09-2 | 25.545 | 0.28 | 25.552 | 0.43 |
| 2-methyl-6-methylene-2-octanol | 18479-59-9 | 33.419 | 0.13 | — | — |
| z,z-2,5-pentadecadien-1-ol | 139185-79-8 | 33.724 | 0.06 | — | — |
| 3,7,11,15-tetramethyl-2-hexadecen-1-ol | 102608-53-7 | 34.298 | 0.14 | — | — |
| 4-cyclooctene-1-methanol | 13366-81-9 | — | — | 17.61 | 0.03 |
| 2-methyl-2-(4-methyl-3-pentenyl)-cyclopropanemethanol | 98678-70-7 | — | — | 25.978 | 0.11 |
| trans,trans-2,6-dimethyl-2,6-octadiene-1,8-diol | 26488-97-1 | — | — | 26.083 | 0.09 |
| 2-butyl-1-octanol | 3913-2-8 | — | — | 26.552 | 0.07 |
| phytol | 150-86-7 | — | — | 31.946 | 0.19 |
| aromatics | |||||
| 1,3-bis(1,1-dimethylethyl)-benzene | 1014-60-4 | 20.486 | 0.15 | 20.486 | 0.3 |
| 2,4-di-tert-butylphenol | 96-76-4 | 29.334 | 0.55 | — | — |
| 5-pentyl-1,3-benzenediol | 500-66-3 | 29.459 | 0.62 | 29.466 | 0.15 |
| asarone | 2883-98-9 | 31.174 | 0.49 | — | — |
| 5-butyl-6-hexyloctahydro-1h-indene | 55044-36-5 | 40.623 | 0.01 | — | — |
| 2,3,6,7-tetrahydro-3a,6-methano-3ah-indene | 98640-29-0 | — | — | 21.792 | 0.35 |
| 5-ethyl-5-methyl-2-phenyl-2-oxazoline | 91875-70-6 | — | — | 23.518 | 0.05 |
| butylated hydroxytoluene | 128-37-0 | — | — | 29.18 | 0.05 |
| ß-asarone | 5273-86-9 | — | — | 31.175 | 1.64 |
| 1,2,3-trimethoxy-5-(2-propenyl)-benzene | 487-11-6 | — | — | 32.133 | 0.18 |
| ketones | |||||
| 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-one | 6901-97-9 | 27.293 | 0.14 | — | — |
| trans-ß-ionone | 79-77-6 | 28.635 | 1.53 | — | — |
| 4-cyclohexylidene-3,3-diethyl-2-pentanone | 313253-65-5 | 32.093 | 0.2 | — | — |
| 1-phenyl-1-propanone | 93-55-0 | 16.33 | 1.01 | 16.346 | 0.6 |
| (z)- 6,10-dimethyl-5,9-undecadien-2-one | 3879-26-3 | 27.897 | 0.26 | 27.897 | 0.17 |
| (r)-5,6,7,7a-tetrahydro-4,4,7a-trimethyl-2(4h)-benzofuranone | 17092-92-1 | 29.56 | 0.27 | 29.567 | 0.24 |
| 6,10,14-trimethyl-2-pentadecanone | 502-69-2 | 34.413 | 1.34 | 34.413 | 0.38 |
| (1-oxa-2-aza-spiro [2.5]oct-2-yl)-phenyl methanone | 2289-83-0 | — | — | 12.351 | 0.17 |
| 3-nonen-2-one | 14309-57-0 | — | — | 20.208 | 0.03 |
| 2,4,4-trimethyl-3-(3-methylbutyl)cyclohex-2-enone | 88725-82-0 | — | — | 24.81 | 0.19 |
| α-ionone | 127-41-3 | — | — | 27.294 | 0.14 |
| 4-(2,2,6-trimethyl-7-oxabicyclo [4.1.0]hept-1-yl)-3-buten-2-one | 23267-57-4 | — | — | 28.636 | 1.59 |
| others | |||||
| dicyclopentadiene diepoxide | 81-21-0 | 23.921 | 0.74 | — | — |
| 3-methyl-2-(3,7,11-trimethyldodecyl) furan | 166773-55-3 | 35.617 | 0.61 | 35.627 | 0.14 |
