Multiple Functions of Malpighian Tubules in Insects: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
| Product | Function | Taxonomy | References |
|---|---|---|---|
| Mucopolysaccharides and proteins | Construction of foam nests | Rhynchota: Homoptera: Aphrophoridae, Cercopidae | [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] |
| Mucofibrils | Construction of dwelling tubes | Rhynchota: Homoptera: Clastopteridae, Machaerotidae | [35,47,48,49,50,51,52,53] |
| Adhesive secretions | Construction of oothecae | Coleoptera: Chrysomelidae | [54,55] |
| Facilitation of locomotion | Coleoptera: Chrysomelidae Neuroptera: Chrysopidae | [56,57,58,59] | |
| Brochosomes | Protection of eggs, juveniles, and adults | Rhynchota: Homoptera: Cicadellidae (ascertained), Psylloidea Heteroptera: Berytidae, Lygaeidae, Miridae, Plataspidae, Rhopalidae, Saldidae, Tingidae Diptera: Culicidae | [8,15,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85] |
| Other organic secretions | |||
| Carbohydrates and proteins | Building of hallways and chambers | Rhynchota: Homoptera: Cicadidae | [43,86,87,88,89] |
| Glycoproteins and lipoproteins | Reinforcing of shields | Rhynchota: Homoptera: Coccoidea | [90,91,92] |
| Glycosaminoglycans, polysaccharides, and proteins | Unclear (related to cocoons covering) | Hymenoptera: Apidae, Megachilidae | [93,94,95,96,97] |
| Inorganic salts | |||
| Calcium (calcospherites and granules) | Hardening of puparium | Diptera: Muscidae, Tephritidae | [98,99,100,101,102,103,104,105,106] |
| Calcium (lime glands) | Precipitation of excessive salts | Diptera: Ephydridae | [107] |
| Calcium and phosphorus | Acquisition of freeze tolerance | Diptera: Tephritidae | [108] |
| Calcium | Hardening of eggs chorion | Phasmatodea: Heteronemiidae | [109,110,111,112,113,114] |
| Calcium | Hardening of cocoons | Lepidoptera: Erebidae, Lasiocampidae | [115,116,117,118,119] |
| Calcium | Construction and closure of pupal cells | Coleoptera: Cerambycidae | [88,120,121,122] |
| Bioluminescence | Attraction of prey and partners | Diptera: Keroplatidae | [16,123,124,125,126,127,128,129,130,131,132,133,134] |
| Silk-like fibers | Construction of cocoons | Coleoptera: Carabidae, Curculionidae (: Hyperinae) | [135,136,137,138,139] |
| Construction of cocoons | Hymenoptera: Eulophidae | [140,141,142,143,144] | |
| Adhesive for debris | Neuroptera: Chrysopidae | [145,146] | |
| Construction of cocoons | Neuroptera: Ascalaphidae, Chrysopidae, Coniopterygidae, Myrmeleontidae, Sisyridae | [58,147,148,149,150,151,152,153,154,155,156,157,158] | |
| Construction of cocoons | Thysanoptera: Aeolothripidae, Melanthripidae, Thripidae | [159,160,161,162,163,164,165,166] |
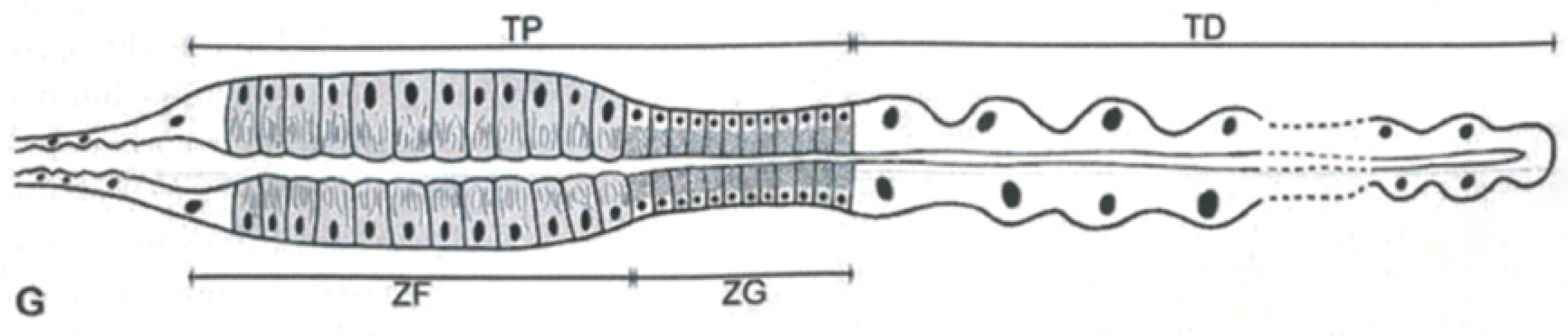
2. Specialized Functions of Malpighian Tubules
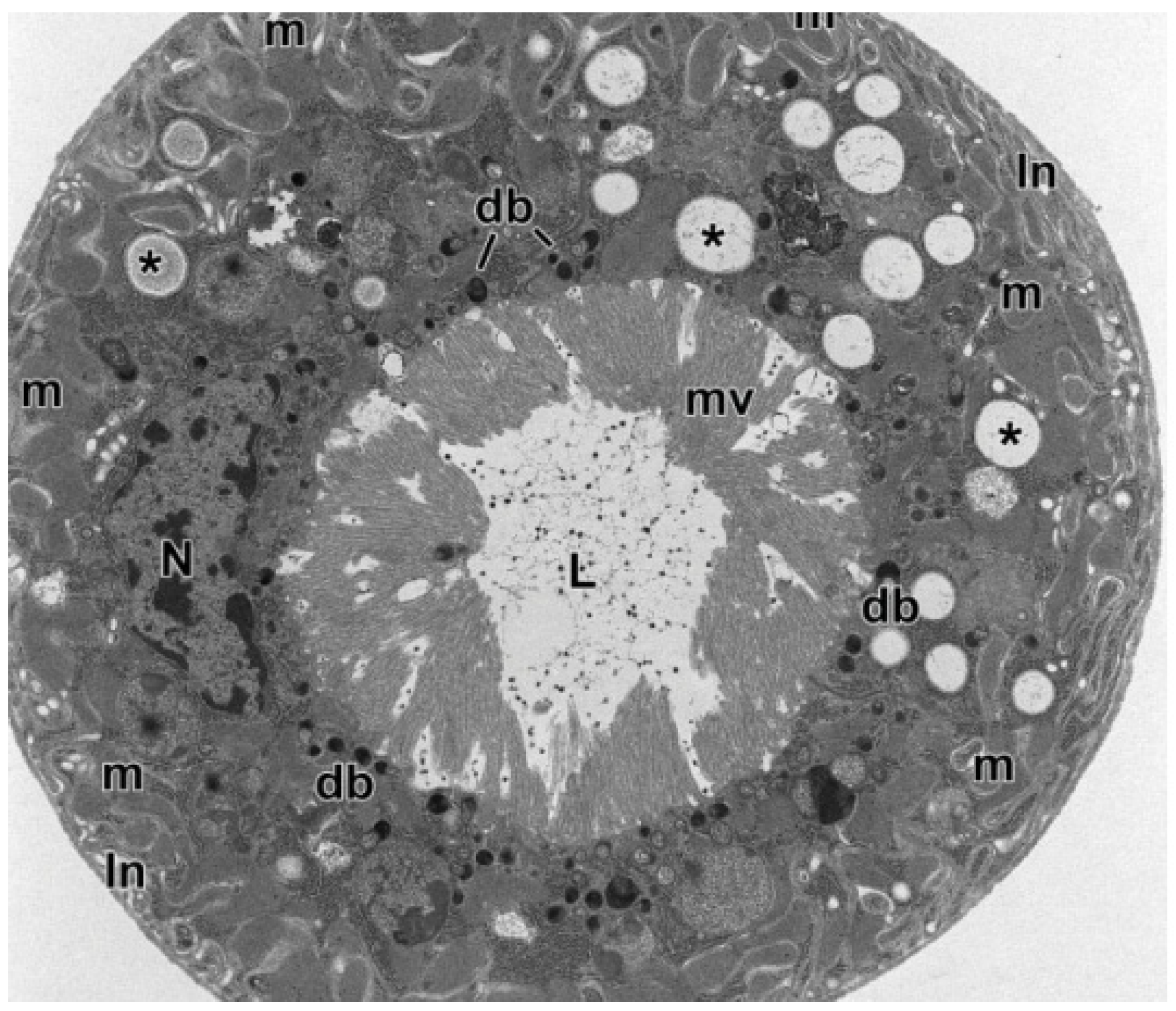
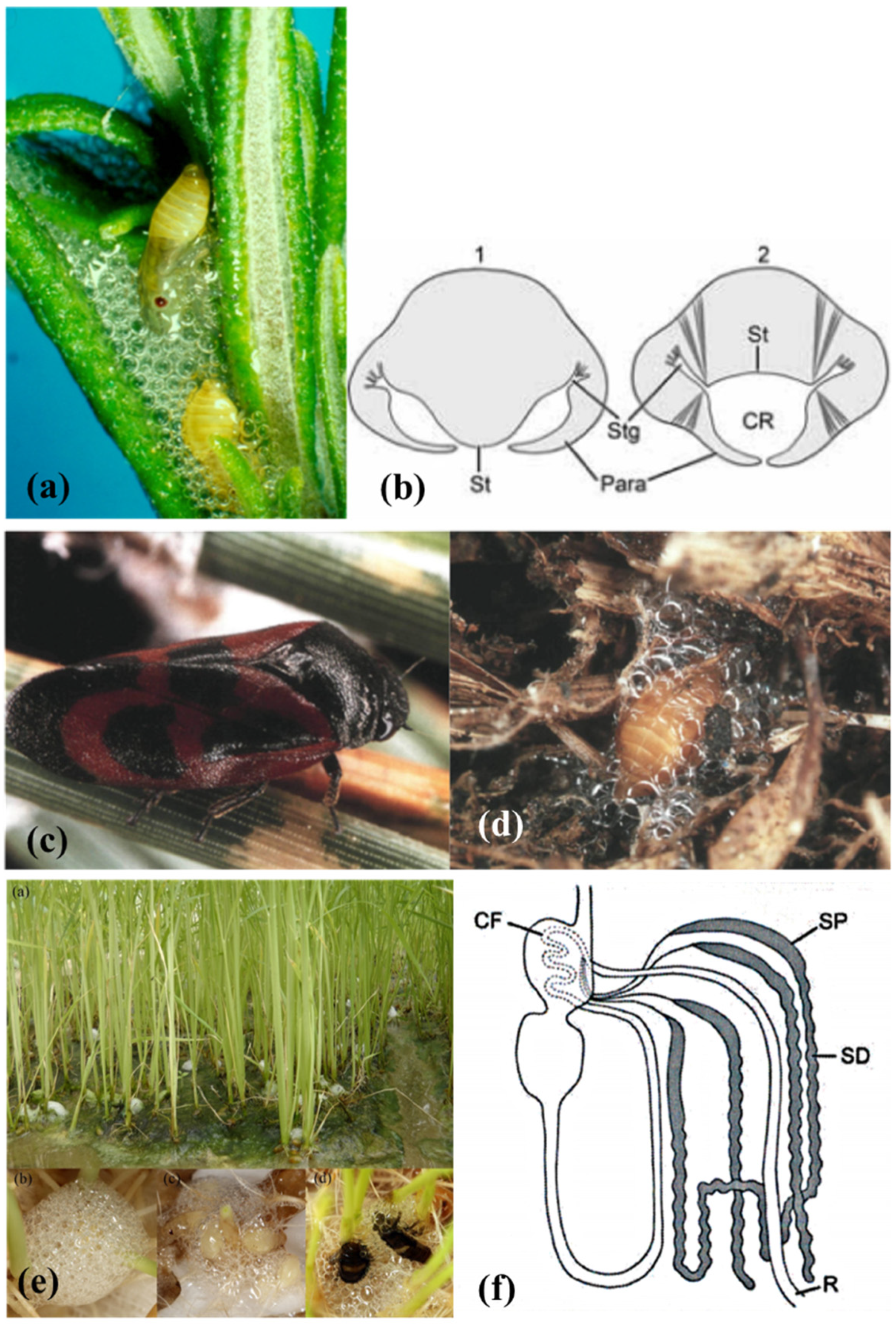
2.1. Mucopolysaccharides and Proteins
2.2. Mucofibrils
2.3. Adhesive Secretions
2.4. Brochosomes
2.5. Other Organic Secretions
2.6. Inorganic Salts
2.7. Bioluminescence
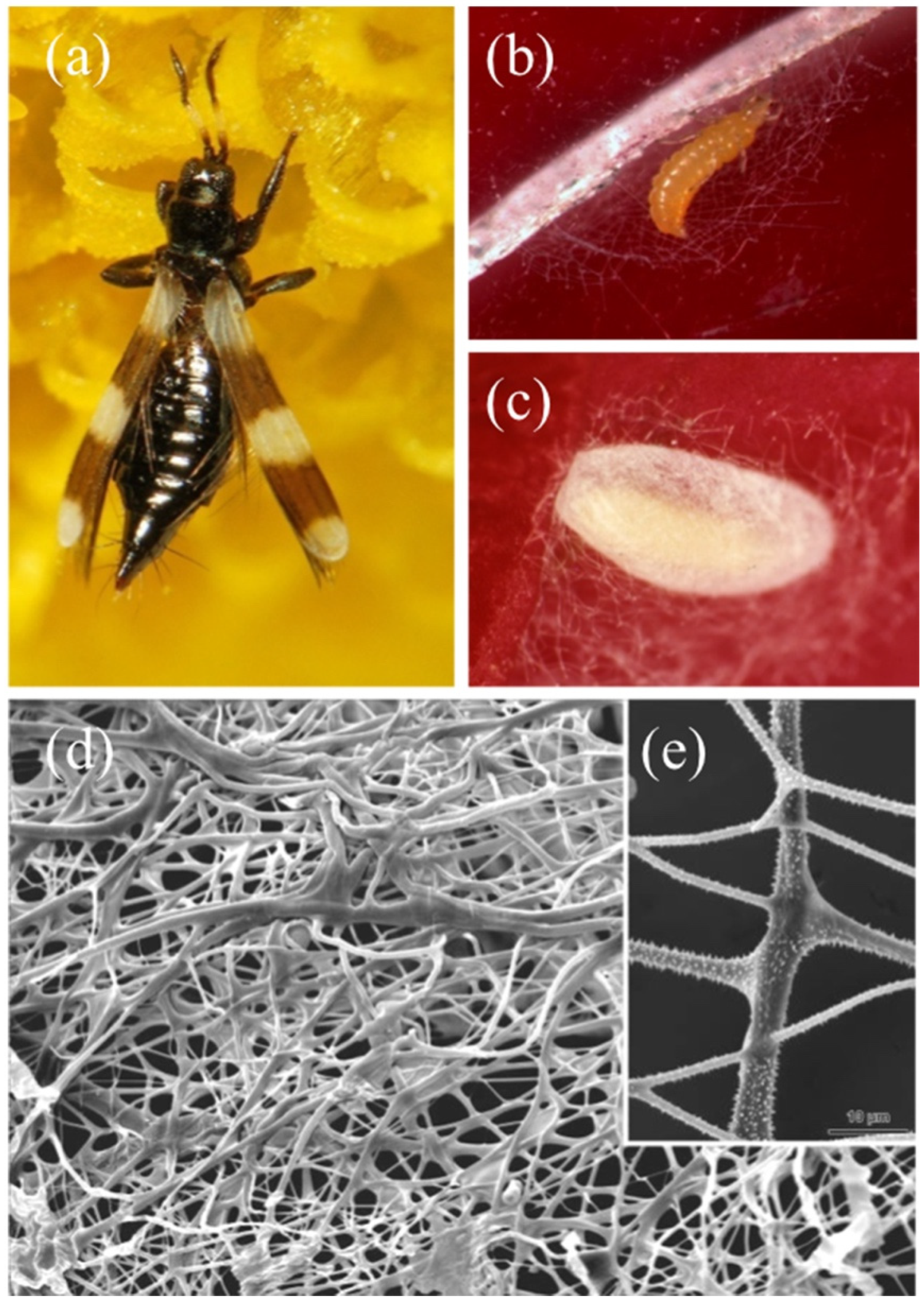
2.8. Silk-like Fibers
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raccaud-Schoeller, J. Les Insectes: Physiologie, Developpement; Masson: Paris, France, 1980; p. 296. [Google Scholar]
- Klowden, M.J. Excretory Systems. In Physiological Systems in Insects; Academic Press: Cambridge, MA, USA, 2002; pp. 403–431. [Google Scholar]
- Bradley, T.J. Excretion. In Encyclopedia of Insects; Resh, V.H., Cardé, R.T., Eds.; Academic Press: Orlando, FL, USA, 2003; pp. 380–387. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function; Cambridge University Press: Cambridge, UK, 2012; p. 770. [Google Scholar] [CrossRef]
- Conti, B.; Giusti, F.; Mercati, D.; Gottardo, M.; Dallai, R. Le funzioni complementari e alternative dei Tubuli Malpighiani degli insetti. Atti Accad. Naz. Ital. Entomol. 2011, 59, 193–210. [Google Scholar]
- Konopová, B.; Kolosov, D.; O’Donnell, M.J. Water and ion transport across the eversible vesicles in the collophore of the springtail Orchesella cincta. J. Exp. Biol. 2019, 222, jeb200691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, X.; White, T.A.; Yang, X.; Douglas, A.E. The molecular correlates of organ loss: The case of insect Malpighian tubules. Biol. Lett. 2015, 11, 20150154. [Google Scholar] [CrossRef]
- Smith, D.S.; Littau, V.G. Cellular specialization in the excretory epithelia of an insect, Macrosteles fascifrons Stål (Homoptera). J. Cell Biol. 1960, 8, 103–133. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J. Comparative physiology of insect renal function. Am. J. Physiol. 1981, 241, 241–257. [Google Scholar] [CrossRef]
