Simple Summary
In this study, a new mealybug species from the Cape Verde Islands, Mirococcus capeverdensis Łagowska and Hodgson sp. n., collected on Campylanthus glaber Benth. (Scrophulariaceae) is described and illustrated based on the adult female. A key to the mealybugs from the Afrotropical Region that lack cerarii is provided. In addition, seven scale insect species are recorded for the first time from the Cape Verde Islands. An updated checklist of Coccomorpha species from this region, along with their known island distributions and valid sources, is also appended. Prior to this study, 38 scale insect species in 7 families and 28 genera are known from the Cape Verde Islands.
Abstract
In this study, a new species of mealybug from the Cape Verde Islands, Mirococcus capeverdensis Łagowska and Hodgson sp. n., collected on Campylanthus glaber Benth. (Scrophulariaceae), a native plant to these islands, is described and illustrated based on the adult female. A taxonomic key to the mealybugs from the Afrotropical Region that lack cerarii is provided. In addition, seven scale insect species, namely Aulacaspis tubercularis Newstead, Hemiberlesia cyanophylli (Signoret), Pseudaonidia trilobitiformis (Green), Icerya aegyptiaca (Douglas), Maconellicoccus hirsutus (Green), Palmicultor palmarum (Ehrhorn), and Pseudococcus comstocki (Kuwana) are recorded for the first time from the Cape Verde Islands and an updated checklist of Coccomorpha species known from this region, along with their known island distributions and valid sources, is appended.
1. Introduction
The Afrotropical region has a unique and diverse scale insect fauna with 1458 species distributed on the African continent and its many oceanic islands, including the Cape Verde archipelago [1].
The Cape Verde volcanic archipelago is a group of 10 major islands plus several smaller uninhabited islets located in the central Atlantic Ocean, approximately 570 km off the western coast of the African continent, to the west of Senegal, Gambia, and Mauritania. It lies between 14° to 18° N latitude and 22° to 26° W longitude and forms part of the Macaronesia ecoregion, along with the Azores, the Canary Islands, Madeira, and the Savage Isles. The total land area is approximately 4033 km2, with the islands of Santiago (785.0 km2) and Santo Antão (785 km2) being the largest. Three of the Cape Verde islands, Sal, Boa Vista, and Maio, are fairly flat, sandy, and dry; the others are generally rockier with more vegetation. Cape Verde’s climate is milder than that of the African mainland, with average daily high temperatures ranging from 26 °C in February to 31 °C in September, whilst the average annual rainfall ranges from less than 100 mm in the arid coastal areas to more than 1000 mm in the humid mountains. Vegetation in the islands is similar to that of the savannah or steppe type. Some of the plant species are considered to be endemic, having evolved over millions of years of isolation [2,3].
According to ScaleNet, an open-source database for scale insects [1], prior to this study, 38 scale insect species in 7 families and 28 genera had been recorded from the Cape Verde Islands. These data were the result of studies by only a few scientists, notably Fernandes [4,5,6,7], Schmutterer et al. [8], and Van Harten et al. [9]. In February 2018, the first author had the opportunity to travel to three of the Cape Verde islands (Sal, Santo Antão, and São Vicente) and collected scale insects of 5 families and 18 genera, among them an undescribed species, Mirococcus capeverdensis Łagowska and Hodgson sp. n.
In this paper, we describe and illustrate the adult female of this new species of Mirococcus, which was collected on Campylanthus glaber Benth. (Scrophulariaceae), a native plant to the Cape Verde Islands. In addition, we include a key to the mealybugs known from the Afrotropics that lack cerarii. Of the other species collected, seven are new species records for the Cape Verde Islands and an updated checklist of Coccomorpha from this archipelago is also included.
