Development and Fecundity of Oriental Fruit Moth (Lepidoptera: Tortricidae) Reared on Various Concentrations of Amygdalin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Artificial Diet Preparation
2.3. Larval Feeding: Multiple Choice Test
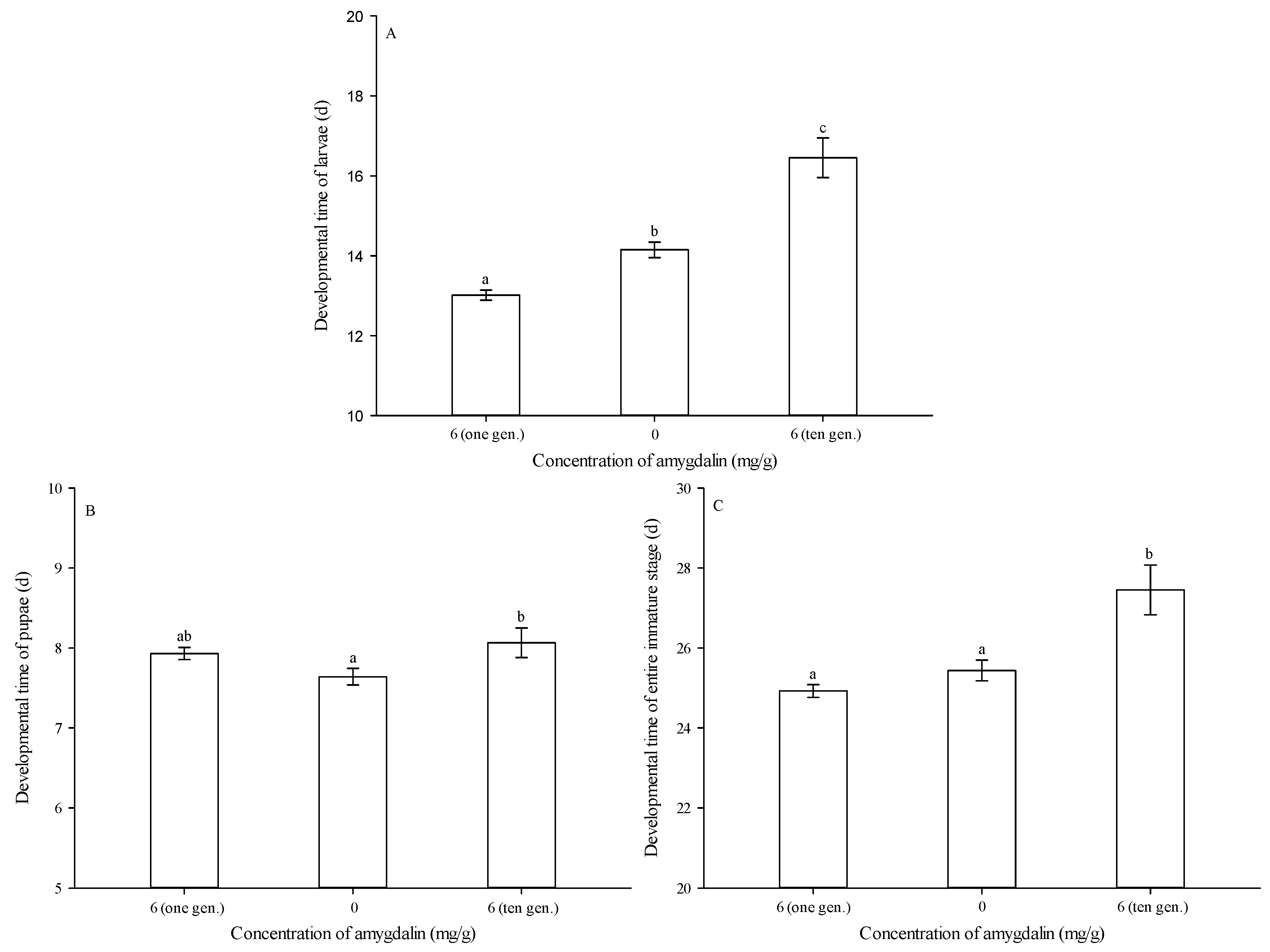
2.4. Performance Measurement over One or Ten Generations
2.5. Statistical Analysis
3. Results
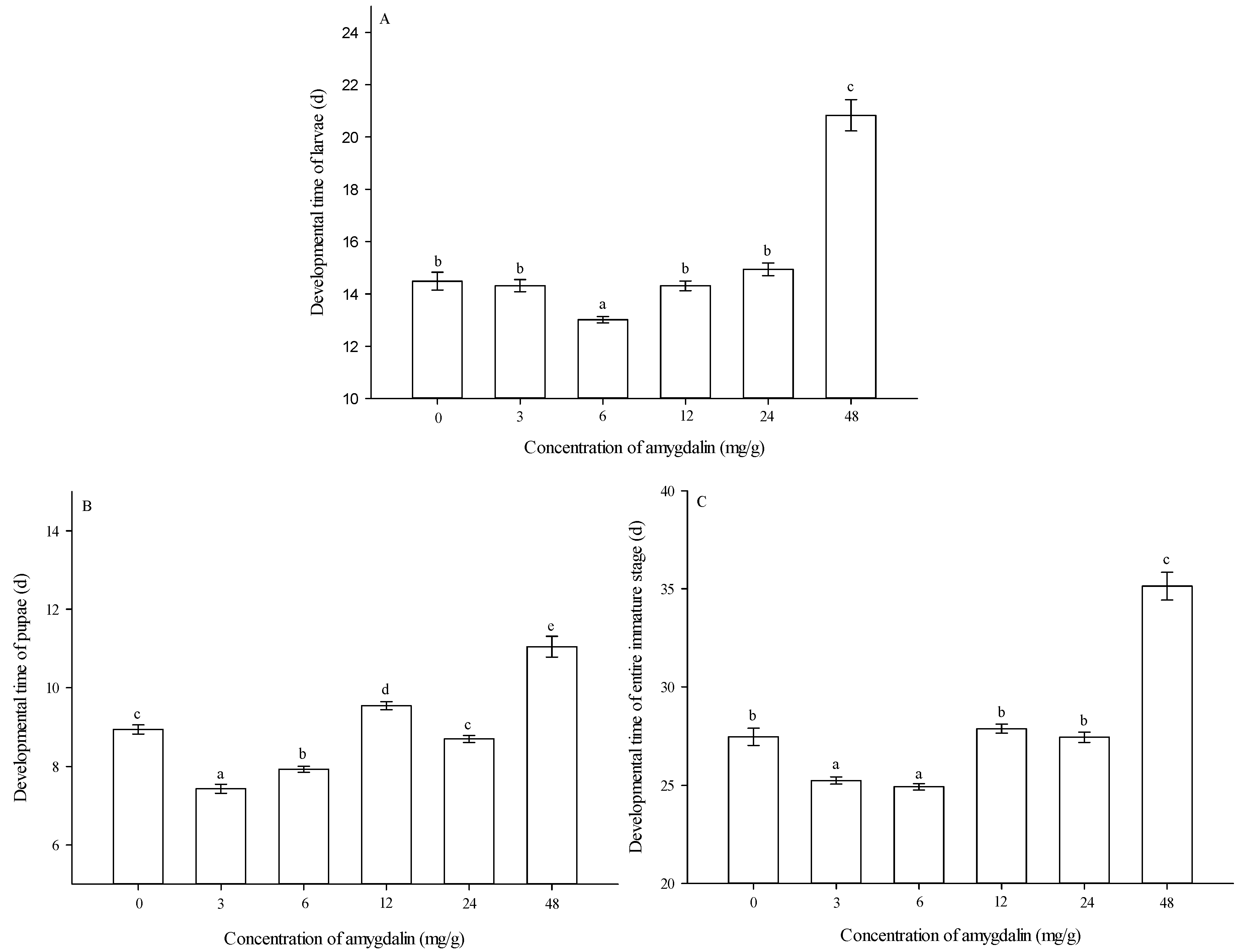
3.1. Larval Feeding on Diets and Developmental Times of Immature Stages
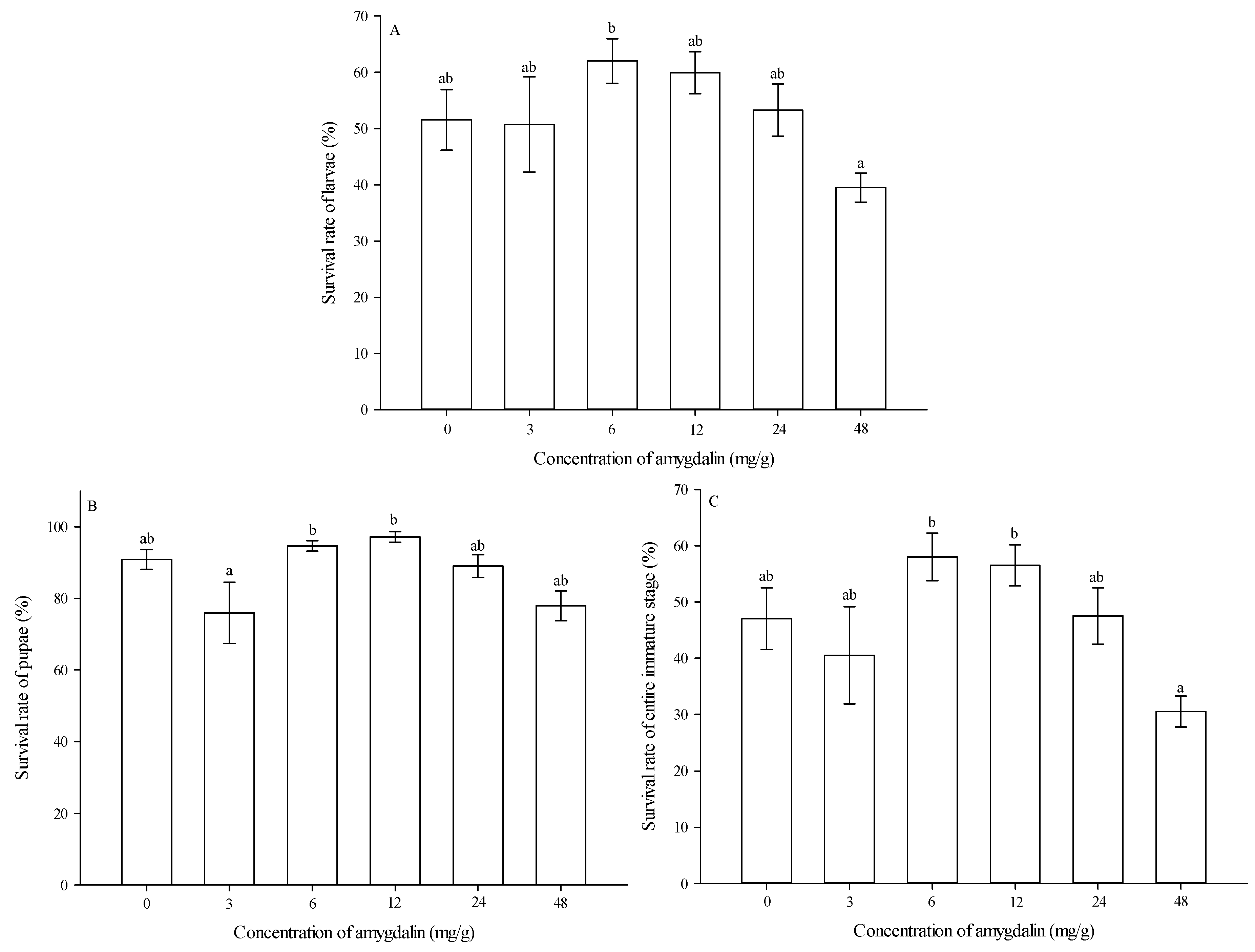
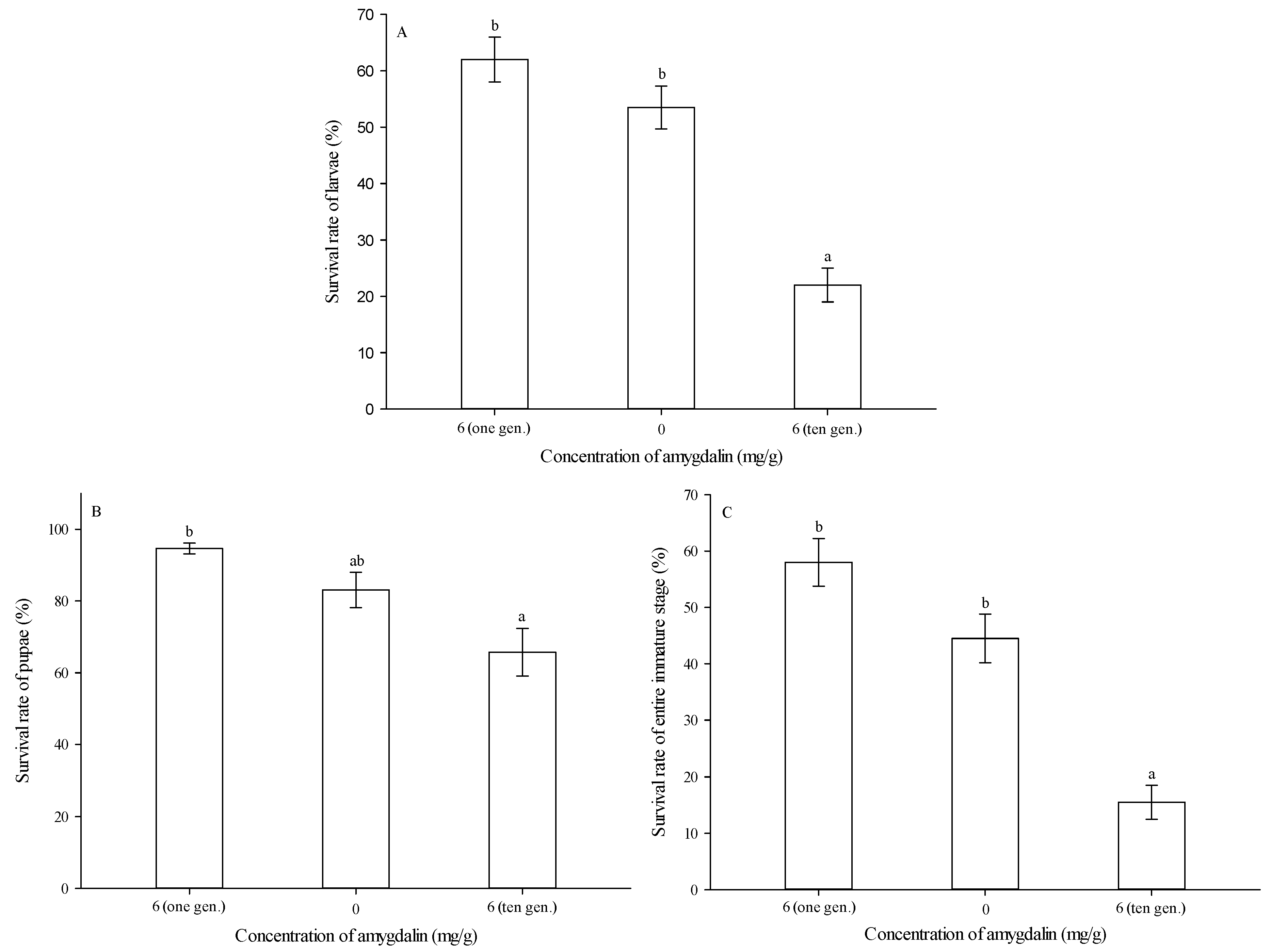
3.2. Survival Rates of Immature Stages
3.3. Pupal Weights, Fecundity of Adult Stage and Population Parameters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Myers, C.T. Orchard Host Plant Effects on the Survival, Development, Reproduction, and Behavior of the Oriental Fruit Moth, Grapholita molesta (Busck). Ph.D. Thesis, Pennsylvania State University, State College, PA, USA, 2005. [Google Scholar]
- Wang, Y.; Kong, W.N.; Zhao, L.L.; Xiang, H.M.; Zhang, L.J.; Li, J.; Ridsdill-Smith, J.; Ma, R.Y. Methods to measure performance of Grapholitha molesta on apples of five varieties. Entomol. Exp. Appl. 2018, 166, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.N.; Wang, Y.; Guo, Y.F.; Chai, X.H.; Li, J.; Ma, R.Y. Importance of preovipositional period of an oligophagous moth in predicting host suitability. J. Econ. Entomol. 2020, 113, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Bae, S.W.; Son, Y.R.; Park, J.A. Analysis of migration of the oriental fruit moth, Grapholita molesta, in apple-cultivating areas based on population monitoring using sex pheromone and RAPD molecular marker. Korean J. Appl. Entomol. 