- Millet-Boureima, C.; Porras Marroquin, J.; Gamberi, C. Modeling renal disease “on the Fly”. Biomed. Res. Int. 2018, 2018, 5697436. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, M. Insect excretory mechanisms. Adv. Insect Phys. 2008, 35, 1–122. [Google Scholar] [CrossRef]
- Dow, J.A.T.; Davies, S.A. The Malpighian tubule: Rapid insights from post-genomic biology. J. Insect Physiol. 2006, 52, 365–378. [Google Scholar] [CrossRef]
- Dow, J.A.T. Insights into the Malpighian tubule from functional genomics. J. Exp. Biol. 2009, 212, 435–445. [Google Scholar] [CrossRef]
- Casartelli, M. I Tubuli Malpighiani nei processi di detossificazione e difesa. Atti Accad. Naz. Ital. Entomol. 2011, 59, 183–187. [Google Scholar]
- Zhong, H.; Zhang, Y.; Wei, C. Morphology and ultrastructure of the Malpighian tubules in Kolla paulula (Hemiptera: Cicadellidae). Zool. Anz. 2015, 257, 22–28. [Google Scholar] [CrossRef]
- Silva, J.R.; Amaral, D.T.; Hastings, J.W.; Wilson, T.; Viviani, V.R. A transcriptional and proteomic survey of Arachnocampa luminosa (Diptera: Keroplatidae) lanterns gives insights into the origin of bioluminescence from the Malpighian tubules in Diptera. Luminescence 2015, 30, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Shelomi, M. De novo transcriptome analysis of the excretory tubules of Carausius morosus (Phasmatodea) and possible functions of the midgut ‘appendices’. PLoS ONE 2017, 12, e0174984. [Google Scholar] [CrossRef] [Green Version]
- Maddrell, S.H.; Gardiner, B.O. Excretion of alkaloids by Malpighian tubules of insects. J. Exp. Biol. 1976, 64, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.; Linton, S.M.; Francis, P.S.; O’Donnell, M.J.; Toop, T. Accumulation and excretion of morphine by Calliphora stygia, an Australian blow fly species of forensic importance. J. Insect Physiol. 2011, 57, 62–73. [Google Scholar] [CrossRef]
- Zhu, J.; Tian, K.; Reilly, C.A.; Qiu, X. Capsaicinoid metabolism by the generalist Helicoverpa armigera and specialist H. assulta: Species and tissue differences. Pestic. Biochem. Physiol. 2020, 163, 164–174. [Google Scholar] [CrossRef]
- Rheault, M.R.; Plaumann, J.S.; O’Donnell, M.J. Tetraethylammonium and nicotine transport by the Malpighian tubules of insects. J. Insect Physiol. 2006, 52, 487–498. [Google Scholar] [CrossRef]
- Saremba, B.M.; Murch, S.J.; Tymm, F.J.M.; Rheault, M.R. The metabolic fate of dietary nicotine in the cabbage looper, Trichoplusia ni (Hübner). J. Insect Physiol. 2018, 109, 1–10. [Google Scholar] [CrossRef]
- Ruiz-Sanches, E.; Van Walderveen, M.; Livingston, A.; O’Donnell, M.J. Transepithelial transport of salicylate by the Malpighian tubules of insects from different orders. J. Insect Physiol. 2007, 53, 1034–1045. [Google Scholar] [CrossRef]
- Yang, J.; McCart, C.; Woods, D.J.; Terhzaz, S.; Greenwood, K.G.; Ffrench-Constant, R.H.; Dow, J.A.T. A Drosophila systems approach to xenobiotic metabolism. Physiol. Genom. 2007, 30, 223–231. [Google Scholar] [CrossRef]
- Bogwitz, M.R.; Chung, H.; Magoc, L.; Rigby, S.; Wong, W.; O’Keefe, M.; Mckenzie, J.A.; Batterham, P.; Daborn, P.J. Cyp12a4 confers lufenuron resistance in a natural population of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2005, 102, 12807–12812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terhzaz, S.; Cabrero, P.; Brinzer, R.A.; Halberg, K.A.; Dow, J.A.; Davies, S.A. A novel role of Drosophila cytochrome P450-4e3 in permethrin insecticide tolerance. Insect Biochem. Mol. Biol. 2015, 67, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGettigan, J.; McLennan, R.K.; Broderick, K.E.; Kean, L.; Allan, A.K.; Cabrero, P.; Regulski, M.R.; Pollock, V.P.; Gould, G.W.; Davies, S.A.; et al. Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect Biochem. Mol. Biol. 2005, 35, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Tapadia, M.G. Epithelial immune response in Drosophila Malpighian Tubules: Interplay between Diap2 and ion channels. J. Cell. Physiol. 2014, 229, 1078–1095. [Google Scholar] [CrossRef]
- Zhong, X.-W.; Zou, Y.; Liu, S.-P.; Yi, Q.-Y.; Hu, C.M.; Wang, C.; Xia, Q.-Y.; Zhao, P. Proteomic-based insight into Malpighian Tubules of silkworm Bombyx mori. PLoS ONE 2013, 8, e75731. [Google Scholar] [CrossRef] [Green Version]
- Licent, P.E. Récherches d’anatomie et de physiologie comparées sur le tube digestif des Homoptères superieurs. Cellule 1912, 28, 7–161. [Google Scholar]
- Evans, J.W. Tube-building cercopids (Homoptera: Machaerotidae). Trans. R. Soc. South Aust. 1940, 64, 70–75. [Google Scholar]
- Pesson, P. Sécrétion d’une mucoprotéine par les tubes de Malpighi des larves de Cercopides. Son rôle dans la formation de l’abri spumeux. Boll. Lab. Zool. Gen. Agr. Portici 1956, 33, 341–349. [Google Scholar]