2. Materials and Methods
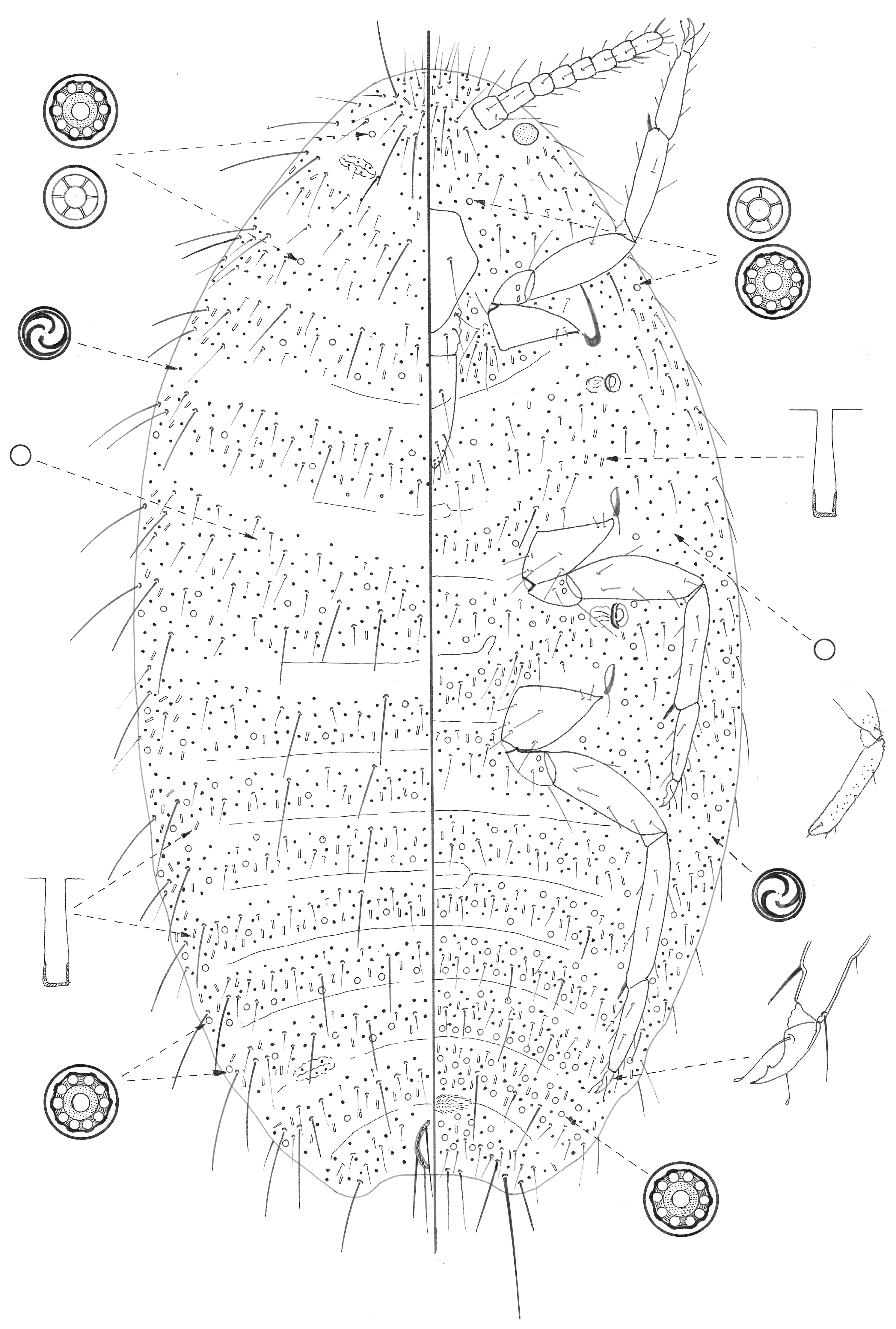
The material studied was collected during February 2018 on Sal, Santo Antão, and the São Vicente islands, mainly on wild plants. In total, approximately 100 lots of scale insect material were collected and studied to select suitable specimens for slide-mounting. These were stained and mounted in Canada Balsam on glass slides, mainly using the methodology in Hodgson and Henderson [10], except the specimens were left in cold KOH for one or two days before clearing. The figure of M. capeverdensis Łagowska and Hodgson sp. n. shows the dorsum on the left side and the venter on the right, with vignettes of important structures (not drawn to scale) around the margin. The measurements provided for the legs, spiracles, clypeolabral shield, and anal plates are for their greatest width or length. The morphological terms for Pseudococcidae follow those of Williams [11].
The slides are stored in the Department of Plant Protection, at the University of Life Sciences in Lublin, except for the holotype and paratype of the new species, which were deposited in the Natural History Museum, Cromwell Road, London, UK (BMNH).
3. Results
- Taxonomy
- Pseudococcidae
- Mirococcus Borchsenius, 1947: 142 [12]
- Type species: Phenacoccus inermis Hall, 1925 by original designation.