2009, 48, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Amat, C.; Bosch-Serra, D.; Avilla, J.; Escudero, C.L.A. Different population phenologies of Grapholita molesta (Busck) in two hosts and two nearby regions in the NE of Spain. Insects 2021, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [Green Version]
- War, A.R.; Buhroo, A.A.; Hussain, B.; Ahmad, T.; Sharma, H.C. Plant defense and insect adaptation with reference to secondary metabolites. In Co-Evolution of Secondary Metabolites; Merillon, J.M., Ramawat, K.G., Eds.; Springer: Berlin, Germany, 2020; pp. 795–822. [Google Scholar]
- Wang, C.Z. Effects of gossypol and tannic acid on the growth and digestion physiology of cotton bollworm larvae. J. Plant Prot. 1997, 24, 13–18. [Google Scholar]
- Guo, X.M.; Zhang, Y.S.; Yao, J.B. Studies on the natural insect resistant germplasm from interspecific hybridization of G. hirsutum and G. hirsutum var. latifolium. Cotton Sci. 2004, 16, 126–128. [Google Scholar]
- Wang, X.L.; Wang, Y.T.; Duan, L.Q.; Li, H.P.; Feng, S.J. Effects of four plant phenolics on the growth and development and fecundity of the gypsy moth, Lymantria dispar (Lepidoptera: Lymantriidae). Acta Entomol. Sinica 2014, 57, 831–836. [Google Scholar]
- Li, L.S. Feeding Preference of Hyphantria cunea (Drury) to Host Plants and Its Metabolic Mechanisms Against the Secondary Metabolites. Master’s Thesis, Beijing Forestry University, Beijing, China, 2018. [Google Scholar]
- Liu, X.X. Effects of Three Plant Secondary Substances on the Growth and Detoxification Ability of Hyphantria cunea. Master’s Thesis, Northeast Forestry University, Harbin, China, 2020. [Google Scholar]