- Kato, K. The origin and composition of the cuckoo spit. Sci. Rep. Saitama Univ. Ser. B 1958, 3, 33–53. [Google Scholar]
- Marshall, A.T. Spittle-production and tube-building by cercopoid nymphs (Homoptera). 1. The cytology of the Malpighian tubules of spittle-bug nymphs. Q. J. Microsc. Sci. 1964, 105, 257–262. [Google Scholar] [CrossRef]
- Marshall, A.T. Protein synthesis and secretion by Malpighian tubules of cercopoid larvae (Homoptera). J. Insect Physiol. 1973, 19, 2317–2326. [Google Scholar] [CrossRef]
- Mello, M.L.S.; Pimentel, E.R.; Yamada, A.T.; Storopoli-Neto, A. Composition and structure of the froth of the spittlebug, Deois sp. Insect Biochem. 1987, 17, 493–502. [Google Scholar] [CrossRef]
- Roversi, P.F.; Baccetti, B. On the ecology and ethology of Haematoloma dorsatum (Ahrens) (Homoptera, Cercopidae). Redia 1994, 77, 133–150. [Google Scholar]
- Turner, J.S. Anomalous water loss rates from spittle nests of spittlebugs Aphrophora saratoga (Homoptera: Cercopidae). Comp. Biochem. Physiol. 1994, 107, 679–683. [Google Scholar] [CrossRef]
- Maksyutova, N.N.; Tarchevsky, I.A.; Yusupova, D.V.; Gvozdeva, E.L.; Valueva, T.A.; Yakovleva, V.G. Enzymatic activity of aphroproteins. Biochemistry 1999, 64, 680–682. [Google Scholar]
- Yamada, H.; Igarashi, Y.; Takasu, Y.; Tsubouchi, K.; Kato, Y. Proteinaceous component in the froth produced by nymphs of the spittle insect Aphrophora intermedia. Int. J. Wild Silkmoth Silk 2003, 8, 25–28. [Google Scholar]
- Del Campo, M.L.; King, J.T.; Gronquist, M.R. Defensive and chemical characterization of the froth produced by the cercopid Aphrophora cribrata. Chemoecology 2011, 21, 1–8. [Google Scholar] [CrossRef]
- Auad, A.M.; Martins, M.F.; Fonseca, I.; Paula-Moraes, S.V.; Kopp, M.M.; Cordeiro, M.C. Spittle protein profile of Mahanarva spectabilis (Hemiptera: Cercopidae) fed various elephant grass genotypes. Genet. Mol. Res. 2012, 11, 3601–3606. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhonga, H.; Yalin, Z.; Wei, C. Comparative morphology of the distal segments of Malpighian tubules in cicadas and spittlebugs, with reference to their functions and evolutionary indications to Cicadomorpha (Hemiptera: Auchenorrhyncha). Zool. Anz. 2015, 258, 54–68. [Google Scholar] [CrossRef]
- Chen, X.; Meyer-Rochow, V.B.; Fereres, A.; Morente, M.; Liang, A.P. The role of biofoam in shielding spittlebug nymphs (Insecta, Hemiptera, Cercopidae) against bright light. Ecol. Entomol. 2018, 43, 273–281. [Google Scholar] [CrossRef]
- Tonelli, M.; Gomes, G.; Silva, W.D.; Magri, N.T.C.; Vieira, D.M.; Aguiar, C.L.; Bento, J.M.S. Spittlebugs produce foam as a thermoregulatory adaptation. Sci. Rep. 2018, 8, 4729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.C.; Shih, H.T.; Lu, K.H. Antifungal effect and chitinase activities of the froth of spittlebug Poophilus costalis (Walker) (Hemiptera: Cercopoidea: Aphrophoridae). J. Asia-Pac. Entomol. 2019, 22, 269–273. [Google Scholar] [CrossRef]
- Marshall, A.T. Spittle-production and tube-building by cercopoid nymphs (Homoptera). 2. The cytology and function of the granule zone of the Malpighian tubules of tube-building nymphs. Q. J. Microsc. Sci. 1964, 105, 415–422. [Google Scholar] [CrossRef]
- Marshall, A.T. Spittle-production and tube-building by cercopoid nymphs (Homoptera). 3. The cytology and function of the fibril zone of the Malpighian tubules of tube-building nymphs. Q. J. Microsc. Sci. 1965, 106, 37–44. [Google Scholar] [CrossRef]
- Marshall, A.T. The chemical nature of Malpighian Tubule mucofibrils in cercopoid dwelling-tubes. J. Insect Physiol. 1968, 14, 1435–1444. [Google Scholar] [CrossRef]
- Marshall, A.T.; Marshall, P.M. The life history of a tube-dwelling cercopoid: Machaerota coronata Maa (Homoptera: Machaerotidae). Proc. R. Entomol. Soc. Lond. A 1966, 41, 17–20. [Google Scholar] [CrossRef]
- Marshall, A.T.; Cheung, W.W.K. Calcification in insects: The dwelling-tube and midgut of machaerotid larvae (Homoptera). J. Insect Physiol. 1973, 19, 963–972. [Google Scholar] [CrossRef]
- Bell, A.J.; Svenson, G.J.; Cryan, J.R. The phylogeny and revised classification of Machaerotidae, the tube-making spittlebugs (Hemiptera: Auchenorrhyncha: Cercopoidea). Syst. Entomol. 2014, 39, 474–485. [Google Scholar] [CrossRef]
- Hamilton, A.K.G. A new tribe and species of Clastopterinae (Hemiptera: Cercopoidea: Clastopteridae) from Africa, Asia and North America. Zootaxa 2015, 3946, 151–189. [Google Scholar] [CrossRef] [Green Version]
- Kleine, R. Cassida nebulosa L. und ihr Fraßbild. Eine biologische Betrachtung. Stett. Entomol. Zeit. 1916, 77, 187–216. [Google Scholar]
- Kleine, R. Cassidenstudien III. Über Cassida rubiginosa Müll. Entomol. Blätt. 1917, 13, 63–73. [Google Scholar]