- Generic diagnosis. Members of the genus Mirococcus can be diagnosed by the following combination of features: antennae eight or nine segmented (rarely seven); legs usually normally developed; claw usually with a denticle; anal apparatus complete in type species, but much simplified in other species; posterior pair of ostioles always present, anterior pair sometimes absent; multilocular and trilocular pores present; quinquelocular pores present or absent; oral collar tubular ducts usually simple; oral rim ducts absent; cerarii absent, and conical setae absent [13].
- Comment.Mirococcus is one of the few genera of mealybugs that completely lack cerarii and conical setae. Species found within the African continent lacking cerarii can be separated using the key below.
- Mirococcus capeverdensis Łagowska and Hodgson sp. n.
- Material studied: Holotype: Left label: Cape Verde Is / Sal, St. Maria / Campylanthus glaber / 14.ii.2018 / B. Łagowska; right label: Mirococcus / capeverdensis / Łagowska & / Hodgson / Holotype (one adult female in fair–good condition, body slightly twisted). Paratype: Data are the same as for the holotype, a single adult female but broken in two, with each half mounted on separate slides. The anterior half is poor, and the posterior half is good. All three slides were deposited in BMNH, London.
Description of adult female (Figure 1). Data for paratype in brackets. Appearance in life not recorded. Mounted adult female 1.3 mm long and 0.5 mm wide. Anal lobes small, each with a long apical seta 118–126 (118) µm long, plus several other setae, each 50–76 (55) µm long; anal lobe bar absent. Antennae eight (nine) segmented, each approximately 223 (252) µm long. Legs well developed, with lengths (iii) in µm: coxa 90–95 (116–120), trochanter + femur 168 (189); tibia 144 (151), and tarsus 84–86 (84); tarsal digitules setose; claw approximately 26–28 (27) um long, with a strong denticle; claw digitules knobbed, longer than claw; translucent pores present on distal end of femur and on tibia. Labium approximately 100 µm long; clypeolabral shield with a single pair of long setae. Spiracles with anterior peritreme 25 (32–34) µm wide, posterior peritreme 32 (42) µm wide. Circulus well developed, approximately 50 (92) µm wide, possibly divided by an intersegmental line. Ostioles present, with few trilocular pores on lips and no setae. Cerarii absent but with very long setose setae (lengths ranging from 35 µm to 60 µm) in groups of one to three along margin, located approximately segmentally. Each group also with one to three shorter setae, each approximately 25–35 µm long.
Figure 1.
Adult female of Mirococcus capeverdensis Łagowska and Hodgson sp.n.
Dorsal surface with rows of setae, some very short (only about 8 µm long) but also with a few setae similar to long marginal setae, each 40–50 (40–50) µm long with a few on all segments. Multilocular disc pores, each 6–8 µm wide, frequent throughout but most abundant posteriorly; where present mainly along posterior margin of each segment; those posteriorly all with 10 loculi, but number of loculi decreasing more anteriorly, with five or six loculi in some smaller pores. Quinquelocular pores otherwise absent. Trilocular pores slightly larger than usual, almost round, each 3–4 µm wide, and present throughout. Simple pores, each approximately 1.5 µm wide, present sparsely throughout. Oral collar tubular ducts, each 8–10 µm long, present throughout but most abundant posteriorly and marginally. Oral rim ducts absent.
Ventral surface with mainly slender setose setae but with an occasional quite long seta, as on dorsum. Multilocular disc pores as on dorsum, throughout but most abundant on abdomen; where present mainly along both anterior and posterior margins. Trilocular pores, as on dorsum, evenly distributed. Simple pores as on dorsum. Oral collar tubular ducts present, as on dorsum, but more abundant and present more or less throughout. Oral rim ducts absent.
Etymology. The name of the species is derived from Cape Verde Islands, the archipelago from which this species was collected, with the adjectival suffix -ensis, indicating the place of origin.
Comment. Adult female M. capeverdensis sp. n. can be diagnosed by a combination of the following features: (i) all cerarii and conical setae absent; (ii) dorsum, margin, and venter with very long setae, each up to 60 µm long; (iii) small anal lobes; (iv) legs with translucent pores; (v) each claw with a strong denticle; (vi) multilocular pores present on both dorsum and venter; (vii) oral collar tubular ducts all about the same size, present on dorsum and venter, and; (viii) trilocular pores slightly larger than usual, almost round.