- Wang, X.J.; Lu, H.; Feng, Y.J.; Zong, S.X. Detoxification and digestive enzyme activities in Streltzoviella insularis (Staudinger) larvae (Lepidoptera: Cossidae) in response to host trees. J. Environ. Entomol. 2020, 42, 480–492. [Google Scholar]
- Feng, N. The Effect of Host Fruit on the Infestation and Growth Development of Grapholita molesta Busck and the Primary Physic-Chemical Mechanism. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2014. [Google Scholar]
- Bai, P. The Damage of Grapholitha molesta to Peach Tree and the Effect of Some Secondary Substances. Master’s Thesis, Shandong Agricultural University, Taian, China, 2015. [Google Scholar]
- Wang, C.Z.; Huang, Q.L. Selection of host plants by phytophagous insect. In Frontiers of Chemical Ecology; Kong, C.H., Lou, Y.G., Eds.; Higher Education Press: Beijing, China, 2010; pp. 143–173. [Google Scholar]
- Dar, S.A.; Wani, A.B.; Wani, M.Y.; Hussain, S.; Majid, M.S. Plant-insect interactions-cyanogenic glucosides. Imp. J. Interdiscipl. Res. 2016, 2, 1107–1118. [Google Scholar]
- Santos, P.L.P.; Schilthuizen, M.; Verpoorte, R.; Choi, Y.H. Quantitative analysis of amygdalin and prunasin in Prunus serotina Ehrh. using H-NMR spectroscopy. Phytochem. Anal. 2014, 25, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem. 2015, 170, 437–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wybouw, N.; Dermauw, W.; Leeuwen, T.V. A gene horizontally transferred from bacteria protects arthropods from host plant cyanide poisoning. eLife 2014, 3, e02365. [Google Scholar] [CrossRef]