- Brass, P. Das 10. Abdominalsegment der Käferlarven als Bewegungsorgan. Doctoral dissertation, Konigliche Universitat Greifswald, Würzburg, Greifswald, 1914. [Google Scholar]
- Spiegler, P.E. The origin and nature of the adhesive substance in larvae of the genus Chrysopa (Neuroptera: Chrysopidae). Ann. Entomol. Soc. Am. 1962, 55, 69–77. [Google Scholar] [CrossRef] [Green Version]
- LaMunyon, C.W. Hindgut changes preceding pupation and related cocoon structure in Chrysoperla comanche Banks (Neuroptera, Chrysopidae). Psyche 1988, 95, 203–209. [Google Scholar] [CrossRef] [Green Version]
- LaMunyon, C.W.; Adams, P.A. Use and effect of an anal defensive secretion in larval Chrysopidae (Neuroptera). Ann. Entomol. Soc. Am. 1987, 80, 804–808. [Google Scholar] [CrossRef]
- Swain, R.B. Notes on the oviposition and life-history of the leafhopper Oncometopia undata Fabr. (Homoptera: Cicadellidae). Entomol. News 1936, 47, 264–266. [Google Scholar]
- Tulloch, G.S.; Shapiro, J.E. Brochosomes. Bull. Brooklyn Entomol. Soc. 1953, 48, 57–63. [Google Scholar]
- Tulloch, G.S.; Shapiro, J.E.; Cochran, G.W. The occurrence of ultramicroscopic bodies with leafhoppers and mosquitoes. Bull. Brooklyn Entomol. Soc. 1952, 47, 41–42. [Google Scholar]
- Gouranton, J.; Maillet, P.L. Sur la production de corpuscules lipoprotéiques par les tubes de Malpighi de certains insectes. CR Soc. Biol. 1966, 160, 1724–1726. [Google Scholar]
- Day, M.F. Brochosomes of Australian Cicadelloidea. In Proceedings of the 8th Auchenorrhyncha Congress, Delphi, Greece, 9–13 August 1993; Drosopoulos, S., Petrakis, P.V., Claridge, M.F., de Vrijer, P.W.F., Eds.; 1993; pp. 10–11. [Google Scholar]
- Arzone, A. Brocosomi: Origine, forma, funzione. Atti Accad. Naz. Ital. Entomol. Rendiconti 1986, 34, 59–71. [Google Scholar]
- Navone, P. Origine struttura e funzioni di escreti e secreti entomatici di aspetto ceroso distribuiti sul corpo mediante zampe. Ann. Fac. Sci. Agric. Univ. Torino 1987, 14, 237–294. [Google Scholar]
- Rakitov, R.A. The covering formed by brochosomes on the cuticle of leafhoppers (Homoptera: Cicadellidae). Entomol. Rev. 1995, 74, 90–103. [Google Scholar]
- Rakitov, R.A. Post-moulting behaviour associated with Malpighian tubule secretions in leafhoppers and treehoppers (Homoptera: Membracoidea). Eur. J. Entomol. 1996, 93, 167–184. [Google Scholar]
- Rakitov, R.A. Secretory products of the Malpighian tubules of Cicadellidae (Hemiptera, Membracoidea): An ultrastructural study. Int. J. Insect Morphol. Embryol. 1999, 28, 179–193. [Google Scholar] [CrossRef]
- Rakitov, R.A. Secretion of brochosomes during the ontogenesis of a leafhopper, Oncometopia orbona (F.) (Insecta, Homoptera, Cicadellidae). Tissue Cell 2000, 32, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Rakitov, R.A. What are brochosomes for? An enigma of leafhoppers (Hemiptera, Cicadellidae). Denisia 2002, 4, 411–432. [Google Scholar]
- Rakitov, R.A. Powdering of egg nests with brochosomes and related sexual dimorphism in leafhoppers (Hemiptera: Cicadellidae). Zool. J. Linn. Soc. 2004, 140, 353–381. [Google Scholar] [CrossRef] [Green Version]
- Rakitov, R.A. Chapter 7—Brochosomal coatings of the integument of leafhoppers (Hemiptera, Cicadellidae). In Functional Surfaces in Biology; Gorb, S.N., Ed.; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2009; Volume 1, pp. 113–137. [Google Scholar] [CrossRef]
- Rakitov, R.A. Contamination as the cause of erroneous records of brochosomes. Psyche 2011, 2011, 767963. [Google Scholar] [CrossRef]
- Humphrey, E.C.; Dworakowska, I. The natural history of brochosomes in Yakuza gaunga (Hemiptera, Auchenorrhyncha, Cicadellidae, Typhlocybinae, Erythroneurini). Denisia 2002, 176, 433–454. [Google Scholar]
- de Azevedo Filho, W.S.; Carvalho, G.S. Brochosomes-for-eggs of the Proconiini (Hemiptera: Cicadellidae, Cicadellinae) species associated with orchards of Citrus sinensis (L.) Osbeck in Rio Grande do Sul, Brazil. Neotrop. Entomol. 2005, 34, 387–394. [Google Scholar] [CrossRef]
- Velema, H.P.; Hemerik, L.; Hoddle, M.S.; Luck, R.F. Brochosome influence on parasitisation efficiency of Homalodisca coagulata (Say) (Hemiptera: Cicadellidae) egg masses by Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae). Ecol. Entomol. 2005, 30, 485–496. [Google Scholar] [CrossRef]
- De Azevedo Filho, W.S.; Botton, M.; Paladini, A.; Carvalho, G.S.; Ringenberg, R.; Spotti Lopes, J.R. Egg brochosomes of Proconiini (Hemiptera: Cicadellidae, Cicadellinae) species associated with cultivation of grapevines. Sci. Agric. 2008, 65, 209–213. [Google Scholar] [CrossRef]