M. capeverdensis sp. n. differs from all other species in this genus in having very long setae on the dorsum and margin.
Of the species currently included in Mirococcus, adult female M. capeverdensis sp. n. are most similar to that of M. inermis (Hall), sharing the following features: (i) cerarii and conical setae absent; (ii) circulus well developed; (iii) both anterior and posterior ostioles present; (iv) legs with translucent pores; (v) simple pores scattered on the dorsum and venter; (vi) multilocular pores present on the dorsum and venter, and (vii) oral collar tubular ducts of one size present on the dorsum and venter. However, it differs as follows (character-states in M. inermis in brackets): (i) many dorsal setae, very long and setose (all dorsal setae short and slender), (ii) claw digitules capitate (claw digitules setose), and (iii) claw with a strong denticle (claw denticle weak).
With the description of M. capeverdensis sp. n., the total number of species in Mirococcus is increased to 15, distributed mainly in the Palaearctic Region (14 species). Currently, only two Mirococcus species are known from the Afrotropics: M. inermis (Hall), recorded from north Africa (Egypt, Sudan, and Tunisia), and M. capeverdensis sp. n. from the Cape Verde Islands.
- Key to mealybug genera and species in the Afrotropical Region that lack cerarii, including cerarii on the anal lobes (mainly after Watson (unpublished) [14] and Millar [15]).
- 1.
- Dorsal setae and ventral marginal body setae very stoutly spiniform with apices rounded or truncated, and some slightly curved. Antennae nine segmented. Hind leg without translucent pores. Claw with a denticle. Quinquelocular pores present … Eriocorys hystrix De Lotto (South Africa).
- -
- Body setae are usually slender, with acute apices. Other characteristics not in this combination … 2
- 2.
- Multilocular disc pores absent from both dorsum and venter … 3
- -
- Multilocular disc pores present, at least on the venter near the vulva … 5
- 3.
- Quinquelocular pores present on both the venter and dorsum. Antennae, each with six segments. Claw without a denticle … Lacombia Goux (two species: L. bouhelieri (Goux) (Morocco), L. dactyloni (Bodenheimer) (Tunisia)).
- -
- Quinquelocular pores are absent. Antennae, each with 5–7 segments. Claw with or without a denticle … 4
- 4.
- Anal ring situated at body apex, bearing eight setae. Antenna with five or six segments. Hind leg fairly slender, without translucent pores. Found living underground in an ant’s nest … Bimillenia plagiolepicola Matile-Ferrero and Ben-Dov (Algeria).
- -
- Anal ring situated on dorsum, bearing six setae. Antenna with seven segments. Hind leg is very robust, with translucent pores on coxa, trochanter, and femur. Found living with ants in above-ground domatia on Acacia … Acaciacoccus hockingi Williams and Matile-Ferrero (Kenya, Tanzania).
- 5.
- Hind coxa enlarged basally, bearing granular bosses. Margins of posterior-most four or five abdominal segments bearing lanceolate spines not grouped into cerarii. Anal opening funnel-shaped, and anal ring recessed … Grewiacoccus gregalis Brain (South Africa).
- -
- Hind coxa not enlarged and without granular bosses. Other characteristics not as above … 6
- 6.
- Multilocular disc-pores frequent throughout dorsum and venter … 7
- -
- Multilocular disc-pores, if present, restricted to abdominal segments only … 8
- 7.
- Claw with a strong denticle. Many dorsal setae very long and setose. Claw digitules capitate … Mirococcus capeverdensis Łagowska and Hodgson sp. n. (Cape Verde Islands).
- -
- Claw with a weak denticle. All dorsal setae short and slender. Claw digitules not capitate … Mirococcus inermis (Hall) (Egypt, Tunisia, Sudan).
- 8.
- Anal ring without setae and with only 0–2 cells. Each trochanter with three sensilla on each surface. Claw without a denticle … Lenania De Lotto (two species: L. africana (Brain), L. prisca De Lotto (South Africa)).
- -
- Anal ring with setae, with or without cells. Each trochanter with two sensilla on each surface. Claws with or without a denticle … 9
- 9.
- Anal lobes are well developed, each with a sclerotized area dorsally. Circulus present … Madeurycoccus bicolor De Lotto (South Africa).
- -
- The anal lobes are undeveloped. Circulus absent … 10
- 10.
- Anterior ostioles absent. Oral rim ducts absent … 11
- -
- Anterior ostioles present. Oral rim ducts present, at least ventrally on head … 12
- 11.
- Anal ring is complete, with a single row of cells and six anal ring setae. Multilocular disc pores absent from dorsum. Antennae each six segmented. Claw digitules setose … Mirococcopsis salsolae (Vayssière) (Tunisia).