- Simon, J.; Gleadow, R.M.; Woodrow, I.E. Allocation of nitrogen to chemical defence and plant functional traits is constrained by soil N. Tree Physiol. 2010, 30, 1111–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernards, M.A.; Ivanov, D.A.; Neculai, M.A.; Nicol, R.W. Ginsenosides: Phytoanticipins or host recognition factors? In The Biological Activity of Phytochemicals, Recent Advances in Phytochemistry; Gang, D.R., Ed.; Springer: New York, NY, USA, 2011; pp. 13–32. [Google Scholar]
- Jensen, N.B.; Zagrobelny, M.; Hjernø, K.; Olsen, E.; Bak, S. Convergent evolution in biosynthesis of cyanogenic defence compounds in plants and insects. Nat. Commun. 2011, 2, 273. [Google Scholar] [CrossRef] [PubMed]
- Ballhorn, D.J.; Elias, J.D. Salinity-mediated cyanogenesis in white clover (Trifolium repens) affects trophic interactions. Ann. Bot. 2014, 114, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in co-evolution. Evol. 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Jones, D.A. Cyanogenesis in animal-plant interactions. Ciba. Found. Symp. 1988, 140, 151–170. [Google Scholar] [PubMed]
- Schoonhoven, L.M.; van Loon, J.J.A. An inventory of taste in caterpillars: Each species its own key. Acta Zool. Acad. Sci. H. 2002, 48, 215–263. [Google Scholar]
- van Loon, J.J.A.; Wang, C.Z.; Nielsen, J.K.; Gols, R.; Qiu, Y.T. Flavonoids from cabbage are feeding stimulants for diamondback moth larvae additional to glucosinolates: Chemoreception and behaviour. Entomol. Exp. Appl. 2002, 104, 27–34. [Google Scholar] [CrossRef]
- Sajitha, T.P.; Siva, R.; Manjunatha, B.L.; Rajani, P. Sequestration of the plant secondary metabolite, colchicine, by the noctuid moth Polytela gloriosae (Fab.). Chemoecology 2019, 29, 135–142. [Google Scholar] [CrossRef]