- Wyniger, D.; Burckhardt, D.; Mühlethaler, R.; Mathys, D. Documentation of brochosomes within Hemiptera with emphasis on Heteroptera (Insecta). Zool. Anz. 2008, 247, 239–341. [Google Scholar] [CrossRef]
- Rakitov, R.A.; Appel, E. Life history of the camelthorn gall leafhopper, Scenergates viridis (Vilbaste) (Hemiptera, Cicadellidae). Psyche 2012, 2012, 930975. [Google Scholar] [CrossRef]
- Dong, H.; Huang, M. Analysis of the anointing and grooming behavior of several adult insects in Typhlocybinae (Hemiptera: Cicadellidae). J. Insect Behav. 2013, 26, 540–549. [Google Scholar] [CrossRef]
- Rakitov, R.A.; Gorb, S.N. Brochosomal coats turn leafhopper (Insecta, Hemiptera, Cicadellidae) integument to superhydrophobic state. Proc. R. Soc. B 2013, 280, 20122391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakitov, R.A.; Gorb, S.N. Brochosomes protect leafhoppers (Insecta, Hemiptera, Cicadellidae) from sticky exudates. J. R. Soc. Interface 2013, 10, 20130445. [Google Scholar] [CrossRef]
- Lin, M.; Vasseur, L.; Yang, G.; Gurr, G.M.; You, M. Avoidance, escape and microstructural adaptations of the tea green leafhopper to water droplets. Sci. Rep. 2016, 6, 37026. [Google Scholar] [CrossRef] [Green Version]
- Rakitov, R.A.; Moysa, A.A.; Kopylov, A.T.; Moshkovskii, S.A.; Peters, R.S.; Meusemann, K.; Misof, B.; Dietrich, C.H.; Johnson, K.P.; Podsiadlowski, L.; et al. Brochosomins and other novel proteins from brochosomes of leafhoppers (Insecta, Hemiptera, Cicadellidae). Insect Biochem. Mol. Biol. 2018, 94, 10–17. [Google Scholar] [CrossRef]
- Fabre, J.H. Le Capricorne. In Souvenirs Entomologiques: Études sur L’instinct et les mœurs des Insects; Librairie Charles Delagrave: Paris, France, 1891; p. 355. [Google Scholar]
- Boulard, M. Notes sur la biologie larvaire des Cigales (Hom. Cicadidae). Ann. Soc. Entomol. Fr. 1965, 1, 503–522. [Google Scholar]
- Schrader, S. Influence of earthworms on the pH conditions of their environment by cutaneous mucus secretion. Zool. Anz. 1994, 233, 211–219. [Google Scholar]
- Rakitov, R.A. Structure and function of the Malpighian tubules and related behaviours in juveniles Cicadas: Evidence and homology with spittlebugs (Hemiptera: Cicadoidea and Cercopoidea). Zool. Anz. 2002, 241, 117–130. [Google Scholar] [CrossRef]
- Pesson, P.; Foldi, I. Fine structure of the tegumentary glands secreting the protective “shield” in a sessile insect (Homoptera, Diaspididae). Tissue Cell 1978, 10, 389–399. [Google Scholar] [CrossRef]
- Foldi, I. Étude structurale et expérimentale de la formation du bouclier chez les Cochenilles Diaspines (Hom. Coccoidea Diaspididae). Ann. Soc. Entomol. Fr. 1982, 18, 317–330. [Google Scholar]
- Foldi, I. The wax glands in scale insects: Comparative ultrastructure, secretion, function and evolution (Homoptera: Coccoidea). Ann. Soc. Entomol. Fr. 1991, 27, 163–188. [Google Scholar]
- Mello, M.L.S. A mucous secretion in the Malpighian Tubes of a Neotropical Bumblebee, Bombus atratus Franklin. Protoplasma 1979, 99, 147–158. [Google Scholar] [CrossRef]
- Mello, M.L.S. Structure of the cocoon of the Neotropical bumblebee Bombus atratus Franklin. Can. J. Zool. 1982, 60, 1017–1023. [Google Scholar] [CrossRef]
- Mello, M.L.S.; Kerr, W.E. Histochemistry of salivary gland and Malpighian tubule secretions contributing to the cocoon in Plebeia droryana and Scaptotrigona postica Hymenoptera Apoidea. Zool. Anz. 1984, 213, 177–189. [Google Scholar]
- Rozen, J.G., Jr.; Mello, M.L.S. Polarization microscopy and topochemistry of the cocoon of Lithurgus chrysurus (Hymenoptera: Megachilidae). Ann. Entomol. Soc. Am. 2014, 107, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Mello, M.L.S.; dos Anjos, E.H.M.; de Campos Vidal, B.; Rozen, J.G., Jr. Topochemistry, optical anisotropy and FT-IR microspectroscopy of the cocoon of Lithurgus chrysurus (Hymenoptera, Megachilidae). Micron 2016, 90, 87–96. [Google Scholar] [CrossRef]
- Harting, P. Recherches de Morphologie Synthétique sur la Production Artificielle de Quelques Formations Calcaires Organiques; Académie Royale Néerlandaise des Sciences: Amsterdam, The Netherlands, 1873; p. 84. [Google Scholar]
- Keilin, D. On the calcium carbonate and the calcospherites in the Malpighian tubes and the fat body of Dipterous larvae and the ecdysial elimination of these products of excretion. Q. J. Microsc. Sci. 1921, 65, 611–625. [Google Scholar]
- Fraenkel, G.; Hsiao, C. Calcification, tanning, and the role of ecdyson in the formation of the puparium of the facefly, Musca autumnalis. J. Insect Physiol. 1967, 13, 1387–1394. [Google Scholar] [CrossRef]
- Gilby, A.R.; Mckellar, J.W. The calcified puparium of a fly. J. Insect Physiol. 1976, 22, 1465–1468. [Google Scholar] [CrossRef]