- -
- Anus reduced to a sclerotized ring lacking cells and setae. Multilocular disc pores present on both dorsum and venter of abdomen. Antennae each seven segmented. Claw digitules capitate … Mirococcopsis ptilura Gavrilov-Zimin (South Africa).
- 12.
- Oral rim ducts numerous on both dorsal and ventral surfaces. Antennae each six segmented. Anal ring complete, with cells and setae, the latter equal in length to diameter of anal ring. Translucent pores present on hind coxae only … Humococcus (Mirococcopsis) mackenziei Ezzat (Egypt).
- -
- Oral rim ducts, if present on both dorsal and ventral surfaces, never numerous. Antennae each seven or eight segmented. Anal ring poorly developed, without cells. Translucent pores either absent or more widespread on hind legs … 13
- 13.
- Trilocular pores of two sizes present, with larger pores forming groups along margin. Multilocular disc pores absent from dorsum. Antennae each seven segmented. Oral rim ducts present on dorsum only. Translucent pores present on hind coxa, femur, and tibia … Iberococcus gomezmenori Matile-Ferrero (Tunisia).
- -
- Trilocular pores of only one size present, never forming groups along margin. Multilocular disc pores present dorsally and ventrally on abdomen. Antennae each eight segmented. Oral rim ducts very few and restricted to ventrally on head. Translucent pores absent from hind legs … Mirococcopsis sphaerica (Balachowsky) (Algeria).
- Checklist of Coccomorpha from Cape Verde Islands
In addition to the new species described above, the samples of scale insects collected on the Cape Verde Islands in 2018 by the first author also included 7 species new to the fauna and 11 species which had been reported earlier by Fernandes [4,5,6,7], Schmutterer et al. [8], and Van Harten et al. [9].
The species new to the Cape Verde islands in the list below include notes regarding their economic importance and worldwide distribution. In addition, an updated checklist of Coccomorpha species known from this archipelago, along with validation sources, is appended (Table 1). Families and species within each family are listed in alphabetical order according to the classification used in the ScaleNet database [1]. The references to species recorded from the Cape Verde Islands reported in ScaleNet have been checked and, where erroneous, corrected in the present checklist.

Table 1.
Checklist of scale insect species from the Cape Verde archipelago. All records from the present study are included in bold.
- Annotated list of scale insect species newly recorded for the Cape Verde Islands
- Family Diaspididae Targioni Tozzetti, 1868
- Aulacaspis tubercularis (Newstead, 1906)
- Material examined: Santo Antão, 6.ii.2018, 7.ii.2018; 5 ♀♀ on the upper leaf surface of mango trees (Anacardiaceae).
- Distribution: A cosmopolitan species endemic to the Asian continent and introduced in other parts of the world together with infested plant material [16].
- Hemiberlesia cyanophylli (Signoret, 1869)
- Material examined: Sal, Santa Maria, 14.ii.2018, ♀♀ common on different wild dicotyledonous plants, including Heliotropium curassavicum L. (Boraginaceae), Synedrella nodiflora (L.) Gaertn. (Asteraceae), and Tetraena fontanesii (Webb and Berthel.) (Zygophyllaceae).
- Distribution: A cosmopolitan, highly polyphagous species widely distributed in the tropical and subtropical regions [17,18,19,20].
- Pseudaonidia trilobitiformis (Green, 1896)
- Material examined: São Vicente, Mindelo, 10.ii.2018, 4 ♀♀ on the upper surface of Nerium oleander (Apocynaceae).
- Distribution: A cosmopolitan species reported in 77 countries in different parts of the world [1]. Its origin is probably southern Asia, where it is common, but it has spread into Africa, the Malagasian area, and tropical America [20].
- Family Monophlebidae Morrison, 1927
- Icerya aegyptiaca (Douglas, 1890)
- Material examined: Sal, Santa Maria, 11.ii.2018, 12.ii.2018, 6 ♀♀ on Euphorbia sp. (Euphorbiaceae); 14.ii.2018, 2♀♀ on Tamarix senegalensis DC. (Tamaricaceae); 15.ii.2018, 1 ♀ on Hibiscus sp. (Malvaceae).
- Distribution: A highly polyphagous species, recorded from the Afrotropical, Australasian, Palaearctic, and Oriental Regions [21,22,23,24].