- Szewczyk, K.; Zidorn, C. Ethnobotany, phytochemistry, and bioactivity of the genus Turnera (Passifloraceae) with a focus on damiana-Turnera diffusa. J. Ethnopharmacol. 2014, 152, 424–443. [Google Scholar] [CrossRef]
- Zagrobelny, M.; Motawia, M.S.; Olsen, C.E.; Bak, S.; Møller, B.L. Male-to-female transfer of 5- hydroxytryptophan glucoside during mating in Zygaena filipendulae (Lepidoptera). Insect Biochem. Mol. Biol. 2013, 43, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Zagrobelny, M.; Motawie, M.S.; Olsen, C.E.; Pentzold, S.; Fürstenberg-Hägg, J. Sequestration, tissue distribution and developmental transmission of cyanogenic glucosides in a specialist insect herbivore. Insect Biochem. Mol. Biol. 2014, 44, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chai, X.H.; Guo, Y.F.; Hu, X.F.; Kong, W.N.; Li, J.; Ma, R.Y. Seasonal variation of amygdalin content in different host plants of Grapholitha molesta. Nor. Horticul. 2022; in press. [Google Scholar]
- Du, J.; Wang, Y.R.; Wu, J.X. Effect of four different artificial diets on development and reproduction of Grapholita molesta (Lepidoptera: Tortricidae). J. Shanxi Agr. Univ. 2010, 30, 229–231. [Google Scholar]
- Kong, W.N.; Wang, Y.; Jia, X.T.; Gao, Y.; Fan, R.J.; Li, J.; Ma, R.Y. Emergence and mating behavior of the oriental fruit moth Cydia molesta (Lepidoptera: Tortricidae) and its potential for reproduction. Ann. Soc. Entomol. Fr. 2019, 55, 446–453. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. One-way classifications: Analysis of variance. In Statistical Methods; Snedecor, G.W., Chochran, W.G., Eds.; Iowa State University Press: Ames, IA, USA, 1967; pp. 258–298. [Google Scholar]
- Hood, G.M. PopTools Version 3.2.5. 2010. Available online: http://www.poptools.org (accessed on 2 September 2017).