- Darlington, M.V.; Meyer, H.J.; Graf, G.; Freeman, T.P. The calcified puparium of the face fly, Musca autumnalis (Diptera:Muscidae). J. Insect Physiol. 1983, 29, 157–162. [Google Scholar] [CrossRef]
- Grodowitz, M.J.; Broce, A.B. Calcium storage in face fly (Diptera: Muscidae) larvae for puparium formation. Ann. Entomol. Soc. Am. 1983, 76, 418–424. [Google Scholar] [CrossRef]
- Grodowitz, M.J.; Broce, A.B.; Kramer, K.J. Morphological and biochemical composition of mineralized granules from the Malpighian tubules of Musca autumnalis de Geer larvae (Diptera: Muscidae). Insect Biochem. 1987, 17, 335–345. [Google Scholar] [CrossRef]
- Krueger, R.A.; Broce, A.B.; Hopkins, T.L. Dissolution of mineralized granules in the Malpighian tubules of the face fly, Musca autumnalis DeGeer, for use in puparium mineralization. J. Insect Physiol. 1987, 33, 255–263. [Google Scholar] [CrossRef]
- Krueger, R.A.; Broce, A.B.; Hopkins, T.L.; Kramer, K.J. Calcium transport from Malpighian tubules to puparial cuticle of Musca autumnalis. J. Comp. Physiol. B 1988, 158, 413–419. [Google Scholar] [CrossRef]
- Herbst, D.B.; Bradley, T.J. A Malpighian tubule lime gland in an insect inhabiting alkaline salt lakes. J. Exp. Biol. 1989, 145, 63–78. [Google Scholar] [CrossRef]
- Mugnano, J.A.; Lee, R.E., Jr.; Taylor, R.T. Fat body cells and calcium phosphate spherules induce ice nucleation in the freeze-tolerant larvae of the gall fly Eurosta solidaginis (Diptera, Tephritidae). J. Exp. Biol. 1996, 199, 465–471. [Google Scholar] [CrossRef]
- de Sinéty, R. Recherches sur la biologie et l’anatomie des Phasmes. Cellule 1901, 19, 117–278. [Google Scholar] [CrossRef]
- Ramsay, J.A. Active transport of water by the Malpighian tubules of the stick insect, Dixippus morosus (Orthoptera; Phasmidae). J. Exp. Biol. 1954, 31, 104–113. [Google Scholar] [CrossRef]
- Ramsay, J.A. The excretory system of stick insect, Dixippus morosus (Orthoptera, Phasmidae). J. Exp. Biol. 1955, 32, 183–199. [Google Scholar] [CrossRef]
- Savage, A.A. The development of the Malpighian tubules of Carausius morosus (Orthoptera). Q. J. Microsc. Sci. 1962, 103, 417–437. [Google Scholar] [CrossRef]
- Monteiro, E.C.; Tamaki, F.K.; Terra, W.R.; Ribeiro, A.F. The digestive system of the “stick bug” Cladomorphus phyllinus (Phasmida, Phasmatidae): A morphological, physiological and biochemical analysis. Arthropod Struct. Dev. 2014, 43, 123–134. [Google Scholar] [CrossRef]
- Shelomi, M.; Kimsey, L.S. Vital staining of the stick insect digestive system identifies appendices of the midgut as novel system of excretion. J. Morphol. 2014, 275, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Tutt, J.W. The Lasiocampids. Proc. Trans. Br. Entomol. Nat. Hist. Soc. 1898, 1899–1900, 1–11. [Google Scholar]
- Dewitz, J. Über die Entstehung der Farbe der Kokons gewisser Lepidopteren (Lasiocampa quercus). Zool. Anz. 1912, 40, 396–399. [Google Scholar]
- Ohnishi, E.A.; Takahashi, S.Y.A.; Sonobe, H.A.; Hayashi, T.B. Crystals from cocoons of Malacosoma neustria testacea. Science 1968, 3829, 783–784. [Google Scholar] [CrossRef]
- Takahashi, S.J.; Suzuki, G.; Ohnishi, E. Origin of oxalic acid in Ca oxalate crystals in the Malpighian tubes of the tent caterpillar, Malacosoma neustria testacea. J. Insect Physiol. 1969, 15, 403–407. [Google Scholar] [CrossRef]
- Naumann, C.M. Rasterelektronenoptische Untersuchungen zur Feinstruktur von Lepidopteren-Gespinsten. Mitteilungen 1977, 67, 27–37. [Google Scholar]
- Mayet, V.M. Une nouvelle function des tubes de Malpighi. Bull. Soc. Entomol. Fr. 1896, 1, 122–127. [Google Scholar] [CrossRef]
- Beeson, C.F.C. The construction of calcareous opercula by longicorn larvae of the group Cerambycini. For. Bull. DehraDun 1919, 38, 1–10. [Google Scholar]
- Schumacher, F. Über einige Fälle von Kalkabscheidung bei Käfern. Deut. Entomol. Z. 1921, 65, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, W.M.; Williams, F.X. The luminous organ of the New Zealand glow-worm. Psyche 1915, 22, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Gatenby, J.B. Notes on the New Zealand Glow-worm, Bolitophila (Arachnocampa) luminosa. Trans. Proc. R. Soc. N. Z. 1959, 87, 291–314. [Google Scholar]
- Ganguly, G. Notes on the histology and anatomy of the larva of Bolitophila (Arachnocampa) luminosa. J. R. Microsc. Soc. 1960, 79, 137–154. [Google Scholar] [CrossRef]
- Richards, A.M. Observations on the New Zealand Glow-worm Arachnocampa luminosa (Skuse) 1890. Trans. Proc. R. Soc. N. Z. 1960, 88, 559–574. [Google Scholar]
- Green, L.B.S. The fine structure of the light organ of the New Zealand glow-worm Arachnocampa luminosa (Diptera: Mycetophilidae). Tissue Cell 1979, 11, 457–465. [Google Scholar] [CrossRef]