- Family Pseudococcidae Westwood, 1840
- Maconellicoccus hirsutus (Green, 1908)
- Material examined: São Vicente, Mindelo, 10.ii.2018, 6 ♀♀ on Hibiscus sp. (Malvaceae); Sal, Santa Maria, 12.ii.2018, 8 ♀♀ on Euphorbia sp. (Euphorbiaceae); 14.ii.2018, 16 ♀♀ on Tetraena fontanesi (Zygophyllaceae); Heliotropium curassavicum (Boraginaceae) and Tamarix senegalensis (Tamaricaceae).
- Distribution: A polyphagous species widespread throughout southern Asia, Australia, and Africa [20].
- Palmicultor palmarum (Ehrhorn, 1916)
- Material examined: Sal, Santa Maria, 5.ii.2018, 17.ii.2018, 11 ♀♀ on palms; Santo Antão, 6.ii.2018, 5 ♀♀ on an unidentified plant.
- Distribution: Widely distributed in the Australasian, Oriental, and Palaearctic regions. It has also been reported from the Nearctic and Neotropics [1,11,18,25,26,27]. This is the first report from the Afrotropical Region.
- Pseudococcus comstocki (?) (Kuwana, 1902)
- Material examined: Sal, Santa Maria, 14.ii.2018, 1♀ on an unidentified plant.
- Distribution: A polyphagous species widely distributed in the Nearctic and Palaearctic regions and also reported from a few (1–6) countries in the Neotropical, Australasian, Oriental, and Afrotropical regions [1].
4. Discussion
Following the current study, 48 scale insect species are known from the Cape Verde Islands: 18 Diaspididae, 11 Pseudococcidae, 13 Coccidae, 2 Asterolecaniidae, 2 Monophlebidae, 1 Dactylopiidae, and 1 Ortheziidae (Table 1). This updated checklist includes all the scale insect species recorded from the Cape Verde Islands according to ScaleNet [1], plus the eight species reported here for the first time. However, Orthezia urticae (L.) is erroneously recorded in the ScaleNet catalogue, citing Van Harten et al. [9], but neither they nor any of the other authors who have studied the fauna of these islands, such as Fernandes [4,5,6,7] and Schmutterer et al. [8], mention it as being present. The list above also includes a further three diaspidid species (Aonidomytilus albus (Cockerell), Duplachionaspis natalensis (Maskell), and Pinnaspis buxi (Bouché)) which have been omitted from the ScaleNet database, although there are published records in Schmutterer et al. [8] and Van Harten et al. [9].
In addition to the new Mirococcus species described above, there may be other undescribed species in the various lots of material previously collected on these islands. Van Harten et al. [9] noticed an undescribed species of Planococcoides (which would now be placed in Formicococcus), which he considered had probably been introduced from mainland Africa. Van Harten et al. [9] also collected a Ceroplastes species, which they considered similar to but different from C. rusci (L.). It may have been these specimens from the Cape Verde Islands that were seen by Hodgson and Peronti [28], who also considered the specimens not to be C. rusci (L.) but possibly referable to one of the other cryptic species in this group.
Apart from M. capeverdensis, the newly recorded scale insects in the present study are all widely distributed, polyphagous species and considered to be plant pests. Thus, the cosmopolitan Aulacaspis tubercularis is a serious pest of mangos in many parts of the world [34,35,36]; Hemiberlesia cyanophylli causes damage to various ornamentals and avocado trees, while Pseudaonidia trilobitiformis was recorded as a pest of cocoa in The Democratic Republic of the Congo [1]. Another species, Icerya aegyptiaca, is considered a pest of Artocarpus altilis (Breadfruit) in the Pacific Region [23]. In addition, all the newly recorded mealybug species are known to cause some damage; thus, Maconellicoccus hirsutus is a potentially invasive pest of several crops, particularly pineapple [1]. Palmicultor palmarum attacks the leaves of oil palm and can kill young germinating plants [1,11], and Pseudococcus comstocki is a pest of many fruit and ornamental trees in the U.S.A. and Japan [1]. Although none of the above mentioned species were very abundant, each could potentially be important both ecologically and economically on the Cape Verde Islands.
Author Contributions
Conceptualization, B.Ł. and C.J.H.; investigation: B.Ł.; methodology, B.Ł., C.J.H., and K.G.; slide-mounting, K.G.; formal analysis, B.Ł. and C.J.H.; writing—original draft preparation, B.Ł. and C.J.H.; visualization, K.G.; resources, K.G.; funding acquisition, K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Life Sciences in Lublin, grant number OKK/S/44, in 2019–2022.
Data Availability Statement
All the required data relevant to the presented study are included in the manuscript.