- Lu, P.F.; Huang, L.Q.; Wang, C.Z. Semiochemicals used in chemical communication in the oriental fruit moth (Grapholitha molesta) (Lepidoptera: Tortricidae). Acta Entomol. Sinica 2010, 53, 1390–1403. [Google Scholar]
- Lu, P.F.; Huang, L.Q.; Wang, C.Z. Identification and field evaluation of pear fruit volatiles attractive to the oriental fruit moth, Cydia molesta. J. Chem. Ecol. 2012, 38, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, G.; Xu, X.; Wu, J. Development and fecundity performance of oriental fruit moth (Lepidoptera: Tortricidae) reared on shoots and fruits of peach and pear in different seasons. Environ. Entomol. 2015, 44, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Piskorski, R.; Ineichen, S.; Dorn, S. Ability of the oriental fruit moth Grapholita molesta (Lepidoptera: Tortricidae) to detoxify juglone, the main secondary metabolite of the non-host plant walnut. J. Chem. Ecol. 2011, 37, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Piskorski, R.; Dorn, S. How the oligophage codling moth Cydia pomonella survives on walnut despite its secondary metabolite juglone. J. Insect Physiol. 2011, 57, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, R.L.; Hemming, J. Responses of the gypsy moth (Lepidoptera: Lymantriidae) to tremulacin, an aspen phenolic glycoside. Environ. Entomol. 1990, 19, 842–847. [Google Scholar] [CrossRef]
- Zagrobelny, M.; Bak, S.; Ekstrøm, C.T.; Olsen, C.E.; Møller, B.L. The cyanogenic glucoside composition of Zygaena filipendulae (Lepidoptera: Zygaenidae) as effected by feeding on wild-type and transgenic lotus populations with variable cyanogenic glucoside profiles. Insect Biochem. Mol. Biol. 2007, 37, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Jafary-Jahed, M.; Razmjou, J.; Nouri-Ganbalani, G.; Naseri, B.; Hassanpour, M.; Leppla, N.C. Life table parameters and oviposition preference of Plutella xylostella (Lepidoptera: Plutellidae) on six brassicaceous crop plants. J. Econ. Entomol. 2019, 112, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Clissold, F.J.; Simpson, S.J. Temperature, food quality and life history traits of herbivorous insects. Curr. Opin. Insect Sci. 2015, 11, 63–70. [Google Scholar] [CrossRef]
- Kuczyk, J.; Raharivololoniaina, A.; Fischer, K. Population-specific responses of an insect herbivore to variation in host-plant quality. Ecol. Evol. 2021, 11, 17963–17972. [Google Scholar] [CrossRef]
- Rania, S.R.; Doaa, M.H. Toxic effect of Spirulina platensis and Sargassum vulgar as natural pesticides on survival and biological characteristics of cotton leaf worm Spodoptera littoralis. Sci. Afr. 2020, 8, e00323. [Google Scholar]
- Ayyanath, M.M.; Scott-Dupree, C.D.; Cutler, G.C. Effect of low doses of precocene on reproduction and gene expression in green peach aphid. Chemosphere 2015, 128, 245–251. [Google Scholar] [CrossRef]