| ethinamate | 126-52-3 | — | — | 10.652 | 0.1 |
| Compounds | CAS Number | Female Flowers | Male Flowers | ||
|---|---|---|---|---|---|
| Retain Time (min) | Relative Content (%) | Retain Time (min) | Relative Content (%) | ||
| alkenes | |||||
| docosanoic acid, ethyl ester | 5908-87-2 | 36.978 | 0.55 | 33.688 | 0.05 |
| aldehydes | |||||
| e-2-hexenal | 6728-26-3 | 4.566 | 7.13 | 4.619 | 3.22 |
| benzaldehyde | 100-52-7 | 7.952 | 48.83 | 7.938 | 74.39 |
| benzeneacetaldehyde | 122-78-1 | 11.268 | 1.06 | 11.203 | 1.06 |
| 1-nonanal | 124-19-6 | 13.62 | 0.24 | 13.653 | 1.4 |
| benzenepropanal | 104-53-0 | 16.094 | 2.12 | 16.1 | 0.45 |
| pentadecanal- | 2765-11-9 | 32.562 | 0.4 | 34.003 | 0.04 |
| aromatics | |||||
| 1,3-bis(1,1-dimethylethyl)-benzene | 1014-60-4 | 20.486 | 0.15 | 20.486 | 0.3 |
| 5-pentyl-1,3-benzenediol | 500-66-3 | 29.459 | 0.62 | 29.466 | 0.15 |
| ketones | |||||
| 1-phenyl-1-propanone | 93-55-0 | 16.33 | 1.01 | 16.346 | 0.6 |
| (z)-6,10-dimethyl-5,9-undecadien-2-one | 3879-26-3 | 27.897 | 0.26 | 27.897 | 0.17 |
| (r)-5,6,7,7a-tetrahydro-4,4,7a-trimethyl-2(4h)-benzofuranone | 17092-92-1 | 29.56 | 0.27 | 29.567 | 0.24 |
| 6,10,14-trimethyl-2-pentadecanone | 502-69-2 | 34.413 | 1.34 | 34.413 | 0.38 |
| alcohols | |||||
| 3,6,6-trimethyl-2-norpinanol | 29548-09-2 | 25.545 | 0.28 | 25.552 | 0.43 |
| others | |||||
| 3-methyl-2-(3,7,11-trimethyldodecyl) furan | 166773-55-3 | 35.617 | 0.61 | 35.627 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, J.; Gao, F.; Chen, M.; Jiang, Y.; Zhao, H.; Ma, W. Electrophysiological and Behavioral Responses of Apis mellifera and Bombusterrestris to Melon Flower Volatiles. Insects 2022, 13, 973. https://doi.org/10.3390/insects13110973
Zhang J, Liu J, Gao F, Chen M, Jiang Y, Zhao H, Ma W. Electrophysiological and Behavioral Responses of Apis mellifera and Bombusterrestris to Melon Flower Volatiles. Insects. 2022; 13(11):973. https://doi.org/10.3390/insects13110973
Chicago/Turabian StyleZhang, Jiangchao, Jinjia Liu, Fei Gao, Min Chen, Yusuo Jiang, Huiting Zhao, and Weihua Ma. 2022. "Electrophysiological and Behavioral Responses of Apis mellifera and Bombusterrestris to Melon Flower Volatiles" Insects 13, no. 11: 973. https://doi.org/10.3390/insects13110973
APA StyleZhang, J., Liu, J., Gao, F., Chen, M., Jiang, Y., Zhao, H., & Ma, W. (2022). Electrophysiological and Behavioral Responses of Apis mellifera and Bombusterrestris to Melon Flower Volatiles. Insects, 13(11), 973. https://doi.org/10.3390/insects13110973