- Broadley, R.A.; Stringer, A.N. Prey attraction by larvae of the New Zealand glowworm, Arachnocampa luminosa (Diptera: Mycetophilidae). Invertebr. Biol. 2001, 120, 170–177. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. Glowworms: A review of Arachnocampa spp. and kin. Luminescence 2007, 22, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.H.; Graham, G.C.; Scott, K.D.; Cameron, S.L.; Yeates, D.K.; Merritt, D.J. Distribution and phylogenetic relationships of Australian glow-worms Arachnocampa (Diptera, Keroplatidae). Mol. Phylogenetics Evol. 2008, 48, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.L.; Dearden, P.K.; Gimenez, G.; Krause, K.L. Comparative RNA seq analysis of the New Zealand glowworm Arachnocampa luminosa reveals bioluminescence-related genes. BMC Genom. 2015, 16, 825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trowell, S.C.; Dacres, H.; Dumancic, M.M.; Leitch, V.; Rickards, R.W. Molecular basis for the blue bioluminescence of the Australian glow-worm Arachnocampa richardsae (Diptera: Keroplatidae). Biochem. Biophys. Res. Commun. 2016, 478, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Von Byern, J.; Dorrer, V.; Merritt, D.J.; Chandler, P.; Stringer, I.; Marchetti-Deschmann, M.; McNaughton, A.; Cyran, N.; Thiel, K.; Noeske, M.; et al. Characterization of the Fishing Lines in Titiwai (=Arachnocampa luminosa Skuse, 1890) from New Zealand and Australia. PLoS ONE 2016, 12, e0162687. [Google Scholar] [CrossRef] [Green Version]
- Watkins, O.C.; Sharpe, M.L.; Perry, N.B.; Krause, K.L. New Zealand glowworm (Arachnocampa luminosa) bioluminescence is produced by a firefly-like luciferase but an entirely new luciferin. Sci. Rep. 2018, 8, 3278. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, F. Contribuzione alla conoscenza della metamorfosi e dei costumi della Lebia scapularis Fourc. con descrizione dell’apparato sericiparo della larva. Redia 1904, 2, 68–84. [Google Scholar]
- Lebedew, K. Über die als Sericterien funktionieren den Malpighischen Gefäße der Phytonomus larven. Zool. Anz. 1914, 44, 49–56. [Google Scholar]
- Mazzi, V.; Baccetti, B. Prime osservazioni sui tubi malpighiani e la secrezione di seta in Donus crinitus Boh. Ital. J. Zool. 1956, 23, 137–148. [Google Scholar] [CrossRef]
- Kenchington, W. The larval silk of Hypera spp. (Coleoptera: Curculionidae). A new example of the cross-β protein conformation in an insect silk. J. Insect Physiol. 1983, 29, 355–361. [Google Scholar] [CrossRef]
- Skuhrovec, J.; Bogusch, P. The morphology of the immature stages of Metadonus vuillefroyanus (Capiomont, 1868) (Coleoptera, Curculionidae, Hyperini) and notes on its biology. Zookeys 2016, 589, 123–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, M. Some observations on the biology and anatomy of a cocoon-making Chalcid larva, Euplectrus bicolor Swed. Saert. Vid. Medd. Dansk. Naturh. Foren. 1927, 84, 73–90. [Google Scholar]
- Ferrière, C.H. New species of Euplectrini (Hym. Chalcidoidea) from Europe, Africa and Asia. Bull. Entomol. Res. 1941, 32, 17–48. [Google Scholar] [CrossRef]
- Schauff, M.E.; Janzen, D.H. Taxonomy and ecology of Costa Rican Euplectrus (Hymenoptera: Eulophidae), parasitoids of caterpillars (Lepidoptera). J. Hymenopt. Res. 2001, 10, 181–230. [Google Scholar]
- Bellon, P.P.; Favero, K.; Teixeira Tavares, M.; Nonato De Oliveira, H. First record of Euplectrus floryae (Hymenoptera: Eulophidae) parasitizing Erinnyis ello (Lepidoptera: Sphingidae) in Brazil. Rev. Colomb. Entomol. 2013, 39, 166–167. [Google Scholar]
- Hansson, C.; Smith, M.A.; Janzen, D.H.; Hallwachs, W. Integrative taxonomy of New World Euplectrus Westwood (Hymenoptera, Eulophidae), with focus on 55 new species from Area de Conservación Guanacaste, northwestern Costa Rica. Zookeys 2015, 485, 1–236. [Google Scholar] [CrossRef]
- Jones, D.T. Further notes on the snail-collecting aphis-lion larva (Neuroptera: Chrysopidae). Entomol. News 1941, 52, 39–44. [Google Scholar]
- Tauber, C.A.; Sosa, F.; Albuquerque, G.S.; Tauber, M.J. Adults and larvae of two Leucochrysa (Leucochrysa) species (Neuroptera: Chrysopidae): Descriptions, biological notes, and relationships. Zootaxa 2013, 3750, 101–129. [Google Scholar] [CrossRef] [Green Version]
- Hagen, H. Die Entwicklung und der innere Bau von Osmylus. Linnaea Entomol. 1852, 7, 368–418. [Google Scholar]
- Meinert, F. Contribution à l’anatomie de Fourmilions. In Oversigt over Det Kongelige Danske Videnskabernes Selskabs Forhandlinger; Bianco Lunos Bogtrykkeri: Copenaghen, Denmark, 1889; pp. 43–66. [Google Scholar]
- Anthony, M.H. The Metamorphosis of Sisyra. Am. Nat. 1902, 36, 615–631. [Google Scholar] [CrossRef] [Green Version]