- Tang, Q.; Xiang, M.; Hu, H.; An, C.; Gao, X. Evaluation of sublethal effects of sulfoxaflor on the green peach aphid (Hemiptera: Aphididae) using life table parameters. J. Econ. Entomol. 2015, 108, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Rix, R.R.; Ayyanath, M.M.; Christopher Cutler, G. Sublethal concentrations of imidacloprid increase reproduction, alter expression of detoxification genes, and prime Myzus persicae for subsequent stress. J. Pest Sci. 2016, 89, 581–589. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, Y.; Zhang, Z.; Xu, C.; Mu, W. Sublethal and hormesis effects of clothianidin on the black cutworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 2018, 111, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Gabre, R.M.; Adham, F.K.; Chi, H. Life table of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae). Acta Oecol. 2005, 27, 179–183. [Google Scholar] [CrossRef]
- Siemens, D.H.; Mitchell-Olds, T. Glucosinolates and herbivory by specialists (Coleoptera: Chrysomelidae, Lepidoptera: Plutellidae): Consequences of concentration and induced resistance. Environ. Entomol. 1996, 25, 1344–1353. [Google Scholar] [CrossRef]
- Tanton, M.T. Agar and chemostimulant concentrations and their effect on intake of synthetic food by larvae of the mustard beetle, Phaedon cochleariae. Entomol. Exp. Appl. 1965, 8, 74–82. [Google Scholar] [CrossRef]
- El Sayed, G.; Louveaux, A.; Mavratzotis, M.; Rollin, P.; Quinsac, A. Effects of glucobrassicin, epiprogoitrin and related breakdown products on locusts feeding: Schouwia purpurea and desert locust relationships. Entomol. Exp. Appl. 1996, 78, 231–236. [Google Scholar] [CrossRef]
- Li, Q.; Eigenbrode, S.D.; Stringham, G.R.; Thiagarajah, M.R. Feeding and growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with varying glucosinolate concentrations and myrosinase activities. J. Chem. Ecol. 2000, 26, 2401–2419. [Google Scholar] [CrossRef]
- Müller, C.; Schulz, M.; Pagnotta, E.; Ugolini, L.; Yang, T.; Matthes, A.; Lazzeri, L.; Agerbirk, N. The role of the glucosinolate-myrosinase system in mediating greater resistance of Barbarea verna than B. vulgaris to Mamestra brassicae larvae. J. Chem. Ecol. 2018, 44, 1190–1205. [Google Scholar] [CrossRef]
- Gutbrodt, B.; Dorn, S.; Unsicker, S.B.; Mody, K. Species-specific responses of herbivores to within-plant and environmentally mediated between-plant variability in plant chemistry. Chemoecology 2012, 22, 101–111. [Google Scholar] [CrossRef]
- Cutler, G.C.; Guedes, R.N.C. Occurrence and significance of insecticide-induced hormesis in insects. In Pesticide Dose: Effects on the Environment and Target and Non-Target Organisms; Duke, S.O., Kudsk, P., Solomon, K., Eds.; American Chemical Society: New York, NY, USA, 2017; pp. 101–119. [Google Scholar]