Acknowledgments
The authors are deeply grateful to Douglas J. Williams (Natural History Museum, London) and Giuseppina Pellizzari (University of Padua, Italy) for providing specialized comments and suggestions. We give special thanks to Maria Cristina Duarte (Centre for Ecology, Evolution and Environmental Changes (cE3c), Faculdade de Ciências, Universidade de Lisboa) for her great help with plant identification.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morales, M.G.; Denno, B.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy, N.B. ScaleNet, a literature-based model of scale insect biology and systematics. Database 2016, 2016, bav118. Available online: http//scalenet.info (accessed on 24 February 2022).
- Pim, J.; Peirce, C.; Watts, A.B.; Grevemeyer, I.; Krabbenhoeft, A. Crustal structure and origin of the Cape Verde Rise. Earth Planet. Sci. Lett. 2008, 272, 422–428. [Google Scholar] [CrossRef]
- Griffiths, M. Cape Verde Travel Guide: Where to Go & What to Do; Independently Published: Chicago, IL, USA, 2019; p. 32. [Google Scholar]
- Fernandes, I.M. Contribuiçᾶo para o conhecimento da quermofauna do arquipélago de Cabo Verde (2a). Garcia Orta. Ser. Zool. 1972, 1, 11–16. [Google Scholar]
- Fernandes, I.M. Alguns coccoidea (Homoptera) do Arquipélago de Cabo Verde. Mem. Junta Investig. Ultramar 1973, 58, 251–269. [Google Scholar]
- Fernandes, I.M. Homoptera (Coccoidea) do arquipélago de Cabo Verde. Garcia Orta. Ser. Zool. Lisboa 1975, 4, 41–46. [Google Scholar]
- Fernandes, I.M. Novos dados para o conhecimento da quermofauna do Arquipelago de Cabo Verde. García Orta Ser. Zool. 1999, 23, 85–89. [Google Scholar]
- Schmutterer, H.; Pires, A.; Klein Koch, C. Zur Schädlingsfauna der Kapverdischen Inseln. Z. Angew. Entomol. 1978, 86, 320–336. [Google Scholar] [CrossRef]
- Van Harten, A.; Cox, J.M.; Williams, D.J. Scale insects of the Cape Verde Islands (Homoptera: Coccoidea). Cour. Forsch. Senckenberg 1990, 129, 131–137. [Google Scholar]
- Hodgson, C.J.; Henderson, R. Coccidae (Insecta: Hemiptera: Coccoidea). Fauna N. Z. 2000, 41. [Google Scholar] [CrossRef]
- Williams, D.J. Mealybugs of Southern Asia; The Natural History Museum & Southdene Sdn. Bhd.: Kuala Lumpur, Malaysia, 2004; p. 896. [Google Scholar]
- Borchsenius, N.S. Two New Mealybug Genera and a New Diaspididae Species (Homoptera-Coccoidea) from Armenia. Dokl. Akad. Nauk Arm. SSR 1947, 7, 141–143. [Google Scholar]
- Danzig, E.M.; Gavrilov-Zimin, I.A. Palaearctic Mealybugs (Homoptera: Coccinea: Pseudococcidae); ZIAS: St. Petersburg, Russia, 2014; Volume 148, p. 678. [Google Scholar]
- Watson, G.W. Towards the identification of the scale insects (Hemiptera: Coccomorpha) of continental Africa: Pseudococcidae. unpublished.