- McDunnough, J. Über den Bau des Darms und seiner Anhänge von Chrysopa perla L. Arch. Naturgesch. 1909, 75, 313–360. [Google Scholar]
- Lozinski, P. Über die Malpighischen Gefäße der Myrmeleonidenlarven als Spinndrüsen. Zool. Anz. 1911, 38, 401–417. [Google Scholar]
- Turner, C.H. Notes on the behavior of the ant-lion with emphasis on the feeding activities and letisimulation. Biol. Bull. 1915, 29, 277–307. [Google Scholar] [CrossRef]
- Withycombe, C.L. XV. Some aspects of the biology and morphology of the Neuroptera. With special reference to the immature stages and their possible phylogenetic significance. Trans. R. Entomol. Soc. Lond. 1925, 72, 303–411. [Google Scholar] [CrossRef]
- Henry, C.S. The behavior and life histories of two North American Ascalaphids. Ann. Entomol. Soc. Am. 1977, 70, 179–195. [Google Scholar] [CrossRef]
- Weisman, S.; Trueman, H.E.; Mudie, S.T.; Church, J.S.; Sutherland, T.D.; Haritos, V.S. An unlikely silk: The composite material of Green Lacewing cocoons. Biomacromolecules 2008, 9, 3065–3069. [Google Scholar] [CrossRef]
- Hamada, N.; Pes, A.M.O.; Fusari, L.M. First record of Sisyridae (Neuroptera) in Rio de Janeiro state, Brazil, with bionomic notes on Sisyra panama. Fla. Entomol. 2014, 97, 281–284. [Google Scholar] [CrossRef]
- Pacheco, C.A.; Alevi, K.C.C.; Silva, T.L.; Azerdo-Oliveira, M.T.V.; Ceron, C.R.; Kobayashi, M.K.H. Nucleolar activity during larval development of Myrmeleon uniformis Navas, 1920 (Neuroptera, Myrmeleontidae). Genet. Mol. Res. 2014, 13, 5154–5158. [Google Scholar]
- Gupta, A.; Badano, D. Larval morphology and life history of Ascalaphus dicax Walker, 1853 (Neuroptera: Myrmeleontidae, Ascalaphinae). Fragm. Entomol. 2021, 53, 1–8. [Google Scholar] [CrossRef]
- Kurdjumov, N.V. Synopsis der Familien nach Nymphenstadien. Poltava Trud. Selisk. Choz. Stancii. 1913, 18, 11–32. [Google Scholar]
- Reijne, A. A cocoon spinning thrips. Tijdschr. Voor Entomol. 1920, 63, 40–45. [Google Scholar]
- Karny, H.H. Beitrage zur Malayischen Thysanopterenfauna. Treubia 1926, 9, 6–10. [Google Scholar] [CrossRef]
- Bailey, S.F. Cocoon-spinning Thysanoptera. Pan-Pac. Entomol. 1940, 16, 77–79. [Google Scholar]
- Bailey, S.F. A review of the genus Ankothrips D. L. Crawford (Thysanoptera). Pan-Pac. Entomol. 1940, 16, 97–106. [Google Scholar]
- Izzo, T.J.; Pinent, S.M.J.; Mound, L.A. Aulacothrips dictyotus (Heterothripidae), the first ectoparasitic thrips (Thysanoptera). Fla. Entomol. 2002, 85, 281–283. [Google Scholar] [CrossRef]
- Conti, B. Notes on the presence of Aeolothrips intermedius in northwestern Tuscany and on its development under laboratory conditions. Bull. Insectol. 2009, 62, 107–112. [Google Scholar]
- Conti, B.; Berti, F.; Mercati, D.; Giusti, F.; Dallai, R. The ultrastructure of Malpighian tubules and the chemical composition of the cocoon of Aeolothrips intermedius Bagnall (Thysanoptera). J. Morphol. 2010, 271, 244–254. [Google Scholar] [CrossRef]
- White, J.; Strehl, C.E. Xylem feeding by periodical cicada nymphs on tree roots. Ecol. Entomol. 1978, 3, 323–327. [Google Scholar] [CrossRef]
- Henneguy, L.F. Note sur l’existence des calcospherites dans les corps graisseux des larves des Dipteres. Arch. Anat. Microsc. 1897, 1, 5–8. [Google Scholar]
- Schellinger, J.N.; Rodan, A.R. Use of the Ramsay assay to measure fluid secretion and ion flux rates in the Drosophila melanogaster malpighian tubule. J. Vis. Exp. 2015, 25, 53144. [Google Scholar] [CrossRef] [Green Version]
- New, T.R. A review of the biology of Neuroptera Planipennia. In Neuroptera International; Association Mondiale des Névroptéristes: Nice, France, 1986; p. 57. [Google Scholar]
- Rudall, K.M.; Kenchington, W. Arthropod silks: The problem of fibrous proteins in animal tissue. Annu. Rev. Entomol. 1971, 16, 73–96. [Google Scholar] [CrossRef]
- Akai, H. The ultrastructure and functions of the silk gland cells of Bombyx mori. In Insect Ultrastructure; King, R.C., Akai, H., Eds.; Springer: Boston, MA, USA, 1984; pp. 323–364. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, P.; Bedini, S.; Conti, B. Multiple Functions of Malpighian Tubules in Insects: A Review. Insects 2022, 13, 1001. https://doi.org/10.3390/insects13111001
Farina P, Bedini S, Conti B. Multiple Functions of Malpighian Tubules in Insects: A Review. Insects. 2022; 13(11):1001. https://doi.org/10.3390/insects13111001
Chicago/Turabian StyleFarina, Priscilla, Stefano Bedini, and Barbara Conti. 2022. "Multiple Functions of Malpighian Tubules in Insects: A Review" Insects 13, no. 11: 1001. https://doi.org/10.3390/insects13111001
APA StyleFarina, P., Bedini, S., & Conti, B. (2022). Multiple Functions of Malpighian Tubules in Insects: A Review. Insects, 13(11), 1001. https://doi.org/10.3390/insects13111001