- Silva, M.C.; Terra, W.R.; Ferreira, C. Absorption of toxic beta-glucosides produced by plants and their effect on tissue trehalases from insects. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2006, 143, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, R.L. Differential esterase activity in Papilio glaucus subspecies: Absence of cross-resistance between allelochemicals and insecticides. Pestic. Biochem. Phys. 1989, 35, 185–191. [Google Scholar] [CrossRef]
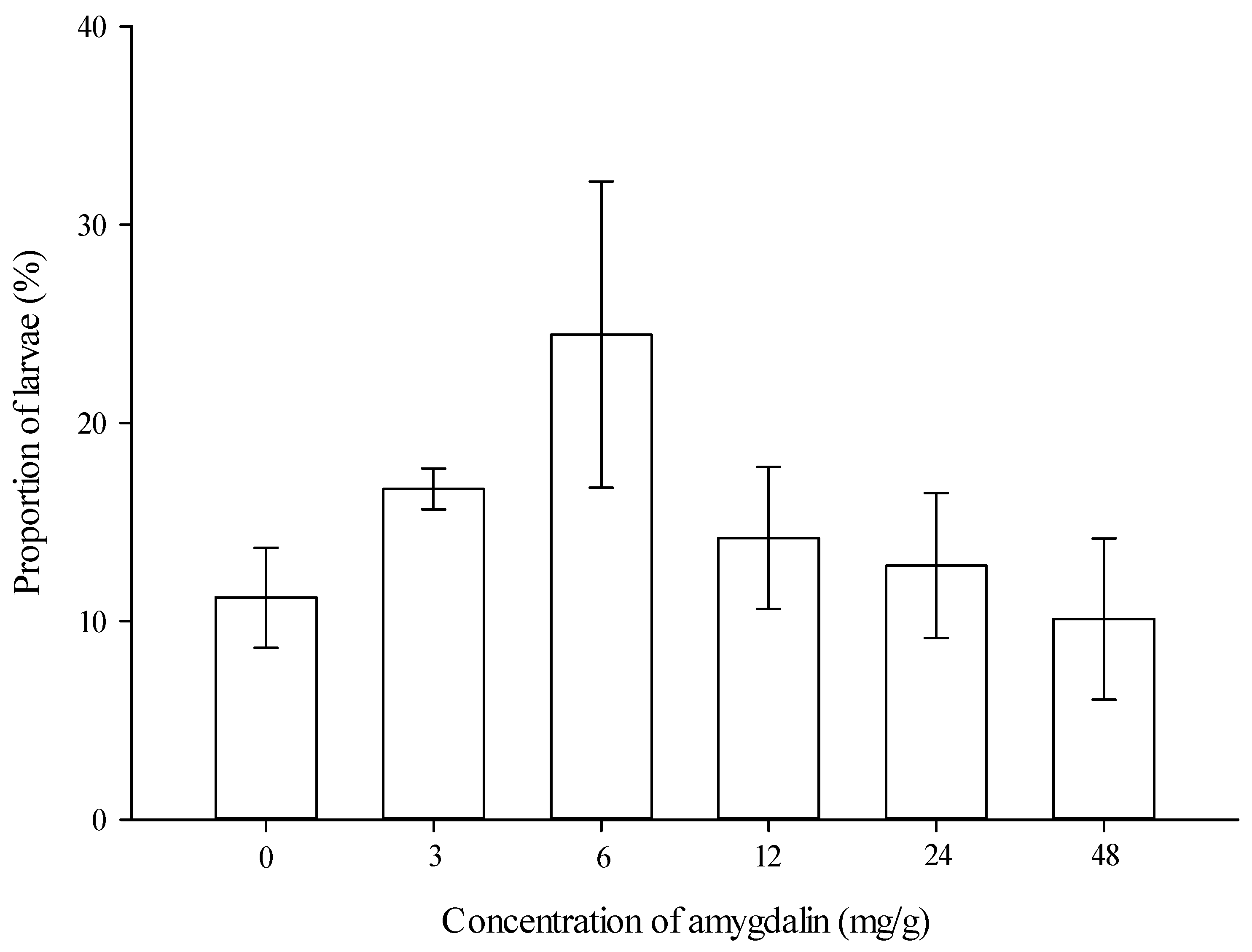
| Amygdalin (mg Compound/g Diet) | Pupal Weight (mg) | Total Eggs (Number of Emerged Females in Group) | Number of Eggs Laid/Female |
|---|---|---|---|
| 0 | 8.70 ± 0.16 b | 2274 (46) | 49.43 |
| 3 | 8.10 ± 0.30 ab | 2599 (40) | 64.98 |
| 6 | 8.80 ± 0.18 b | 3598 (62) | 58.03 |
| 12 | 8.60 ± 0.19 ab | 3453 (62) | 55.69 |
| 24 | 7.90 ± 0.20 a | 2595 (44) | 58.98 |
| 48 | 9.00 ± 0.27 b | 676 (38) | 17.79 |
| Amygdalin (mg Compound/g Diet) | Pupal Weight (mg) | Total Eggs Laid (Number of Emerged Females in Group) | Number of Eggs Laid/Female |
|---|---|---|---|
| 6 mg/g (1 gen.) | 8.80 ± 0.18 a | 3598 (62) | 58.03 |
| 0 mg/g | 10.20 ± 0.23 b | 2043 (39) | 52.38 |
| 6 mg/g (10 gen.) | 7.90 ± 0.41 a | 922 (12) | 76.83 |
| Amygdalin (mg Compound/g Diet) | rm | λ | R0 | T |
|---|---|---|---|---|
| 0 | 0.101 | 1.107 | 35.431 | 34.147 |
| 3 | 0.106 | 1.112 | 28.756 | 30.708 |
| 6 | 0.105 | 1.111 | 38.838 | 33.212 |
| 12 | 0.095 | 1.100 | 37.633 | 36.990 |
| 24 | 0.094 | 1.099 | 27.920 | 34.267 |
| 48 | 0.061 | 1.063 | 14.930 | 43.632 |
| Amygdalin (mg Compound/g Diet) | rm | λ | R0 | T |
|---|---|---|---|---|
| 6 mg/g (1 gen.) | 0.105 | 1.111 | 38.838 | 33.212 |
| 0 mg/g | 0.107 | 1.113 | 28.635 | 30.596 |
| 6 mg/g (10 gen.) | 0.106 | 1.112 | 23.978 | 29.178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, J.; Chai, X.; Hu, X.; Li, X.; Kong, W.; Ma, R. Development and Fecundity of Oriental Fruit Moth (Lepidoptera: Tortricidae) Reared on Various Concentrations of Amygdalin. Insects 2022, 13, 974. https://doi.org/10.3390/insects13110974
Wang Y, Li J, Chai X, Hu X, Li X, Kong W, Ma R. Development and Fecundity of Oriental Fruit Moth (Lepidoptera: Tortricidae) Reared on Various Concentrations of Amygdalin. Insects. 2022; 13(11):974. https://doi.org/10.3390/insects13110974
Chicago/Turabian StyleWang, Yi, Jie Li, Xiaohan Chai, Xuefeng Hu, Xianwei Li, Weina Kong, and Ruiyan Ma. 2022. "Development and Fecundity of Oriental Fruit Moth (Lepidoptera: Tortricidae) Reared on Various Concentrations of Amygdalin" Insects 13, no. 11: 974. https://doi.org/10.3390/insects13110974