- Millar, I.M. Mealybug genera (Hemiptera: Pseudococcidae) of South Africa: Identification and review. Afr. Entomol. 2002, 10, 185–233. [Google Scholar]
- Takagi, S. The tubercularis species group of Aulacaspis (Sternorrhyncha: Coccoidea: Diaspididae). Insecta Matsumurana (New Ser.) 2010, 66, 57–114. [Google Scholar]
- Balachowsky, A.S. Les cochenilles de la Guadeloupe et de la Martinique (première liste). Rev. Pathol. Végétale D’entomologie Agric. Fr. 1957, 36, 198–208. [Google Scholar]
- Williams, D.J.; Watson, G.W. The Scale Insects of the Tropical South Pacific Region, Part 2, The Mealybugs (Pseudococcidae); CAB International Institute of Entomology: Wallingford, UK, 1988; p. 260. [Google Scholar]
- Matile-Ferrero, D.; Oromí, P. Hemiptera. Coccoidea. [List of Wild Species from the Canaries (Mushrooms, Plants and Land Animals).]; Consejería de Política Territorial y Medio Ambiente Gobierno de Canarias Tenerife: Tafira, Spain, 2001; pp. 186–196. [Google Scholar]
- Germain, J.-F.; Minatchy, J.; Pastou, D.; Bagny, P.; Mérion, S.; Pallas, R.; Quilici, S.; Matile-Ferrero, D. An Updated Checklist of the Scale Insects from Réunion Island (Indian Ocean). Acta Zool. Bulg. 2014, 6, 21–27. [Google Scholar]
- Morrison, H. The nondiaspine Coccidae of the Philippine Islands, with descriptions of apparently new species. Philipp. J. Sci. 1920, 17, 147–202. [Google Scholar] [CrossRef]
- Beardsley, J.W., Jr. Insects of Micronesia. Homoptera Coccoidea. Insects Micrones. 1966, 6, 377–562. [Google Scholar]
- Williams, D.J.; Miller, D.R. Scale insects (Hemiptera: Sternorrhyncha: Coccoidea) of the Krakatau Islands including species from adjacent Java. Zootaxa 2010, 2451, 43–52. [Google Scholar] [CrossRef]
- Uesato, T.; Kondo, T.; Unruh, C.; Williams, D.J. Establishment and host records of Icerya aegyptiaca (Douglas) (Hemiptera: Coccoidea: Monophlebidae) in the Sakishima Islands of the Ryukyu Archipelago, Japan, with notes on its worldwide distribution. Entomol. Sci. 2011, 14, 49–55. [Google Scholar] [CrossRef]
- Williams, D.J.; de Willink, M.C.G. Mealybugs of Central and South America; CAB International: London, UK, 1992; p. 635. [Google Scholar]
- Ben-Dov, Y. A Systematic Catalogue of the Mealybugs of the World (Insecta: Homoptera: Coccoidea: Pseudococcidae and Putoidae) with Data on Geographical Distribution, Host Plants, Biology and Economic Importance; Intercept Limited: Andover, UK, 1994; p. 686. [Google Scholar]
- Williams, D.J.; Martin, J.H. A palm mealybug, Palmicultor palmarum (Ehrhorn) (Hem. Pseudococcidae), now found in the Canary Islands. Entomol. Mon. Mag. 2003, 139, 178. [Google Scholar]
- Hodgson, C.J.; Peronti, A.L.B.G. A revision of the wax scale insects (Hemiptera: Sternorrhyncha: Coccoidea: Ceroplastinae) of the Afrotropical Region. Zootaxa 2012, 3372, 1–265. [Google Scholar] [CrossRef]
- Chevalier, A. Les îles du Cap Vert. Flore de l‘Archipel. Rev. Int. Bot. Appl. D’agriculture Trop. 1935, 15, 733–1090. [Google Scholar]
- Nakahara, S. Checklist of the Armored Scales (Homoptera: Diaspididae) of the Conterminous United States; Animal and Plant Health Inspection Service, Plant Protection and Quarantine, United States Department of Agriculture: Washington, DC, USA, 1982; p. 110.
- Miller, D.R.; Davidson, J.A. Armored Scale Insect Pests of Trees and Shrubs; Cornell University Press: Ithaca, NY, USA, 2005; p. 442. [Google Scholar]
- Watson, G.W. Arthropods of Economic Importance: Diaspididae of the World. (Series Title: World Biodiversity Database); ETI Information Services (Expert Center for Taxonomic Identification), University of Amsterdam: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Veiga, M.L. Notas Entomológicas. II. A conchonilha (“Lepidosaphes beckii” Newm.) das laranjeiras de Cabo Verde e seu combate. Garcia Orta 1967, 15, 501–506. [Google Scholar]
- Dos Santos Wolff, V.R.; Corseuil, E. Diaspididae occurring on mango in Brazil with characterization of Aulacaspis tubercularis Newst., 1906 (Hom.: Coccoidea) recorded in Rio Grande do Sul, Brazil. Biociências 1993, 1, 151–161. [Google Scholar]
- Kondo, T.; Kawai, S. Scale insects (Homoptera: Coccoidea) on mango in Colombia. Japan J. Trop. Agric. 1995, 39, 57–58. [Google Scholar]
- Otieno, H.M.O. A Review of White Mango Scale (Aulacaspis tubercularis Newstead; Hemiptera: Diaspididae) in Sub-Saharan Africa: Distribution, Impact and Management Strategies. Pak. J. Agric. Res. 2021, 34, 227–238. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).